Abstract
Background
The effects of progressive resistance exercise (PRE) on the motor signs of Parkinson’s disease have not been studied in controlled trials. Our aim was to compare 6, 12, 18, and 24 month outcomes of patients with Parkinson’s disease who received PRE to a stretching, balance, and strengthening exercise program.
Methods
We conducted a randomized controlled trial between September 2007 and July 2011. Pairs of patients, matched by sex and off-medication Unified Parkinson’s Disease Rating Scale, motor subscale (UPDRS-III), were randomly assigned to the interventions with a 1:1 allocation ratio. The PRE group performed a weight lifting program. The Modified Fitness Counts (mFC) group performed a stretching, balance, and strengthening exercise program. Patients exercised two days per week for 24 months at a gym. A personal trainer directed both weekly sessions for the first six months and one weekly session after six months. The primary outcome was the off-medication UPDRS-III score. Patients were followed for 24 months at six-month intervals.
Results
Of 51 patients, 20 in PRE and 18 in mFC completed the trial. At 24 months, the mean off-medication UPDRS-III score decreased more with PRE than with mFC (mean difference: - 7·3 points; 95% CI: -11·3 to -3·6; P < 0·001). The PRE group had ten adverse events. The mFC group had seven adverse events.
Conclusions
PRE demonstrated a statistically and clinically significant reduction in UPDRS-III scores compared to mFC and is recommended as a useful adjunct therapy to improve Parkinsonian motor signs.
Keywords: Parkinson’s disease, progressive resistance exercise, strength training, randomized controlled trial, Unified Parkinson’s Disease Rating Scale motor subscale (UPDRS-III)
INTRODUCTION
Parkinson’s disease is a neurodegenerative disease that leads to severely reduced function. There is mounting evidence that exercise is beneficial for Parkinson’s disease.1-4 However, neither the effects of exercise on the motor signs of Parkinson’s disease beyond six months, nor the type of exercise that should be recommended for symptom modification are known. Patients with Parkinson’s disease are slow and weak,5, 6 and progressive resistance exercise (PRE) improves muscle strength,7, 8 gait initiation,9 and gait speed.9, 10 PRE in combination with other exercise modalities improves strength,11, 12 decreases postural sway and decreases falls,13 improves whole body bradykinesia,12 and improves quality of life.12 A combination of exercise modalities including resistance, aerobic, and balance and stretching exercises is most likely to be optimal for patients with Parkinson’s disease.14 However, before prescribing a combination of exercise modalities, it is important to understand the independent effects of each mode of exercise intervention in Parkinson’s disease in the context of improving the signs of the disease.
The current clinical trial determined the effects of PRE in improving the signs of Parkinson’s disease. This trial tested the primary hypothesis that 24 months of PRE is superior to a non-progressive stretching, balance, and strengthening program (Modified Fitness Counts (mFC))15 at improving the off-medication Unified Parkinson’s Disease Rating Scale, motor subscale (UPDRS-III) scores in patients with Parkinson’s disease. Secondary outcome measures included on-medication UPDRS-III scores, medication dosage, movement speed and strength, functional ability, and quality of life.
METHODS
Study Design and Participants
We conducted a prospective, parallel-group, single-center, randomized controlled trial between September 2007 and July 2011. Patients with idiopathic Parkinson’s disease, confirmed by a Movement Disorders specialist as outlined by the Parkinson’s Disease Society Brain Bank criteria,16 were self-referred or recruited from Rush University Medical Center (RUMC). Patients were evaluated at the University of Illinois at Chicago (UIC). We targeted patients with moderate disease severity since these patients are sufficiently impaired so that a treatment effect can be observed, and they are not so impaired that there are major safety issues. Patients were eligible if they were 50 to 67 years; on stable dopaminergic therapy; and able to walk for six minutes. Patients were ineligible if they had a neurological history other than Parkinson’s disease; significant arthritis; failed the Physical Activity Readiness Questionnaire;17 had cognitive impairment as indicated by a Mini-Mental State Examination score < 23;18 were already exercising; or had surgery for Parkinson’s disease. Patients were followed for 24 months or until they withdrew from the study. The institutional review boards at RUMC and UIC approved the study. Patients provided written informed consent. Race and ethnicity data were recorded as required by NIH’s policy (PHS 398).
Interventions
We chose mFC because it is an exercise program recommended by the National Parkinson Foundation. The modifications to Fitness Counts were minimal (see Supplementary Material). We chose the PRE program to determine if PRE not only increases strength but also reduces the signs of Parkinson’s disease. The programs were identical in all aspects (duration of exercise, number of exercise sessions, and time with the personal trainer) except for the specific exercises. Patients participated in their respective interventions twice a week19 for 24 months. One-on-one exercise with a certified personal trainer was provided for both weekly exercise sessions during the first six months; the trainer-assisted sessions reduced to once per week after six months (see Supplementary Table 1 for details about trainers). Patients were instructed not to engage in additional exercise.
Modified Fitness Counts Exercise
The mFC exercise program focused on stretches, balance exercises, breathing, and non-progressive strengthening (manual chapters two and three).15
Progressive Resistance Exercise
The PRE program consisted of 11 strengthening exercises: chest press, latissimus pull downs, reverse flys, double leg press, hip extension, shoulder press, biceps curl, rotary calf (ankle plantar flexion), triceps extension, seated quadriceps extension, and back extension.19
See Supplementary Material for details on both of the exercise programs.
Study Procedures
All assessments were performed at the Clinical Motor Control Laboratory at UIC after 12-hour overnight withdrawal of dopaminergic medication.20 Off-medication assessment was completed in the morning. Patients then took their medication, had lunch, and 60 minutes later repeated the assessments (on-medication). Motor signs were evaluated using the UPDRS-III by a single rater, who had passed the Movement Disorder Society’s standardized training program.
A manipulandum fitted with a torque and position transducer was used to measure elbow flexor muscle strength and movement speed.21, 22 We also assessed functional ability using the modified Physical Performance Test (mPPT)23 and quality of life using the Parkinson’s Disease Questionnaire (PDQ-39).24 See Supplementary Material for details about measuring muscle strength, movement speed, the mPPT and the PDQ-39.
