Abstract
Objective
CRP is a serum pattern recognition molecule that binds to apoptotic cells and nucleoprotein autoantigens and FcγR. In SLE IC containing nucleoprotein autoantigens activate plasmacytoid dendritic cells (pDC) to produce type I IFN, which contributes to disease pathogenesis. Autoantibody IC are taken up by pDC through FcγRIIa into endosomes where the nucleic acid components activate TLR7 or TLR9. The objective of this study was to investigate the effect of CRP on pDC and monocyte responses to nucleoprotein autoantigens and IC.
Methods
Peripheral blood mononuclear cells (PBMC), purified monocytes and pDC were isolated from healthy volunteers and stimulated with autoantibody IC containing apoptotic cells, snRNPs, or DNA or direct TLR7 and TLR9 agonists. Supernatants were analyzed for IFN-α and cytokines by ELISA and multiplex assays. snRNPs were fluorescently labeled and the effect of CRP on binding, uptake and intracellular localization of autoantibody snRNP complexes was measured by flow cytometry and confocal microscopy.
Results
CRP bound to autoantigen did not induce IFN-α in PBMC or pDC, whereas complexes formed with autoantibody did. Significantly, CRP inhibited the IFN-α response to both α-U1 RNP-snRNPs and α-DNA-DNA, but not to other TLR7 and TLR9 agonists. CRP directly inhibited pDC IFN-α release, promoted pDC differentiation, and increased late endosome localization of autoantigen in pDC and monocytes.
Conclusion
CRP is a regulator of the type I IFN response to SLE IC. CRP increased the intracellular processing of IC in late endosomes, which is associated with decreased synthesis of type I IFN after intracellular TLR activation.
Plasmacytoid dendritic cells (pDC) play an important role in host defense against viral infection by producing large quantities of type I IFN (including IFN-α, IFN-β and other subtypes that bind to the IFN-α receptor, IFNAR) (1, 2). Production of type I IFN by pDC is triggered by recognition of viral nucleic acids through intracellular sensors located in the cytoplasm and in endosomes. In the pDC endosomal pathway TLR7 recognizes RNA and TLR9 recognizes DNA resulting in MyD88-dependent signaling that culminates in the phosphorylation of interferon regulatory factor (IRF) 7, and transcription of type I IFN and other genes (2, 3). The intracellular sequestration of TLR7 and TLR9 prevents activation of this pathway by self RNA and DNA. However, activation can occur when IC containing nucleoprotein antigens derived from apoptotic cells are taken up by pDC through FcγRIIa (4, 5).
The generation of autoantibodies against nucleoprotein autoantigens and subsequent formation of IC is characteristic of SLE. The majority of patients with SLE show an IFN signature of gene expression in their peripheral blood cells as the result of chronic overproduction of type I IFN and activation of IFN-inducible genes (6, 7). The IFN signature of gene expression is associated with recent onset of SLE and with a more severe clinical picture including renal and hematological disease (8). Type I IFN stimulates other immune cells including monocytes, dendritic cells, NK cells, T and B lymphocytes and it has been postulated that the activation of pDC by SLE IC may set up a self-perpetuating cycle of autoimmunity (7, 9). This makes the type I IFN pathway an attractive therapeutic target for SLE and several other autoimmune diseases (10).
CRP is a soluble pattern recognition molecule and a major acute phase serum protein (11). Serum levels of CRP increase from less than 5 μg/ml at baseline to several hundred μg/ml following trauma, infection or inflammation. The majority of circulating CRP is produced in the liver in response to IL-6. Structurally CRP is a cyclic pentamer, which binds to microbial and host ligands through five identical calcium-dependent binding sites on one face. The other face interacts with the immune system through FcγR and FcαRI on cells (12) or C1q of the classical complement pathway. CRP ligands include apoptotic cells and protein components of the major SLE autoantigens, snRNPs and chromatin (13-15), as well as microbial polysaccharides and phospholipids. We and others have proposed that CRP contributes to the non-inflammatory removal of dead and damaged cells and prevents immunization with nucleoprotein autoantigens. Although there are no known CRP deficiencies or variations in amino acid sequence, a number of polymorphisms affect levels of CRP and single nucleotide polymorphisms (SNPs) in the CRP gene have been associated with SLE risk (16-19). In addition, IFN-α suppresses CRP synthesis (20) and CRP levels are inappropriately low in SLE flares in the absence of serositis or infection (21, 22).
CRP administered by injection was first shown to have therapeutic benefit in chromatin-accelerated disease in the (NZB × NZW) F1 female mouse (23). Subsequently CRP expressed from a transgene or injected was found to prolong survival and decrease renal disease in the spontaneous (NZB × NZW) F1 female and the MRL/MpJ-Faslpr mouse models of SLE (24-26). The most pronounced effect of CRP in these models was the prevention and suppression of proteinuria and glomerulonephritis. Experiments in other autoimmune and IC disease models including nephrotoxic nephritis, immune thrombocytopenia and experimental autoimmune encephalomyelitis implicate macrophages, FcγR, and IL-10 as key elements in CRP suppression of autoimmune disease (24, 27, 28). Effects of CRP on the type I IFN pathway have not been reported previously.
In this study we examined the effect of CRP on the IFN-α response of human peripheral blood mononuclear cells (PBMC) to IC containing nucleoprotein autoantigens. The results demonstrate a regulatory role for CRP in the type I IFN pathway and provide an additional mechanism for the therapeutic effects of CRP in autoimmune disease models.
MATERIALS AND METHODS
Reagents
Purified snRNPs and serum containing autoantibodies were generously provided by Dr. Rufus Burlingame (Inova Diagnostics). Type A CpG oligodeoxynucleotide (ODN 2216, CpG-A) and endotoxin-free E. coli DNA were purchased from Invivogen. Inactivated influenza A (strain Texas H3N2) was purchased from Microbix Biosystems. IgG was purified from sera containing autoantibodies to U1 RNP or dsDNA on protein G Sepharose and used at a concentration of 200 μg/ml. Only trace amounts of C1q (0.01%) were present in the IgG preparations. snRNPs were labeled with Alexa Fluor (AF) 488 or AF647 using Microscale Protein Labeling Kits from Invitrogen according to the manufacturer’s protocols. Labeled snRNPs combined with α-U1 RNP induced IFN-α in the assay described below.
The following antibodies were used: anti-FcγRIIa (CD32, clone IV.3, Stem Cell Technologies); phycoerythrin (PE) and AF488 anti-LAMP-1, PerCP-Cy5.5 anti-CD123, allophycocyanin (APC) and fluorescein isothiocyanate (FITC) anti-CD14 (BD Biosciences); PE anti-CD303 (Miltenyi); PE anti-ILT-7, PE anti-CD80, AF488 anti-CD86 (eBioscience); horseradish peroxidase (HRP) goat anti-human IgG (Jackson Immunoresearch); HRP goat anti-human CRP (Bethyl Laboratories). AF488 transferrin was from Invitrogen.
CRP was purified from pleural fluid by affinity and ion exchange chromatography, as previously described (29). Purified CRP ran as a single band on overloaded SDS-PAGE gels and contained < 1 unit of endotoxin per mg of protein.
Cells
All human studies were in compliance with the Helsinki Declaration and were approved by the Institutional Review Board at the University of New Mexico Health Science Center. Blood was drawn from healthy volunteers into heparinized tubes and PBMC were isolated on Ficoll-Paque PLUS (GE Healthcare). Monocytes were purified from PBMC using positive selection on anti-CD14 magnetic beads (Miltenyi). Purity was >95% determined using PE anti-CD14. pDC were purified from PBMC by negative selection followed by positive selection on anti-BDCA4 magnetic beads (Miltenyi, Diamond pDC kit). Purity of pDC was >90% determined by flow cytometry with PerCP-Cy5.5 anti-CD123 and either PE anti-CD303 or PE anti-ILT7.
The U-937 cell line, derived from a histiocytic leukemia, was obtained from the ATCC and grown in RPMI with 5% fetal bovine serum. Apoptosis was induced in U-937 cells by exposure to UV for 10 min followed by 4 h culture (30). The cells were stained with FITC annexin and propidium iodide, and found to be >60% annexin positive and <10% propidium iodide positive, indicating that the majority were apoptotic.
Cytokine and IFN-α Assays
PBMC were cultured at 2 × 105 cells per 100 μl well in triplicate in RPMI with 5% fetal bovine serum and 2 mM CaCl2. CRP, IC, and TLR agonists were added at the following concentrations: CRP (100 – 200 μg/ml); α-U1 RNP sera (2 - 12.5%); IgG α-U1 RNP and IgG α-DNA (200 μg/ml); apoptotic U-937 cells (5 × 104 per 100 μl well); snRNPs (5 μg/ml); CpG (5 μg/ml); DNA (5 μg/ml); influenza virus (1 pg/ml RNA). Supernatants were collected after 20 h for cytokine assays. IFN-α was measured by ELISA (R&D Systems). TNF-α, and IL-10 were measured using OptEIA kits (BD Bioscience). Cytokines (IFN-γ, IL-1β, IL-6, IL-10, IL-12, IL-1RA, MCP-1, and IL-12) were also measured using Multiplex assays (Milliplex, Millipore). pDC were cultured at 1 – 2 × 105 per 100 μl well in round bottom plates with 10 ng/ml IL-3 (PeproTech) added for overnight cultures.
Anti-DNA ELISA
Antibody and CRP binding to DNA and CpG was tested by ELISA. Microtiter wells were coated with 2 μg/ml poly(Lys,Phe) (Sigma Aldrich), washed and incubated overnight with 10 μg/ml bacterial DNA or CpG-A. After blocking with 0.1% gelatin, wells were incubated with serum, IgG or CRP, washed, and developed with HRP anti-IgG or HRP anti-CRP and substrate (TMB substrate reagent, BD Bioscience).
Flow Cytometry
To analyze binding of IC, PBMC were incubated for 1 h on ice with AF488 or AF647 snRNPs (5 μg/ml), CRP (200 μg/ml) and α-U1 RNP (IgG) (200 μg/ml) in PAB (PBS containing 0.05% sodium azide, 0.1% BSA). Incubation mixtures included APC or FITC anti-CD14 to identify monocytes and PerCP-Cy5.5 anti-CD123 together with either PE anti-CD303 or PE anti-ILT7 to identify pDC. Cells were fixed in 1% paraformaldehyde and binding of snRNPs to monocytes and pDC was analyzed using an LSR Fortessa flow cytometer (BD Bioscience) and FlowJo Software (Tree Star). To assess differentiation, purified pDC were incubated for 20 h in culture medium with snRNPs (5 μg/ml), and CRP (200 μg/ml), α-U1 RNP (IgG) (200 μg/ml) or both, washed and stained with PE anti-CD80 and AF488 anti-CD86. Expression of CD80 and CD86 were measured by flow cytometry.
Confocal Microscopy
Purified monocytes or pDC were incubated for 30 min or 1 h at 37° with AF647 snRNPs (5 μg/ml), and CRP (200 μg/ml), α-U1 RNP (IgG) (200 μg/ml) or both. To determine early endosome colocalization, AF488 transferrin (50 μg/ml) was added for the last 10 min of incubation (31). After the incubation cells were adhered to poly-L-lysine coated coverslips for 15 min, washed with PBS, stained with 4′, 6-diamidino-2-phenylindole (DAPI) and mounted with Prolong Gold Anti-Fade Reagent (Invitrogen). For late endosome/lysosome colocalization cells were allowed to adhere to poly-L-lysine coated coverslips for 15 min, washed with PBS, fixed with 1% paraformaldehyde in PBS, permeabilized with 0.25% saponin, 1% BSA in PBS. Samples were blocked using Image-iT FX signal enhancer (Invitrogen) and stained with AF488 anti-LAMP-1 in 0.25% saponin, 1% BSA in PBS, followed by DAPI and mounted with Prolong Gold Anti-Fade Reagent (32). Cells were imaged using a Zeiss LSM510 META Confocal microscope and images were analyzed for colocalization using SlideBook software. At least 50 monocytes and at least 30 pDC were analyzed for each treatment in each experiment.
Statistical analysis
Graphing and data analysis were done using Prism v6.0 (GraphPad Software). Two-tailed t-tests or one-way ANOVA with Bonferroni’s multiple comparison correction were used to compare means. Differences with a p-value < 0.05 were considered significant.