Follow-up
Patients returned to the laboratory at 6, 12, 18, and 24 months for follow-up. The entire baseline assessment procedure was repeated. The same rater who evaluated the baseline UPDRS-III evaluated all subsequent off-medication UPDRS-III evaluations. Current medications and adverse events were recorded at each visit.
Randomization and Blinding
The statistician matched the enrolled patients in pairs by sex and off-medication UPDRS-III scores, and randomly assigned one member of each pair to PRE and the other member to mFC. The first assignment in each pair was generated according to a random-length permuted block design so that the assignment sequence would have been difficult to guess.25 Randomization resulted in a parallel group design with a 1:1 allocation ratio. Patients started exercising within a month of randomization. The statistician maintained the randomization sequence in a private file on a password protected account. As patients were assigned, the statistician informed the exercise coordinator of the assigned treatment and the study coordinator that randomization had occurred. On the study team, only the statistician, the exercise coordinator who recruited the trainers for the patients, and the trainers knew the treatment assignment. None of these individuals had a role in data collection. Research personnel involved in data collection, including the rater of the UPDRS-III were blinded to group assignment. The patients knew their treatment assignment but were unaware of the study hypothesis and were explicitly instructed not to discuss their exercise program with the raters.
Statistical Analysis
The primary outcome was the change in the off-medication UPDRS-III score from baseline to 6, 12, 18, and 24 months. Secondary outcomes were changes in levodopa equivalent medication dosage (LED),26 off-medication elbow strength, movement speed, and physical performance. We repeated the analysis using the on-medication data and assessed quality of life on medication. To estimate treatment effects, planned between-group contrasts were performed using a mixed effects regression model for the off-medication UPDRS-III score, muscle strength, and movement speed. LED, mPPT, and the PDQ-39 did not meet the distributional assumptions for parametric analysis. Therefore, we used the Wilcoxon rank-sum test to examine between group differences in change scores. To reduce type I errors, for all non-parametric analyses, we used only the 6- and 24-month change from baseline scores.
The study was initially powered to detect a difference of five points in the six-month UPDRS-III change score, with a power of 80%, assuming a standard deviation of four, at an alpha level of 0·05. The power analysis determined that 17 patients per group were needed. We projected an attrition rate of 30% that resulted in our target sample size of 24 patients per group. Statistical analyses were performed with the use of SAS software, version 9·1. All statistical tests were two-sided, and we used a P value < 0·05.
RESULTS
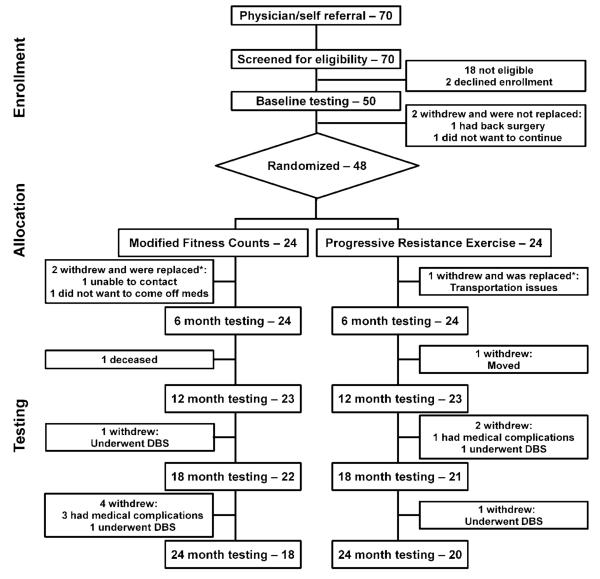
Forty-eight patients with Parkinson’s disease were randomly assigned to mFC or PRE (Figure 1). To ensure that we had 24 patients in each group complete at least six months of exercise, we replaced patients who withdrew after randomization but before testing at six months. Two withdrew from mFC after randomization: one was lost to further contact before starting the exercise program, and one withdrew immediately before the six-month testing session due to discomfort at the prospect of coming off medication for testing. One patient in PRE withdrew due to difficulty with transportation to the exercise facility. These three patients did not complete six-month evaluations and were replaced. Each new patient replaced a patient who had previously been randomized. Thereafter, no patients were replaced. The treatment groups did not differ significantly at baseline (Table 1). Supplementary Table 2 lists by treatment group, the number of patients that withdrew, and the reason for withdrawing from the study. The primary analysis did not impute missing data and assumed data to be missing at random. We repeated the analysis with the last available value carried forward for patients who withdrew from the study and the results were similar (see Supplementary Table 3 and 4).
Figure 1. Trial Profile.
* Patients who were matched for sex and off UPDRS-III score replaced the three patients who withdrew before six-month testing. Patients who withdrew after six months were not replaced. DBS = Deep Brain Stimulation.
Table 1.