RESULTS
CRP inhibits the IFN-α response to α-U1 RNP IC
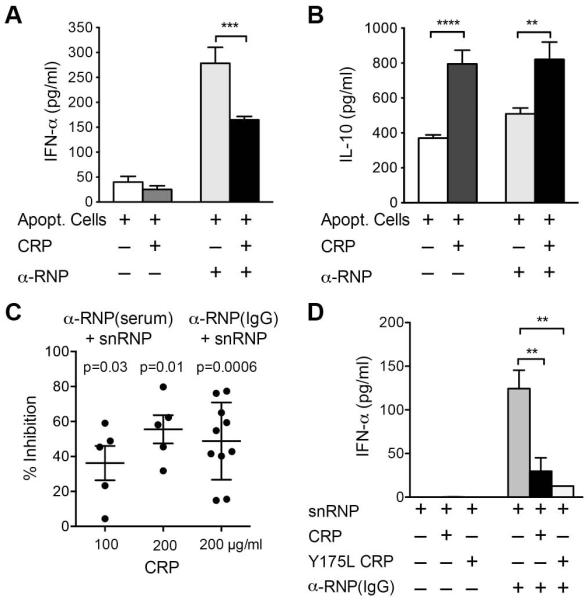
CRP binds to the 70K and SmD proteins of snRNPs and, when used to stain HEp-2 cells, CRP produces a nuclear staining pattern similar to that of α-U1 RNP antibody (13). CRP also binds to membrane phospholipids on late apoptotic and necrotic cells (15, 33). We used apoptotic U-937 cells, as other investigators have used these as a source of interferogenic autoantigen (30). By flow cytometry, CRP bound to apoptotic U-937 cells with saturation at 50 μg/ml (data available upon request from the author). We compared the IFN-α response of PBMC stimulated with apoptotic U-937 cells together with CRP, α-U1 RNP serum or both (Figure 1A). IFN-α was produced in response to α-U1 RNP and apoptotic cells as previously reported (30). In an additional experiment only 9 pg/ml IFN-α and 62 pg/ml IL-10 were produced in response to α-U1 RNP serum in the absence of apoptotic cells. CRP either in the presence or absence of apoptotic cells did not induce IFN-α. Notably, CRP significantly inhibited IFN-α release by cells stimulated with α-U1 RNP and apoptotic cells. In dose response studies CRP inhibition was similar at 50, 100 and 200 μg/ml consistent with the observed saturation of CRP binding to apoptotic cells at 50 μg/ml. CRP, in the presence of apoptotic cells, also increased IL-10 (Figure 1B).
Figure 1.
CRP inhibits the IFN-α response to IC formed with α-U1 RNP. For all panels PBMC were incubated 20 h, and cytokines were measured in supernatants by ELISA. A and B. PBMC were incubated with apoptotic U-937 cells, CRP (100 μg/ml) and α-U1 RNP (12.5% serum). IFN-α and IL-10 were measured. Means ± SEM of triplicate wells are shown for 1 representative of 4 experiments. ** p < 0.01, *** p < 0.001, **** p < 0.0001. Less than 1 pg/ml IFN-α and IL-10 were detected in supernatants from cells incubated with CRP alone. C. PBMC were incubated with snRNPs (5 μg/ml), CRP (100 or 200 μg/ml) and α-U1 RNP (2% serum or 200 μg/ml IgG). IFN-α was measured. Results shown are the % inhibition of IFN-α in cultures with CRP and α-U1 RNP-snRNPs compared to α-U1 RNP-snRNPs alone for 5-10 experiments with different donors. p-values were determined by paired t tests on log-transformed data. D. PBMC were incubated with snRNPs, CRP (200 μg/ml), recombinant mutant CRP (200 μg/ml Y175L CRP) and α-U1 RNP (200 μg/ml IgG). IFN-α was measured by ELISA. Means ± SEM of triplicate wells are shown for 1 representative of 3 experiments. ** p < 0.01.
We next examined the effect of CRP on the response to IC prepared with purified snRNPs. We tested IFN-α release from PBMC stimulated with snRNPs and two different α-U1 RNP sera and found a substantial response using 2 - 12.5% serum or 200 μg/ml purified IgG. The IFN-α response to α-U1 RNP-snRNPs was eliminated by RNase treatment consistent with a TLR7-dependent pathway. No IFN-α was produced by PBMC incubated with snRNPs and serum from healthy control subjects (data available upon request from the author). We showed by surface plasmon resonance that both CRP and α-U1 RNP IgG bound to snRNPs and that there was no competition for binding (data available upon request from the author). Similar to the results using apoptotic cells, adding CRP to α-U1 RNP-snRNPs inhibited the IFN-α response. Figure 1C shows the percent inhibition seen in 10 experiments with 5 different donors, which were 36.2 ± 9.9 for 100 μg/ml CRP and 55.5 ± 8.1 for 200 μg/ml CRP with α-U1 RNP serum. In dose response studies, 50 μg/ml was the minimum inhibitory concentration of CRP. These concentrations of CRP are within the range found during the acute phase response.
Initial experiments were done using IC made with serum as an autoantibody source. Since C1q in serum has been shown by others to inhibit IFN-α responses to SLE IC (34, 35), we tested the requirement for C1q or other serum components by purifying IgG from α-U1 RNP serum. We found that CRP was equally inhibitory for IC made with purified IgG (percent inhibition 48.8 ± 7.0 for 200 μg/ml CRP and α-U1 RNP IgG) (Figure 1C). The lack of involvement of C1q was confirmed by showing inhibition of IFN-α induction with a recombinant mutant CRP (Y175L) that does not bind C1q (Figure 1D) (36).
The culture supernatants from 3 experiments using 200 μg/ml CRP and α-U1 RNP IgG were analyzed for 8 additional cytokines using a multiplex assay (data available upon request from the author). Cytokine responses to α-U1 RNP (IgG)-snRNPs complexes were higher than to CRP-snRNP complexes, and included both pro-inflammatory and anti-inflammatory cytokines. When CRP was added with α-U1 RNP (IgG)-snRNPs, there was increased release of proinflammatory cytokines TNF-α (2.1 ± 1.7 fold), IL-1β (2.2 ± 1.3 fold), IL-6 (1.4 ± 0.6 fold) as well as the anti-inflammatory cytokine IL-10 (1.7 ± 0.7 fold). MCP-1 and IL-1RA were produced, but adding CRP did not change levels. IFN-γ and IL-12 levels were very low. Fold increases represent the mean ± SEM from 3 donors tested in triplicate wells.
CRP inhibits the IFN-α response to α-DNA IC, but not to CpG
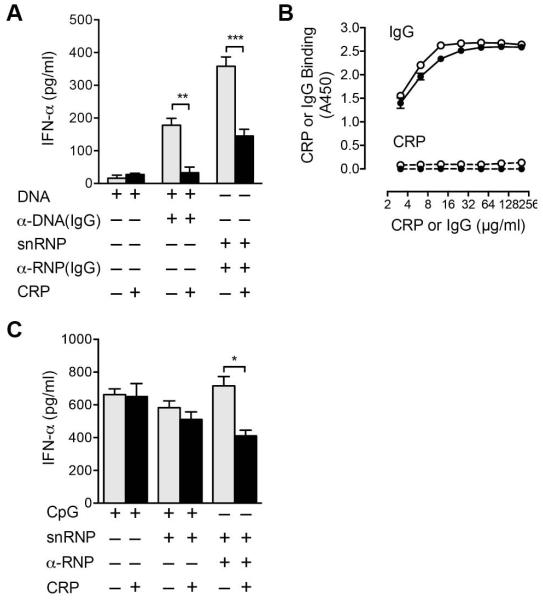
We next analyzed whether CRP inhibition of the IFN-α response was restricted to α-U1 RNP IC. IgG was purified from serum containing α-DNA and used to form complexes with bacterial DNA. At this concentration of DNA, minimal IFN-α was produced in the absence of antibody (Figure 2A). PBMC stimulated with α-DNA-DNA induced IFN-α, and CRP inhibited the response (Figure 2A). CRP did not bind to bacterial DNA, α-DNA (IgG)-DNA IC or the TLR9 agonist, CpG-A. In contrast to the results using IC, CRP did not inhibit IFN-α production in response to CpG-A, even in the presence of snRNPs as a ligand for CRP (Figure 2C). CRP inhibited the IFN-α response to influenza virus, a TLR7 agonist an average of 28 ± 5% in three experiments. These results indicate that the inhibitory effect of CRP is selective for IC, including both α-U1 RNP and α-DNA complexes characteristically found in SLE.
Figure 2.
CRP inhibition of the IFN-α response is selective for IC. For panels A and C, PBMC were incubated 20 h, and IFN-α was measured in supernatants by ELISA. A. PBMC were incubated with bacterial DNA (5 μg/ml), α-DNA (200 μg/ml IgG), α-U1 RNP (200 μg/ml IgG), snRNPs (5 μg/ml), and CRP (200 μg/ml). Means ± SEM of triplicate wells are shown for 1 representative of 3 experiments. ** p < 0.01, *** p < 0.001. B. Dilutions of IgG α-DNA (solid lines) and CRP (dashed lines) were added to wells coated with bacterial DNA (open symbols) or CpG-A (closed symbols) and binding measured by ELISA. Means of duplicate wells are shown for 1 representative of 3 experiments. C. PBMC were incubated with CpG-A (5 μg/ml), snRNPs (5 μg/ml), α-U1 RNP (200 μg/ml IgG), and CRP (200 μg/ml). IFN-α was measured. Means ± SEM of triplicate wells are shown for 1 representative of 4 experiments. * p < 0.05.
CRP acts directly on pDC
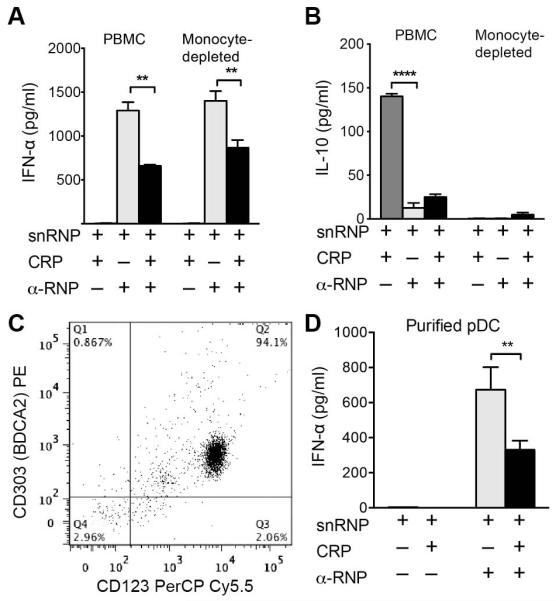
PBMC cultures stimulated with α-U1 RNP-snRNPs produced pro-inflammatory and anti-inflammatory cytokines. Several of these cytokines, particularly TNF-α and IL-10, which were increased by CRP, can inhibit pDC production of IFN-α (30). To determine if CRP inhibition was mediated by monocyte cytokines, we depleted PBMC of monocytes using α-CD14 magnetic beads prior to IC stimulation and analyzed IFN-α (Figure 3A), IL-10 (Figure 3B), and TNF-α (data available upon request from the author) in supernatants. Monocyte-depleted cultures did not release IL-10 or TNF-α, but IFN-α was still present and the amount of IFN-α was decreased in the presence of CRP.
Figure 3.
CRP acts directly on pDC to inhibit the IFN-α response to IC. For panels A, B, and D cells were incubated 20 h, and cytokines in supernatants were measured by ELISA. A. and B. PBMC or PBMC depleted of monocytes using α-CD14 magnetic beads were incubated with snRNPs (5 μg/ml), CRP (100 μg/ml) and α-U1 RNP (12.5% serum). IFN-α and IL-10 were measured. Means ± SEM of triplicate wells are shown for 1 representative of 4 experiments. ** p < 0.01, **** p < 0.0001. C. pDC were purified by negative and positive selection with magnetic beads. The resulting cells were >94% pDC shown by double staining for CD123 and CD303. D. Purified pDC were incubated with snRNPs (5 μg/ml), CRP (200 μg/ml) and α-U1 RNP (200 μg/ml IgG) for 20 h. IFN-α was measured in supernatants by ELISA. Means ± SEM of triplicate wells are shown for 1 representative of 4 experiments. ** p < 0.01.
To determine if CRP acted directly on pDC, we purified pDC from PBMC using a two-step magnetic bead procedure. The purified cells were 94% pDC by two color staining with α-CD123 and α-CD303 (Figure 3C). CRP inhibited the IFN-α response of purified pDC to α-U1 RNP (IgG)-snRNPs (Figure 3D). In four experiments using purified pDC from different donors, CRP inhibited the IFN-α response to α-U1 RNP (IgG)-snRNP IC by 61.3 ± 14.8 %.