Characteristics of Patients at Baseline, by Treatment Group. *
| Characteristic | Treatment group | Difference Between Groups | P value † | |
|---|---|---|---|---|
|
Modified Fitness
Counts (N = 24) |
Progressive
Resistance Training (N = 24) |
(95% Confidence Interval) | ||
| Demographic | ||||
| Age in years | 58·6 ± 5·6 | 59·0 ± 4·6 | −0·4 (−2·6 to 3·4) | 0·78 |
| Sex - no. (%) | 1·00 | |||
| Male | 14 (58·3) | 14 (58·3) | ||
| Female | 10 (41·7) | 10 (41·3) | ||
| Ethnicity - no. (%)Ұ | 0·19 | |||
| Hispanic or Latino | 5 (20·8) | 1 (4·2) | ||
| Not Hispanic or Latino | 19 (79·2) | 23 (95·8) | ||
| Race - no. (%)Ұ | 0·49 | |||
| African American | 0 (0) | 2 (8·3) | ||
| White | 24 (100) | 22 (91·7) | ||
| Handedness - no. (%)Ұ | 1·00 | |||
| Right | 22 (91·7) | 23 (95·8) | ||
| Left | 2 (8·3) | 1 (4·2) | ||
| Clinical | ||||
| Years since diagnosis | 6·5 ± 4·7 | 6·5 ± 4·1 | 0·0 (−2·5 to 2·6) | 0·97 |
| Mini-Mental State Examination | 29·1 ± 1·4 | 29·3 ± 1·1 | 0·2 (−0·5 to 0·9) | 0·56 |
| Most affected side - no. (%) | 0·5 (0·2 to 1·6)ҰҰ | 0·37 | ||
| Right | 17 (70·8) | 13 (54·2) | ||
| Left | 7 (29·2) | 11 (45·8) | ||
| Motor Status | ||||
| Unified Parkinson Disease Rating Scale, part III, motor subscale score (range, 0-108) (primary outcome; off medication) |
34·7 ± 11·5 | 34·5 ± 11·9 | −0·2 (−7·0 to 6·6) | 0·95 |
| Unified Parkinson Disease Rating Scale, part III, motor subscale score (range, 0-108) (primary outcome; on medication) |
20·9 ± 8·0 | 21·6 ± 10·1 | 0·7 (−4·4 to 6·2) | 0·74 |
| Hoehn and Yahr Staging Scale (disability; range, 0-5; off medication) |
2·3 ± 0·53 | 2·2 ± 0·41 | −0·1 (−0·4 to 0·2) | 0·55 |
| Medication **‡ | ||||
| Levodopa equivalent dose | 705 ± 405 | 598 ± 355 | −100 (−125 to 350)‡‡ | 0·37 |
| Strength | ||||
| Elbow flexion torque (Nm; off medication) |
50·2 ± 17·8 | 47·6 ± 15·7 | −2·6 (12·4 to 7·2) | 0·60 |
| Elbow flexion torque (Nm; on medication) |
50·5 ± 19·6 | 49·3 ± 15·9 | −1·2 (−11·6 to 9·2) | 0·47 |
| Movement Speed | ||||
| Elbow flexion velocity (deg/s; off medication) |
330·3 ± 86·3 | 327·2 ± 79·7 | −3·1 (−51·4 to 45·1) | 0·90 |
| Elbow flexion velocity (deg/s; on medication) |
374·9 ± 90·8 | 387·3 ± 79·5 | 12·4 (−37·1 to 62·0) | 0·62 |
| Physical Function ‡ | ||||
| Modified Physical Performance Test (range, 0-36; off medication) |
27·4 ± 6·8 | 26·4 ± 5·0 | −2 (−5·0 to 1·0)‡‡ | 0·27 |
| Modified Physical Performance Test (range, 0-36; on medication) |
31·1 ± 3·9 | 30·7 ± 4·0 | 0·0 (−2·0 to 1·0)‡‡ | 0·69 |
| Quality of Life ‡ | ||||
| Parkinson’s Disease Questionnaire – 39 (range, 0-156; on medication) |
18·5 ± 11·3 | 23·5 ± 14·3 | 3·6 (−2·3 to 10·5)‡‡ | 0·21 |
Plus-minus values are mean ± 1SD
P values calculated with the use of t-tests for continuous variables and Fisher’s exact test for binary variables unless mentioned otherwise
Confidence intervals not estimated for binary variables if total frequency (combined groups) is less than 10
Mantel-Haenszel estimate of the common odds ratio for binary variables. Note: If 1 is contained within the confidence interval, then there is no difference between groups
Medication is measured in levodopa equivalent units in mg/day
P values calculated with the use of Wilcoxon rank-sum test
Hodges-Lehman estimate of location shift
Note: All P values are two-sided
Motor Signs and Medication Status
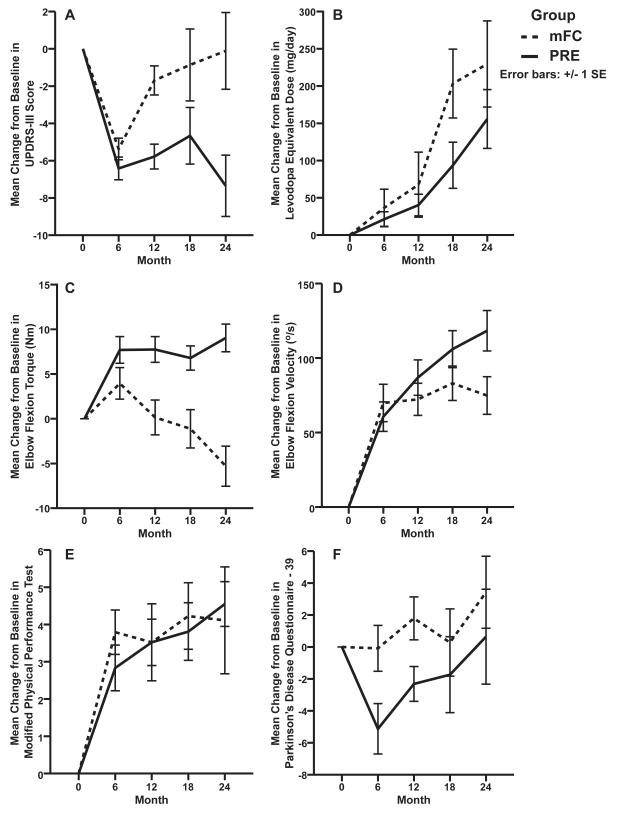
The mean off-medication UPDRS-III score decreased for both mFC (- 5·4; SD ±2·8) and PRE (- 6·4; SD ±3·0) from baseline to 6 months; these changes did not differ by group (mean difference: -1; 95% CI: -4·5 to 2·5; P = 0·55) (Table 2 and Figure 2A). Between group differences in the change scores from baseline were significant at 12 (-4·1; -7·6 to -0·5; P = 0·02), 18 (-3·8; -7·6 to -0·3; P = 0·03), and 24 months (-7·3; - 11·3 to -3·6; P < 0·001). At 24 months, the mFC group had returned to a similar baseline UPDRS-III score (-0·1; SD ±8·7) whereas the PRE group score was - 7·4 (SD ±7·4) points lower.
Table 2.