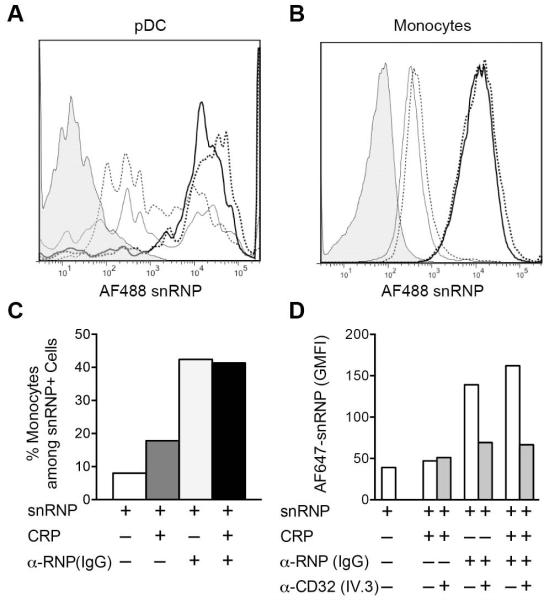
CRP does not block IC binding to pDC or monocytes
FcγRIIa is required for IC uptake by pDC and is also a CRP receptor (4, 29, 37). To determine if CRP was blocking the binding of α-U1 RNP (IgG)-snRNP IC to cells, we fluorescently-labeled snRNPs and analyzed binding to monocytes and pDC by flow cytometry. These experiments were done with PBMC to evaluate whether CRP would alter the distribution of IC, as diversion of IC from pDC to monocytes has been shown to play a role in C1q inhibition of the IFN-α response (34). The results indicate relatively weak binding of CRP-snRNP complexes to both pDC and monocytes. Significantly, CRP did not inhibit the binding of α-U1 RNP (IgG)-snRNP complexes to either monocytes or pDC (Figure 4A and B), and did not change the % of monocytes among the cells that had bound AF488 snRNPs (Figure 4C). We then determined if the presence of CRP changed the receptor used for binding IC. PBMC were incubated with labeled complexes in the presence of the anti-FcγRIIa blocking mAb IV.3. α-U1 RNP (IgG)-snRNP IC binding was blocked by anti-FcγRIIa nearly to the level of snRNPs alone regardless of the presence of CRP, indicating that CRP does not divert IC to a different receptor (Figure 4D).
Figure 4.
CRP does not block IC binding to FcγRIIA on monocytes or pDC. PBMC were incubated for 1 h on ice with AF488 or AF647 snRNPs (5 μg/ml), CRP (200 μg/ml) and α-U1 RNP (200 μg/ml IgG). Cells were stained for monocyte (CD14) and pDC (CD123, CD303) markers, and analyzed by flow cytometry. A and B. Cells from each treatment group were gated on pDC (A) or monocytes (B) and analyzed for AF488 snRNPs binding. Histograms represent the following treatments: untreated cells (shaded); snRNPs only (light dotted line); CRP + snRNPs (light solid line); α-U1 RNP (IgG) + snRNPs (heavy dotted line); CRP + α-U1 RNP (IgG) + snRNPs (heavy solid line). C. AF488 snRNPs positive cells were gated and the percent CD14+ monocytes was determined for the different treatments. D. Inhibition of AF647 snRNPs binding to cells by anti-FcγRIIa blocking mAb IV.3. Cells were incubated with AF647 snRNPs (5 μg/ml), CRP (200 μg/ml) and α-U1 RNP (200 μg/ml IgG) for 60 min on ice in the presence and absence of 5 μg/ml anti-FcγRIIa blocking antibody. snRNPs binding is shown as the geometric mean fluorescence intensity (GMFI).
CRP increases trafficking of IC to late endosomes in monocytes
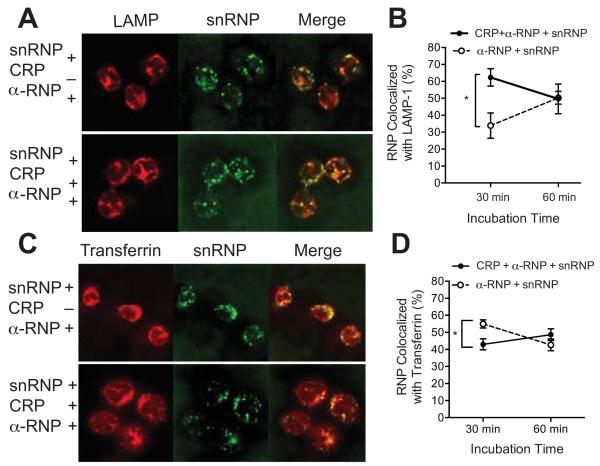
Activation of TLR7 occurs in endosomes so we next examined the effect of CRP on processing of IC. We used confocal microscopy to study the intracellular localization of AF647 snRNPs in complex with α-U1 RNP (IgG) in the presence and absence of CRP. Purified monocytes were incubated for 30 or 60 min with AF647 snRNPs and CRP, α-U1 RNP (IgG) or both. Cells were either fixed, permeabilized and stained for LAMP-1, a late endosome/lysosome marker or AF488 transferrin was added during the last 10 min of incubation to label early recycling endosomes. The results show that after 30 min of incubation with α-U1 RNP (IgG)-snRNPs, CRP increased colocalization of snRNPs with LAMP-1 (Figure 5A and B) and decreased colocalization of snRNPs with transferrin (Figure 5C and D). By 60 min the distribution of snRNPs was not significantly affected by CRP.
Figure 5.
CRP alters intracellular localization of IC in monocytes. Purified monocytes were incubated with AF647 snRNPs (5 μg/ml), CRP (200 μg/ml) and α-U1 RNP (200 μg/ml IgG) for 30 min at 37°, attached to coverslips, stained and analyzed by confocal microscopy. Images show LAMP-1 or transferrin (red), snRNPs (green) and the merged image (yellow). A. Monocytes were fixed, permeabilized and stained with AF488 α-LAMP-1. C. AF488 transferrin was added for the last 10 min of incubation. B and D. Analysis of colocalization of snRNPs with LAMP-1 (B) or transferrin (D) after 30 or 60 min incubation. Mean ± SEM for multiple images. At least 50 cells were analyzed for each. Results are representative of 3 experiments. * p < 0.05.