Motor Signs and Medication Status at Each Visit by Treatment Group.
| Score at visit * | Change from Baseline | Difference (PRE vs mFC) in Change from Baseline (95% CI) |
||||
|---|---|---|---|---|---|---|
| mFC | PRE | mFC | PRE | P value† | ||
| Primary Outcome 1 | ||||||
|
UPDRS-III Off
Medication |
||||||
| Baseline | 34·7 ± 11·5 | 34·5 ± 11·9 | ||||
| 6 Month | 29·3 ± 12·2 | 28·0 ± 10·9 | −5·4 ± 2·8 | −6·4 ± 3·0 | −1·0 (−4·5 to 2·5) | 0·55 |
| 12 Month | 32·8 ± 12·4 | 28·4 ± 10·8 | −1·7 ± 3·7 | −5·8 ± 3·2 | −4·1 (−7·6 to −0·5) | 0·02 |
| 18 Month | 32·8 ± 12·3 | 28·6 ± 9·4 | −0·9 ± 9·1 | −4·7 ± 7·0 | −3·8 (−7·6 to −0·3) | 0·03 |
| 24 Month | 34·0 ± 12·6 | 25·8 ± 10·6 | −0·1 ± 8·7 | −7·4 ± 7·4 | −7·3 (−11·3 to −3·6) | <0·001 |
| Secondary Outcomes 1 | ||||||
|
UPDRS-III On
Medication |
||||||
| Baseline | 20·9 ± 8·0 | 21·6 ± 10·1 | ||||
| 6 Month | 17·7 ± 8·6 | 19·1 ± 9·0 | −3·2 ± 2·9 | −2·5 ± 2·5 | 0·7 (−2·4 to 3·7) | 0·66 |
| 12 Month | 19·1 ± 8·4 | 19·4 ± 8·5 | −1·6 ± 5·1 | −1·9 ± 4·0 | −0·3 (−3·5 to 2·6) | 0·78 |
| 18 Month | 18·1 ± 9·7 | 18·4 ± 6·5 | −2·2 ± 7·1 | −2·0 ± 6·5 | 0·2 (−3·4 to 2·9) | 0·87 |
| 24 Month | 19·3 ± 9·7 | 17·9 ± 8·7 | −1·3 ± 6·6 | −2·3 ± 7·1 | −1·0 (−4·7 to 1·8) | 0·39 |
| Medication (LED) 2 | ||||||
| Baseline | 704·5 ± 405·3 | 597·8 ± 355·2 | ||||
| 6 Month | 741·2 ± 443·5 | 619·0 ± 370·6 | 36·6 ± 122·2 | 21·2 ± 49·2 | 0·0 (0·0 to 0·0)Ұ | 0·96‡ |
| 12 Month | 772·2 ± 472·7 | 638·1 ± 352·2 | 67·7 ± 213·5 | 40·3 ± 71·0 | ||
| 18 Month | 907·9 ± 546·9 | 691·4 ± 384·5 | 203·4 ± 226·2 | 93·6 ± 151·4 | ||
| 24 Month | 934·1 ± 557·2 | 753·6 ± 369·4 | 229·6 ± 283·3 | 155·8 ± 193·3 | −75·0 (−200·0 to 62·0)Ұ | 0·29‡ |
mFC, Modified Fitness Counts; PRE, Progressive Resistance Exercise; CI, Confidence Interval; UPDRS, Unified Parkinson’s Disease Rating Scale, part III, motor subscale; LED, Levodopa Equivalent Dose
Plus-minus values are mean ± SD
P values are based on planned between-group contrasts using a mixed effects regression model unless mentioned otherwise
Negative change scores indicate improvement in UPDRS-III
Positive change scores indicate increase in LED in mg/day
Hodges-Lehman estimate of location shift
P values are based on Wilcoxon rank-sum test
Figure 2. Change from Baseline Scores for All Outcomes in the Two Exercise Groups.
The mean (± SE) change from baseline in the off-medication UPDRS-III score (A), the levodopa equivalent dose (B), off-medication elbow flexion torque (C), off-medication elbow flexion velocity (D), off-medication modified Physical Performance Test (E), and Parkinson’s Disease Questionnaire – 39 (F) at 6, 12, 18, and 24 months. The dashed lines indicate the Modified Fitness Counts group (mFC), and the solid lines indicate the Progressive Resistance Exercise group (PRE). Negative change scores indicate improvement in UPDRS-III score, levodopa equivalent dose, and Parkinson’s Disease Questionnaire – 39. Positive change scores indicate improvement in elbow flexion torque, elbow flexion velocity, and Modified Physical Performance Test.
The mean on-medication UPDRS-III score did not differ by group at 6 ( 0·7; -2·4 to 3·7; P = 0·66), 12 (- 0·3; -3·5 to 2·6; P = 0·78), 18 (0·2; -3·4 to 2·9; P = 0·87), and 24 months (-1·0; -4·7 to 1·8; P = 0·39) (Table 2).
In the first six months of the study, eight patients in mFC and seven patients in PRE required an increase in medication. At 24 months, the change from baseline in levodopa equivalents did not differ significantly between treatment groups (-75 mg; -200 to 62; P = 0·29; Table 2 and Figure 2B), although medication levels increased in each group. The levodopa equivalents increased by 229·6 mg (SD ±283·3) and 155·8 mg (SD ±193·3), for mFC and PRE groups respectively.
Strength and Movement Speed
The mean off-medication elbow flexion torque increased for both mFC (4 Nm; SD ±8·7) and PRE (7·7 Nm; SD ±7·3) from baseline to 6 months; these changes did not differ by group (3·7 Nm; -0·8 to 8·3; P = 0·1) (Table 3 and Figure 2C). Between group differences in the change scores from baseline were significant at 12 (7·6 Nm; 3·2 to 12·5; P = 0·001), 18 (7·9 Nm; 3·7 to 13·2; P < 0·001), and 24 months (14·3 Nm; 9·3 to 19·3; P < 0·001). mFC was weaker than at baseline by -5·3 Nm (SD ±9·5) whereas PRE was stronger by 9 Nm (SD ±6·9). On-medication findings were similar to off-medication findings and were significant at 12 (8·6 Nm; 2·9 to 14·3; P = 0·003), 18 (9·6 Nm; 3·9 to 15·5; P = 0·001), and 24 months (6·2 Nm; 0·3 to 12·3; P = 0·04) (Table 3).
Table 3.
Strength, Movement Speed, Physical Function, and Quality of Life at Each Visit by Treatment Group.
| Score at visit * | Change from Baseline | Difference (PRE vs mFC) in Change from Baseline |
||||
|---|---|---|---|---|---|---|
| mFC | PRE | mFC | PRE | (95% CI) | P value† | |
|
Elbow Flexion Torque 1 |
||||||
| Off Medication | ||||||
| Baseline | 50·2 ± 17·8 | 47·6 ± 15·7 | ||||
| 6 Month | 54·1 ± 21·9 | 55·3 ± 17·2 | 4·0 ± 8·7 | 7·7 ± 7·3 | 3·7 (−0·8 to 8·3) | 0·1 |
| 12 Month | 51·1 ± 20·4 | 54·8 ± 17·5 | 0·1 ± 9·3 | 7·7 ± 6·9 | 7·6 (3·2 to 12·5) | 0·001 |
| 18 Month | 49·9 ± 21·2 | 53·9 ± 13·6 | −1·1 ± 10·0 | 6·8 ± 6·2 | 7·9 (3·7 to 13·2) | <0·001 |
| 24 Month | 43·2 ± 16·2 | 56·3 ± 15·2 | −5·3 ± 9·5 | 9·0 ± 6·9 | 14·3 (9·3 to 19·3) | <0·001 |
| On Medication | ||||||
| Baseline | 54·6 ± 20·3 | 50·8 ± 15·3 | ||||
| 6 Month | 57·2 ± 21·7 | 56·7 ± 17·4 | 2·6 ± 4·4 | 5·9 ± 6·9 | 3·3 (−2·2 to 8·9) | 0·25 |
| 12 Month | 53·2 ± 20·6 | 56·3 ± 15·3 | −2·3± 8·1 | 6·3 ± 7·2 | 8·6 (2·9 to 14·3) | 0·003 |
| 18 Month | 51·8 ± 19·7 | 55·5 ± 15·1 | −3·8± 6·4 | 5·8 ± 9·7 | 9·6 (3·9 to 15·5) | 0·001 |
| 24 Month | 47·2 ± 20·8 | 50·2 ± 20·0 | −6·0± 10·1 | 0·2 ± 16·3 | 6·2 (0·3 to 12·3) | 0·04 |
| Movement Speed 2 | ||||||
| Off Medication | ||||||
| Baseline | 330·3 ± 86·3 | 327·2 ± 79·7 | ||||
| 6 Month | 400·2 ± 99·5 | 387·8 ± 77·9 | 69·8 ± 61·4 | 60·6 ± 48·5 | −9·2 (−34·6 to 16·4) | 0·48 |
| 12 Month | 407·3 ± 95·5 | 413·0 ± 98·9 | 72·3 ± 51·7 | 86·8 ± 57·0 | 14·5 (−11·2 to 40·9) | 0·26 |
| 18 Month | 422·0 ± 90·7 | 424·1 ± 81·2 | 83·1 ± 54·2 | 106·0 ± 56·9 | 22·9 (−5·9 to 47·4) | 0·13 |
| 24 Month | 403·7 ± 73·6 | 438·1 ± 78·9 | 74·8 ± 53·5 | 118·3 ± 60·7 | 43·5 (9·2 to 64·7) | 0·009 |
| On Medication | ||||||
| Baseline | 374·9 ± 90·8 | 387·3 ± 79·5 | ||||
| 6 Month | 436·2 ± 98·4 | 426·1 ± 72·6 | 61·3 ± 60·1 | 38·8 ± 52·8 | −22·5 (−52·7 to 7·6) | 0·14 |
| 12 Month | 443·6 ± 102·1 | 454·2 ± 75·6 | 63·0 ± 76·3 | 69·3 ± 56·7 | 6·3 (−24·4 to 36·8) | 0·69 |
| 18 Month | 462·9 ± 97·8 | 461·0 ± 76·6 | 80·3 ± 69·5 | 82·4 ± 54·5 | 2·1 (−29·4 to 33·2) | 0·91 |
| 24 Month | 450·9 ± 79·0 | 461·2 ± 89·2 | 84·1 ± 64·3 | 80·5 ± 55·9 | −3·6 (−36·9 to 28·4) | 0·80 |
|
Modified Physical Performance Test 3 |
||||||
| Off Medication | ||||||
| Baseline | 27·4 ± 6·8 | 26·4 ± 5·0 | ||||
| 6 Month | 31·2 ± 5·2 | 29·2 ± 4·4 | 3·8 ± 2·9 | 2·8 ± 3·0 | −1·0 (−3·0 to 1·0)Ұ | 0·21‡ |
| 12 Month | 31·2 ± 6·1 | 29·9 ± 5·3 | 3·5 ± 5·0 | 3·5 ± 3·0 | ||
| 18 Month | 32·2 ± 4·0 | 30·5 ± 4·7 | 4·2 ± 4·2 | 3·8 ± 3·5 | ||
| 24 Month | 32·2 ± 2·5 | 31·2 ± 4·1 | 4·1 ± 6·1 | 4·6 ± 2·7 | 0·5 (0·0 to 4·0)Ұ | 0·10‡ |
| On Medication | ||||||
| Baseline | 31·1 ± 3·9 | 30·7 ± 4·0 | ||||
| 6 Month | 32·5 ± 4·1 | 31·2 ± 3·4 | 1·5 ± 1·8 | 0·5 ± 2·7 | −1·0 (−2·0 to 0·0)Ұ | 0·18‡ |
| 12 Month | 34·3 ± 1·8 | 31·6 ± 3·7 | 2·6 ± 2·1 | 1·0 ± 2·8 | ||
| 18 Month | 34·4 ± 2·0 | 32·9 ± 3·3 | 2·7 ± 2·4 | 1·8 ± 3·5 | ||
| 24 Month | 33·8 ± 1·8 | 32·6 ± 5·0 | 2·1 ± 3·5 | 1·7 ± 3·8 | 0·0 (−1·0 to 2·0)Ұ | 0·74‡ |
|
Parkinson’s Disease Questionnaire 4 |
||||||
| On Medication | ||||||
| Baseline | 18·5 ± 11·3 | 23·5 ± 14·3 | ||||
| 6 Month | 18·4 ± 14·2 | 18·4 ± 13·0 | −0·1 ± 7·0 | −5·1 ± 7·7 | −5·0 (−7·4 to −0·6)Ұ | 0·02‡ |
| 12 Month | 20·1 ± 14·4 | 21·4 ± 15·8 | 1·8 ± 6·4 | −2·3 ± 5·2 | ||
| 18 Month | 17·7 ± 15·3 | 20·4 ± 17·4 | 0·3 ± 9·9 | −1·7 ± 10·9 | ||
| 24 Month | 19·6 ± 16·2 | 22·3 ± 18·3 | 3·4 ± 9·6 | 0·6 ± 13·3 | −2·8 (-9·3 to 6·1)Ұ | 0·53‡ |
mFC, Modified Fitness Counts; PRE, Progressive Resistance Exercise; CI, Confidence Interval
Plus-minus values are mean ± SD
P values are based on planned between-group contrasts using a mixed effects regression model unless mentioned otherwise
Positive change scores indicate improvement in elbow flexion torque in Nm
Positive change scores indicate improvement in elbow peak velocity in deg/s
Positive change scores indicate improvement in physical function
Negative change scores indicate improvement in quality of life
Hodges-Lehman estimate of location shift
P values are based on Wilcoxon rank-sum test