CRP increases trafficking of IC to late endosomes in pDC and increases pDC differentiation
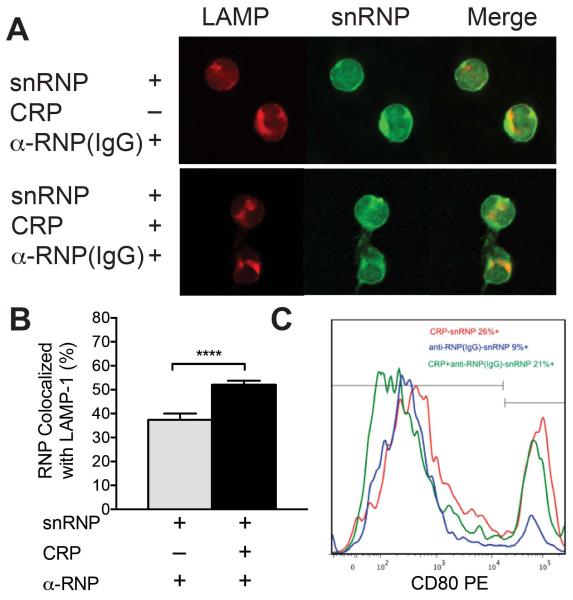
Stimulation of TLR7 or TLR9 can activate two different signaling pathways in pDC, and intracellular localization of the agonist is a primary determinant of which response dominates (32, 38). Early endosome localization stimulates a strong IFN-α response through IRF7 activation, whereas localization to late endosomes activates a nuclear factor κB (NF-κB)-dependent pathway and induces differentiation of pDC. Similar to the results seen with monocytes, pDC incubated with CRP and α-U1 RNP (IgG)-snRNPs showed increased colocalization of snRNPs with LAMP-1 compared to IC alone (Figure 6A and B). We also analyzed expression of pDC maturation markers, CD80 and CD86 after incubation of purified pDC with CRP-snRNPs, α-U1 RNP (IgG)-snRNP complexes or both. The expression of CD80 (Figure 6C) and CD86 (data available upon request from the author) was increased in cells incubated with CRP-snRNPs or CRP with α-U1 RNP (IgG)-snRNPs compared to cells incubated with IC alone.
Figure 6.
CRP alters intracellular localization of IC and increases expression of maturation markers in pDC. A and B. Purified pDC were incubated with AF647 snRNPs (5 μg/ml), CRP (200 μg/ml) and α-U1 RNP (IgG) (200 μg/ml) for 30 min at 37°, attached to coverslips, stained and analyzed by confocal microscopy. A. Images show LAMP-1 (red), snRNPs (green) and the merged image (yellow). B. Analysis of colocalization of snRNPs with LAMP-1 after 30 min incubation. Mean ± SEM for multiple images. At least 30 cells were analyzed for each. Results are representative of 3 experiments. **** p < 0.0001. C. Purified pDC were cultured with snRNPs (5 μg/ml), CRP (200 μg/ml) and α-U1 RNP (IgG) (200 μg/ml) overnight. pDC were stained for CD80 expression and analyzed by flow cytometry. Histograms of pDC expression of CD80 after 20 h culture with CRP + snRNPs (red), α-U1 RNP (IgG) + snRNPs (blue) or CRP + α-U1 RNP (IgG) + snRNPs (green).
DISCUSSION
In this study we examined the effect of CRP on the IFN-α response of human PBMC to IC containing nucleoprotein autoantigens. We demonstrated that CRP acts directly on pDC to inhibit the induction of IFN-α by autoantibody IC containing RNA or DNA. CRP inhibited production of IFN-α in response to these IC but had no significant effect on the response to other IFN inducers. The intracellular compartment in which ligand binding to TLR7 and TLR9 occurs leads to differential activation of IRF7-dependent type I IFN synthesis or NF-κB dependent cytokine synthesis and pDC differentiation (32, 38). Prolonged localization of TLR ligands in early endosomes is required for activation of the IRF7 pathway. Late endosomal localization of TLR ligands results in activation of NF-κB. CRP altered the processing of interferogenic IC in both monocytes and pDC leading to more rapid localization of autoantigen in late endosomal compartments. Consistent with this, CRP increased pDC expression of maturation markers and monocyte cytokines. Type I IFN produced by pDC has a positive feedback effect on autoantibody generation and the activation of this pathway by IC contributes to pathogenesis in SLE (9, 10, 39). CRP-mediated inhibition of this pathway could be of therapeutic benefit in SLE.
CRP is a pentameric soluble pattern recognition molecule with five calcium-dependent binding sites (11). CRP binds to snRNPs and chromatin, two major nucleoprotein autoantigens in SLE (13, 40). We expected to find an effect of CRP on responses to snRNPs, because CRP binds to the 70K and SmD proteins of snRNPs. However, CRP not only inhibited IFN-α induced by α-U1 RNP-snRNP complexes, but also inhibited IFN-α release induced by α-DNA(IgG)-DNA complexes. CRP did not bind to the bacterial DNA used in these experiments or to α-DNA(IgG)-DNA complexes. A recent report found that phosphocholine-specific T15 IgM antibody inhibited cytokine responses of mouse bone marrow-derived DC to IC containing RNA or chromatin IC and also to CpG (41). Although CRP has the same specificity as T15 anti-phosphocholine antibodies, it differs from IgM in that CRP binds to FcγR (42). CRP was not generally suppressive as it did not significantly inhibit IFN-α responses to CpG, a TLR9 agonist or influenza virus, a TLR7 agonist. These results indicate that CRP inhibition does not act at the level of TLR mobilization, processing, activation or the signaling pathways downstream of TLR that result in IFN-α synthesis and release.
CRP and IgG both bind to FcγRIIa, and blocking antibody to FcγRIIA or nonspecific IgG can inhibit IFN-α synthesis by blocking uptake of autoantibody IC (4). We showed that CRP did not block binding of α-U1 RNP (IgG)-snRNP complexes to either pDC or monocytes, and did not alter the distribution of the IC between pDC and monocytes. The complement protein C1q, which has a strong genetic linkage with SLE, was shown in one study to be a non-specific inhibitor of pDC responses to TLR7 and TLR9 agonists (35). In another study, C1q was shown to inhibit pDC responses by increasing monocyte uptake of IgG-RNP complexes (34). CRP, like IgG, binds to C1q and activates the classical complement pathway (43). However, our results showed that C1q was not required for CRP-mediated suppression. The same amount of inhibition of IFN-α was found using purified IgG or autoantibody-containing serum, and a CRP mutant that does not bind C1q was equally effective at suppressing the response (36). Thus, although CRP has the potential to recruit C1q, it also has a direct inhibitory effect on pDC and monocyte responses to autoantibody IC independent of C1q.
pDC are the main source of IFN-α, which was the focus of this study. However, we also observed effects of CRP on cytokine production by monocytes responding to autoantibody IC. We compared a panel of 8 cytokines in supernatants from PBMC stimulated by α-U1 RNP-snRNP complexes in the presence and absence of CRP. CRP increased pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) that are generally produced in response to TLR activation of NF-κB. CRP also increased release of the anti-inflammatory cytokine IL-10. The results are characteristic of the effect of FcγR ligation on macrophage responses to extracellular TLR ligands (44). We have previously shown that CRP, like IgG, increases IL-10 and suppresses IL-12 in mouse macrophages stimulated by LPS (45). The finding of a similar effect of CRP on the human monocyte cytokine response to intracellular TLR activation is new and further characterization of these monocytes is warranted.