The mean off-medication elbow flexion movement speed increased both for mFC (69·8°/s; SD ±61·4) and PRE (60·6°/s; SD ±48·5) from baseline to 6 months. At 24 months, the PRE group was faster than the mFC group (43·5°/s; 9·2 to 64·7; P = 0·009). mFC was 74·8°/s (SD ±53·5) faster than at baseline, whereas PRE was 118·3°/s (SD ±60·7) faster (Table 3 and Figure 2D). The on-medication change scores of the two groups did not differ significantly at any time point.
Physical Function
The mean off-medication mPPT score increased from baseline for both mFC (3·8; SD ±2·9) and PRE (2·8; SD ±3) at 6 months. It also increased at 24 months in mFC (4·1; SD ±6·1) and PRE (4·6; SD ±2·7) (Table 3 and Figure 2E). There were no differences in the change scores between the two groups at 6 (-1; -3 to 1; P = 0·209) or at 24 months (0·5; 0 to 4; P = 0·1).
Parkinson’s Disease Quality of Life
The mean PDQ-39 score remained relatively stable from baseline for mFC (- 0·1; SD ±7) but did decrease for PRE (-5·1; SD ±7·7) at 6 months (Table 3 and Figure 2F). At 6 months, the between group difference in change scores was significant (-5; -7·4 to 0·6; P = 0·02). PRE relative to mFC demonstrated a significantly improved quality of life. There was no difference between the groups at 24 months (-2·8; -9·3 to 6·1; P = 0·53).
Adverse Events
Only one adverse event was directly related to the study: the development of wrist pain during one testing session for a maximal voluntary contraction (Table 4). We also had one serious adverse event possibly related to mFC (back surgery) and six possibly related to the PRE (bilateral hip replacement, two unilateral knee replacements on the same patient, knee surgery to remove old debris, foot surgery, and hospitalization after a fall). All but two of these patients remained in the study following recovery from their events. Seven patients (five from mFC and two from PRE) withdrew from the study with serious adverse events definitely not related to the study and two (one from mFC and one from PRE) withdrew with serious adverse events possibly related to the study.
Table 4.
| Relation to the study | mFC (n=24) |
PRE (n=24) |
|---|---|---|
| Definitely not related | ||
| Passed away† | 1* | 0 |
| DBS Surgery† | 2* | 2* |
| ALS† | 1* | 0 |
| Cancer† | 1* | 0 |
| Hospitalization for bowel obstruction† | 0 | 1 |
| Elbow pain after a fall at home‡ | 0 | 1 |
| Possibly related | ||
| Back surgery - stenosis† | 1* | 0 |
| Bilateral hip replacement† | 0 | 1 |
| Unilateral knee replacement† | 0 | 2 (single patient) |
| Knee surgery to remove old debris† | 0 | 1 |
| Foot surgery† | 0 | 1 |
| Long term hospitalization after a fall at home† | 0 | 1* |
| Definitely related – reported to IRB | ||
| Wrist pain during maximal voluntary contraction testing‡ | 1 | 0 |
| Total | 7 patients & 7 AEs | 9 patients & 10 AEs |
mFC, Modified Fitness Counts; PRE, Progressive Resistance Training; DBS, Deep Brain Stimulation; ALS, Amyotrophic Lateral Sclerosis; IRB, Institutional Review Board; AE, Adverse Event
Serious Adverse Event – an event that required medical intervention and/or hospitalization
Adverse Event - an event that did not require medical intervention
Adverse event prevented further participation in the study and subject withdrew
Completers vs. Non-completers
At baseline, in the mFC group, quality of life was worse for the non-completers relative to the completers (9·8; 0·2 to 22; P = 0·04). No other differences between non-completers and completers were observed in either treatment group. Supplementary Tables 5 and 6 summarize these analyses for each group.
DISCUSSION
We report the results of the first randomized, controlled, blindly assessed clinical trial of PRE for Parkinson’s disease using off-medication UPDRS-III scores. PRE and mFC elicited similar improvements at 6 months. However, at 12, 18, and 24 months the PRE elicited greater improvements than mFC (Table 2 and Figure 2A). At 24 months, the between group difference was -7·3 points, which is a moderate clinically important change on the UPDRS-III scores.27 In addition, off-medication muscle strength and movement speed exhibited greater improvements in response to PRE than mFC (Table 3 and Figure 2C and 2D). This study therefore provides the clearest, most objective evidence that structured exercise in general,28 and structured long-term PRE in particular, improves the signs of patients with Parkinson’s disease.
Our results are consistent with previous studies that have shown that resistance exercise can improve physical performance,11 and quality of life in Parkinson’s disease.12 At 24 months, patients in both groups improved by at least three points in the mPPT scores. The quality of life in both groups at baseline was quite high. However, the quality of life improved to a greater extent (reduced PDQ 39 score) in the Progressive Resistance Group at 6 months possibly suggesting that the more vigorous program did lead to a transient improvement in the quality of life.
We think that PRE was more effective at reducing the UPDRS-III score and improving strength for five reasons. First, the resistance used is much greater in PRE. This was an intentional feature of our experimental design. The two groups did have different doses of exercise, and this difference increased over time. We think that this was a likely determinant of the divergent outcomes of the two treatments. It will be important in future studies to carefully evaluate dose-response effects by varying the frequency, duration, and intensity of an exercise program. Second, PRE progressively increases the resistance over time, whereas the mFC is a non-progressive training program. The beneficial effect of progressive overload on muscle strength has long been recognized.29 A third reason why PRE might be more therapeutic than mFC is that repetitively generating large forces increases neuronal activation in basal ganglia circuits more so than small forces. The blood oxygen level dependent (BOLD) signal increases in specific basal ganglia nuclei, ventral thalamus, and motor cortex with repetitive force generation.30 In patients with Parkinson’s disease, the BOLD signal within all basal ganglia nuclei also increases with force generation, but not to the same level as with control subjects.31 Fourth, reduced corticomotor excitability with force generation has been demonstrated in patients with Parkinson’s disease using transcranial magnetic stimulation.32 Corticomotor excitability has been increased by strength training in healthy controls.33 Thus, we hypothesize that PRE may lead to experience-dependent plasticity in the basal ganglia and corticomotor pathways, which could contribute to improving Parkinsonian signs and enhancing motor performance. Finally, in terms of motivation, PRE was designed to continuously challenge the patients, and they may have found this rewarding and motivating.
Patients tolerated the exercise programs well. There was only one adverse event definitely related to the study and this event did not result in the patient withdrawing from the study (Table 4). One important fact is that 20 out of 25 patients completed the PRE program. This is a retention rate of 80% and shows that patients with Parkinson’s disease can be motivated to adhere to an exercise program for at least two years unless they are confronted with a serious medical condition that severely compromises their ability to exercise. This adherence over two years is of key importance since the benefits of exercise are greatest when an exercise routine is integrated into the lifestyle of an individual. This is entirely feasible for a high percentage of individuals with Parkinson’s disease. The fact that the primary outcome was determined off-medication is important for three reasons. First, it eliminates the confounding effects of variable responses to dopaminergic medication and dampens the effect of changes in daytime medications during the two-year study period.34 Second, at 24 months, averaging between groups the LED increased by 192·7mg/day (SD ±242·8). This increase in medication might mask changes that are caused by exercise on signs of the disease when tested in the on-medication state. Third, the range of possible improvement in the UPDRS-III is increased in the off-medication state since the range of scores is reduced when patients are on medication.
This study had limitations. First, it was not double blind since it is not possible to blind patients with respect to exercising and to the specific exercises they are performing. Participants were aware of their treatment assignment and this may have affected their response. Second, the sample was small and this likely affected our power to detect differences in some measures other than the primary outcome, the UPDRS-III. Third, this was a single-center study with a relatively homogenous patient group with respect to race, age, disease severity and geographic location. Fourth, a no-exercise control group was not used; therefore the net gain of our exercise programs cannot be determined. Fifth, replacing 2 patients in the mFC group and 1 patient in the PRE group before the 6 month testing was also a limitation because these participants were not randomly assigned to a treatment group. However, this was a minor limitation because the participants did not know the group assignment until all screening and baseline testing was completed, and the evaluators did not know until the study was unblinded.
In conclusion, PRE has a greater benefit than mFC on the signs of Parkinson’s disease, upper limb muscle strength, and movement speed at 24 months. PRE has also been shown to reduce falls which are a major concern in the treatment of Parkinson’s disease.4 Based on the findings of our study, and the US Department of Health and Human Services guidelines,35 PRE should be a central component of exercise programs for patients with Parkinson’s disease that also include balance training and aerobic exercise.
Supplementary Material
Acknowledgments
We thank the neurologists and staff at the Section for Movement Disorders in the Department of Neurological Sciences at Rush University Medical Center, Chicago Illinois; the patients for their time and commitment to this research; the Parkinson Disease Foundation; Dr. Janey Prodoehl, PhD, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Illinois, for valuable comments on the manuscript; Lisa Chin-Cottongim, MS, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Illinois, for helping with several aspects of the study; the personal trainers who worked with the patients on their exercise routines; and Ellen Petrick, MS, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Illinois, the exercise study coordinator, who trained all of the personal trainers in how to administer the appropriate exercise program for each patient. Dr. Prodoehl, Ms. Chin-Cottongim, and Ms. Petrick were compensated for their time.
Role of the Funding Source: The sponsors were not involved in the design, conduct, collection, management, analysis, and/or interpretation of the study results and preparation, review, or approval of the manuscript.
Funding Source: Supported by a grant (R01-NS28127-12 to 16) from the National Institute of Neurological Disorders and Stroke.
Footnotes
Author Roles: Dr. Leurgans had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Corcos, Robichaud, Leurgans, Vaillancourt, Kohrt, and Comella.
Acquisition of data: Robichaud, David, and Poon.
Analysis and interpretation of data: Corcos, Robichaud, David, Leurgans, Vaillancourt, Poon, Rafferty, Kohrt, and Comella.
Drafting of the manuscript: Corcos, Robichaud, David, Leurgans, Vaillancourt, Poon, Rafferty, Kohrt, and Comella.
Critical revision of the manuscript for important intellectual content: Corcos, Robichaud, David, Leurgans, Vaillancourt, Poon, Rafferty, Kohrt, and Comella.
Statistical analysis: David, Leurgans.
Obtained funding: Corcos, Robichaud, Vaillancourt, Leurgans, Kohrt.