Studies using different types of CpG oligodeoxynucleotides to activate TLR9 demonstrated two different pDC responses that were determined primarily by the intracellular localization of the stimulus. CpG-A oligodeoxynucleotides, which form large higher order structures, localize in transferrin-positive endosomal compartments and stimulate IRF7 activation and IFN-α synthesis. CpG-B oligodeoxynucleotides, which are smaller, rapidly colocalize with LAMP-1 in late endosomes/lysosomes, and promote pDC differentiation (32). The principle of differential intracellular localization leading to different responses was extended to viral activators of TLR7 in pDC and to CpG in monocytes by recent studies (38). We investigated the effect of CRP on intracellular localization of α-U1 RNP (IgG)-snRNPs in purified monocytes and pDC. CRP accelerated the localization of autoantigen to late endosomes/lysosomes in both monocytes and pDC. CRP caused a reciprocal decrease in localization of autoantigen into early endosomes. These results are consistent with the hypothesis that CRP prevents the early endosomal localization of autoantibody IC, which favors IRF-7 activation and type I IFN synthesis. CRP complexes and CRP with IC increased the expression of pDC maturation markers, CD80 and CD86, consistent with late endosome/lysosome localization. The increase in TNF-α, IL-1β and IL-6 seen in monocytes stimulated with CRP and IC is also consistent with increased late endosome/lysosome localization of snRNPs in the presence of CRP (38). In other studies CRP has been reported to inhibit the differentiation of monocytes into DC (46), which can occur in response to IFN-α (47). This would also block the type I IFN positive feedback pathway. On the other hand CRP activation of monocytes through FcγRI was found to increase BLyS/BAFF release, which promotes B cell differentiation and antibody formation (48).
The overall effect of CRP in our study was to decrease the proinflammatory IFN-α response, increase pDC differentiation and increase synthesis of cytokines characteristic of regulatory monocytes, including IL-10. This represents a novel mechanism of regulation of the cytokine response to autoantibody IC found in patients with autoantibody to two major SLE autoantigens, U1 RNP and DNA that activate pDC through intracellular TLR7 and TLR9.
Our finding, as well as a previous report that IFN-α inhibits CRP synthesis (20), suggests that a regulatory relationship exists between CRP and type I IFN. This suggests an explanation for the low serum CRP levels in patients with SLE as well as the genetic linkage of SNPs that regulate CRP baseline levels with SLE (16, 17). Breaking the IFN positive feedback cycle could lead to increased CRP synthesis and restored homeostasis. CRP has the unique characteristic of specifically targeting IC-induced IFN-α without significantly affecting pDC responses to CpG or influenza virus. This would be an advantage over other therapeutic approaches targeting the type I IFN response, including mAb to IFN-α, immunization against IFN, and synthetic immunoregulatory oligodeoxynucleotides in that host defense functions would be spared (49).
ACKNOWLEDGMENTS
We thank Carol Morris, Audrey Rich, Walter Duran and Kathleen Triplett for technical assistance. Images in this paper were generated in the University of New Mexico & Cancer Center Fluorescence Microscopy Shared Resource, funded as detailed on: http://hsc.unm.edu/crtc/microscopy/Facility.html. We also thank Dr. Rufus Burlingame for generously providing the snRNP preparations.
Supported by grants from the NIH (AI087617, AI085414), the University of New Mexico Clinical and Translational Science Center (DHHS/NIH/NCRR grant #1 UL1 RR031977), and the Department of Veterans Affairs (VA Merit Review, Dr. Du Clos).
REFERENCES
- 1.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 2.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annu Rev Immunol. 2011;29:163–83. doi: 10.1146/annurev-immunol-031210-101345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blasius AL, Beutler B. Intracellular toll-like receptors. Immunity. 2010;32:305–15. doi: 10.1016/j.immuni.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. FcgammaRIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 5.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol. 2004;16:801–7. doi: 10.1016/j.coi.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–92. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Kirou KA, Lee C, George S, Louca K, Peterson MG, Crow MK. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 9.Ronnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54:408–20. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 10.Elkon KB, Wiedeman A. Type I IFN system in the development and manifestations of SLE. Curr Opin Rheumatol. 2012;24:499–505. doi: 10.1097/BOR.0b013e3283562c3e. [DOI] [PubMed] [Google Scholar]
- 11.Peisajovich A, Marnell L, Mold C, Du Clos TW. C-reactive protein at the interface between innate immunity and inflammation. Expert Rev Clin Immunol. 2008;4:379–90. doi: 10.1586/1744666X.4.3.379. [DOI] [PubMed] [Google Scholar]
- 12.Lu J, Marjon KD, Mold C, Du Clos TW, Sun PD. Pentraxins and Fc receptors. Immunol Rev. 2012;250:230–8. doi: 10.1111/j.1600-065X.2012.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du Clos TW. C-reactive protein reacts with the U1 small nuclear ribonucleoprotein. J Immunol. 1989;143:2553–9. [PubMed] [Google Scholar]
- 14.Du Clos TW, Zlock LT, Rubin RL. Analysis of the binding of C-reactive protein to histones and chromatin. J Immunol. 1988;141:4266–70. [PubMed] [Google Scholar]
- 15.Gershov D, Kim S, Brot N, Elkon KB. C-Reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components, and sustains an antiinflammatory innate immune response: implications for systemic autoimmunity. J Exp Med. 2000;192:1353–64. doi: 10.1084/jem.192.9.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edberg JC, Wu J, Langefeld CD, Brown EE, Marion MC, McGwin G, Jr., et al. Genetic variation in the CRP promoter: association with systemic lupus erythematosus. Hum Mol Genet. 2008;17:1147–55. doi: 10.1093/hmg/ddn004. [DOI] [PubMed] [Google Scholar]
- 17.Russell AI, Cunninghame Graham DS, Shepherd C, Roberton CA, Whittaker J, Meeks J, et al. Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet. 2004;13:137–47. doi: 10.1093/hmg/ddh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonsen A, Gunnarsson I, Gullstrand B, Svenungsson E, Bengtsson AA, Nived O, et al. Association between SLE nephritis and polymorphic variants of the CRP and Fc©RIIIa genes. Rheumatology. 2007;46:1417–21. doi: 10.1093/rheumatology/kem167. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes B, Furnrohr BG, Vyse TJ. C-reactive protein in rheumatology: biology and genetics. Nat Rev Rheumatol. 2011;7:282–9. doi: 10.1038/nrrheum.2011.37. [DOI] [PubMed] [Google Scholar]
- 20.Enocsson H, Sjowall C, Skogh T, Eloranta ML, Ronnblom L, Wettero J. Interferon-alpha mediates suppression of C-reactive protein: Explanation for muted C-reactive protein response in lupus flares? Arthritis Rheum. 2009;60:3755–60. doi: 10.1002/art.25042. [DOI] [PubMed] [Google Scholar]
- 21.Becker GJ, Waldburger M, Hughes GR, Pepys MB. Value of serum C-reactive protein measurement in the investigation of fever in systemic lupus erythematosus. Ann Rheum Dis. 1980;39:50–2. doi: 10.1136/ard.39.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams RC, Jr., Harmon ME, Burlingame R, Du Clos TW. Studies of serum C-reactive protein in systemic lupus erythematosus. J Rheumatol. 2005;32:454–61. [PubMed] [Google Scholar]
- 23.Du Clos TW, Zlock LT, Hicks PS, Mold C. Decreased autoantibody levels and enhanced survival of (NZB × NZW) F1 mice treated with C-reactive protein. Clin Immunol Immunopathol. 1994;70:22–7. doi: 10.1006/clin.1994.1005. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez W, Mold C, Kataranovski M, Hutt J, Marnell LL, Du Clos TW. Reversal of ongoing proteinuria in autoimmune mice by treatment with C-reactive protein. Arthritis Rheum. 2005;52:642–50. doi: 10.1002/art.20846. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez W, Mold C, Marnell LL, Hutt J, Silverman GJ, Tran D, et al. Prevention and reversal of nephritis in MRL/lpr mice with a single injection of C-reactive protein. Arthritis Rheum. 2006;54:325–35. doi: 10.1002/art.21556. [DOI] [PubMed] [Google Scholar]
- 26.Szalai AJ, Weaver CT, McCrory MA, van Ginkel FW, Reiman RM, Kearney JF, et al. Delayed lupus onset in (NZB × NZW)F1 mice expressing a human C-reactive protein transgene. Arthritis Rheum. 2003;48:1602–11. doi: 10.1002/art.11026. [DOI] [PubMed] [Google Scholar]
- 27.Marjon KD, Marnell LL, Mold C, Du Clos TW. Macrophages activated by C-reactive protein through FcγRI transfer suppression of immune thrombocytopenia. J Immunol. 2009;182:1397–403. doi: 10.4049/jimmunol.182.3.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szalai AJ, Nataf S, Hu X-Z, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–7. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- 29.Lu J, Marnell LL, Marjon KD, Mold C, Du Clos TW, Sun PD. Structural recognition and functional activation of FcγR by innate pentraxins. Nature. 2008;456:989–92. doi: 10.1038/nature07468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bave U, Vallin H, Alm GV, Ronnblom L. Activation of natural interferon-alpha producing cells by apoptotic U937 cells combined with lupus IgG and its regulation by cytokines. J Autoimmun. 2001;17:71–80. doi: 10.1006/jaut.2001.0519. [DOI] [PubMed] [Google Scholar]
- 31.Zhang CY, Booth JW. Divergent intracellular sorting of FcγRIIA and FcγRIIB2. J Biol Chem. 2010;285:34250–8. doi: 10.1074/jbc.M110.143834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guiducci C, Ott G, Chan JH, Damon E, Calacsan C, Matray T, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narkates AJ, Volanakis JE. C-reactive protein binding specificities: artificial and natural phospholipid bilayers. Ann N Y Acad Sci. 1982;389:172–82. doi: 10.1111/j.1749-6632.1982.tb22135.x. [DOI] [PubMed] [Google Scholar]
- 34.Santer DM, Hall BE, George TC, Tangsombatvisit S, Liu CL, Arkwright PD, et al. C1q deficiency leads to the defective suppression of IFN-alpha in response to nucleoprotein containing immune complexes. J Immunol. 2010;185:4738–49. doi: 10.4049/jimmunol.1001731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lood C, Gullstrand B, Truedsson L, Olin AI, Alm GV, Ronnblom L, et al. C1q inhibits immune complex-induced interferon-alpha production in plasmacytoid dendritic cells: a novel link between C1q deficiency and systemic lupus erythematosus pathogenesis. Arthritis Rheum. 2009;60:3081–90. doi: 10.1002/art.24852. [DOI] [PubMed] [Google Scholar]
- 36.Bang R, Marnell L, Mold C, Stein MP, Du Clos KT, Chivington-Buck C, et al. Analysis of binding sites in human C-reactive protein for Fc©RI, Fc©RIIA, and C1q by site-directed mutagenesis. J Biol Chem. 2005;280:25095–102. doi: 10.1074/jbc.M504782200. [DOI] [PubMed] [Google Scholar]
- 37.Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for C-reactive protein on leukocytes is Fc© receptor II. J Exp Med. 1999;190:585–90. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–4. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 40.Du Clos TW, Zlock LT, Marnell L. Definition of a C-reactive protein binding determinant on histones. J Biol Chem. 1991;266:2167–71. [PubMed] [Google Scholar]
- 41.Vas J, Gronwall C, Marshak-Rothstein A, Silverman GJ. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum. 2012;64:3388–98. doi: 10.1002/art.34537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu J, Marjon KD, Mold C, Du Clos TW, Sun PD. Pentraxins and Fc receptors. Immunological Reviews. 2012;250:230–8. doi: 10.1111/j.1600-065X.2012.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–47. [PubMed] [Google Scholar]
- 44.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mold C, Rodriguez W, Rodic-Polic B, Du Clos TW. C-reactive protein mediates protection from lipopolysaccharide through interactions with FcγR. J Immunol. 2002;169:7019–25. doi: 10.4049/jimmunol.169.12.7019. [DOI] [PubMed] [Google Scholar]
- 46.Zhang R, Becnel L, Li M, Chen C, Yao Q. C-reactive protein impairs human CD14+ monocyte-derived dendritic cell differentiation, maturation and function. Eur J Immunol. 2006;36:2993–3006. doi: 10.1002/eji.200635207. [DOI] [PubMed] [Google Scholar]
- 47.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–3. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Su K, Ji C, Szalai AJ, Wu J, Zhang Y, et al. Immune opsonins modulate BLyS/BAFF release in a receptor-specific fashion. J Immunol. 2008;181:1012–8. doi: 10.4049/jimmunol.181.2.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ronnblom L, Elkon KB. Cytokines as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6:339–47. doi: 10.1038/nrrheum.2010.64. [DOI] [PubMed] [Google Scholar]