Administrative, technical, or material support: Corcos, Robichaud, David, Leurgans, Vaillancourt, Poon, Rafferty, Kohrt, and Comella.
Study supervision: Corcos, Leurgans, Vaillancourt, Kohrt, and Comella.
Full Financial Disclosures: DMC received grant support from NIH and Michael J. Fox, and receives lecture and reviewer fees from NIH. JAR, FJD, and CP received grant support from NIH. SEL was a statistical consultant for this project through the University of Illinios at Chicago. DEV receives grant support from NIH, Michael J. Fox, and consults for projects at UT Southwestern Medical Center and Great Lakes NeuroTechnologies. MRR has scholarship support from the Foundation for Physical Therapy and received grant support from NIH. WMK receives grant support from the NIH and DoD and consulting fees from the NIH. CLC is or has received research support from Allergan Inc., Merz Pharmaceuticals, Ipsen Limited , NIH, and Parkinson Disease Foundation and consulting fees from Neupathe, Allergan Inc., Merz Pharmaceuticals, Ipsen Limited and Medtronic Corporation.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the National Institute of Neurological Disorders and Stroke.
Supplementary Material: Supplementary material is available online.
Clinical Trial Registration: clinicaltrials.gov, NCT00591344.
REFERENCES
- 1.Comella CL, Stebbins GT, Brown-Toms N, Goetz CG. Physical therapy and Parkinson’s disease: a controlled clinical trial. Neurology. 1994;44(3 Pt 1):376–8. doi: 10.1212/wnl.44.3_part_1.376. [DOI] [PubMed] [Google Scholar]
- 2.David FJ, Rafferty MR, Robichaud JA, et al. Progressive resistance exercise and Parkinson’s disease: a review of potential mechanisms. Parkinsons Dis. 2012;2012:124527. doi: 10.1155/2012/124527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petzinger GM, Fisher BE, Van Leeuwen JE, et al. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S141–5. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366(6):511–9. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcos DM, Chen CM, Quinn NP, McAuley J, Rothwell JC. Strength in Parkinson’s disease: relationship to rate of force generation and clinical status. Ann Neurol. 1996;39(1):79–88. doi: 10.1002/ana.410390112. [DOI] [PubMed] [Google Scholar]
- 6.Stevens-Lapsley J, Kluger BM, Schenkman M. Quadriceps muscle weakness, activation deficits, and fatigue with Parkinson disease. Neurorehabil Neural Repair. 2012;26(5):533–41. doi: 10.1177/1545968311425925. [DOI] [PubMed] [Google Scholar]
- 7.Hass CJ, Collins MA, Juncos JL. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: a randomized trial. Neurorehabil Neural Repair. 2007;21(2):107–15. doi: 10.1177/1545968306293449. [DOI] [PubMed] [Google Scholar]
- 8.Schilling BK, Pfeiffer RF, Ledoux MS, Karlage RE, Bloomer RJ, Falvo MJ. Effects of moderate-volume, high-load lower-body resistance training on strength and function in persons with Parkinson’s disease: a pilot study. Parkinsons Dis. 2010;2010:824734. doi: 10.4061/2010/824734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hass CJ, Buckley TA, Pitsikoulis C, Barthelemy EJ. Progressive resistance training improves gait initiation in individuals with Parkinson’s disease. Gait Posture. 2012;35(4):669–73. doi: 10.1016/j.gaitpost.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Scandalis TA, Bosak A, Berliner JC, Helman LL, Wells MR. Resistance training and gait function in patients with Parkinson’s disease. Am J Phys Med Rehabil. 2001;80(1):38–43. doi: 10.1097/00002060-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, LaStayo PC. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson’s disease. Mov Disord. 2006;21(9):1444–52. doi: 10.1002/mds.20997. [DOI] [PubMed] [Google Scholar]
- 12.Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves Quality Of Life in persons with Parkinson’s disease: a preliminary study. Parkinsonism Relat Disord. 2009;15(10):752–7. doi: 10.1016/j.parkreldis.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson’s disease. Arch Phys Med Rehabil. 2003;84(8):1109–17. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 14.Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson’s disease (2001): treatment guidelines. Neurology. 2001;56(11 Suppl 5):S1–S88. doi: 10.1212/wnl.56.suppl_5.s1. [DOI] [PubMed] [Google Scholar]
- 15.Cianci H. Parkinson Disease: Fitness Counts. 3rd ed National Parkinson Foundation; Miami, FL: 2006. [Google Scholar]
- 16.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases [see comments] Journal of Neurology Neurosurgery and Psychiatry. 1992;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canadian Society for Exercise Physiology [accessed 20 August 2012];Physical Activity Readiness Questionnaire - PAR -Q (revised 2002) 2002 http://uwfitness.uwaterloo.ca/PDF/par-q.pdf.
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” : A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Feigenbaum MS, Pollock ML. Prescription of resistance training for health and disease. Med Sci Sports Exerc. 1999;31(1):38–45. doi: 10.1097/00005768-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Langston JW, Widner H, Goetz CG, et al. Core assessment program for intracerebral transplantations (CAPIT) Mov Disord. 1992;7(1):2–13. doi: 10.1002/mds.870070103. [DOI] [PubMed] [Google Scholar]
- 21.Robichaud JA, Pfann KD, Comella CL, Brandabur M, Corcos DM. Greater impairment of extension movements as compared to flexion movements in Parkinson’s disease. Exp Brain Res. 2004;156(2):240–54. doi: 10.1007/s00221-003-1782-0. [DOI] [PubMed] [Google Scholar]
- 22.Vaillancourt DE, Prodoehl J, Verhagen Metman L, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004;127(Pt 3):491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- 23.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55(6):M350–5. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- 24.Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J Neurol. 1998;245(Suppl 1):S10–4. doi: 10.1007/pl00007730. [DOI] [PubMed] [Google Scholar]
- 25.Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. 3rd ed Springer-Verlag; New York: 1998. [Google Scholar]
- 26.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 27.Shulman LM, Gruber-Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson’s disease rating scale. Arch Neurol. 2010;67(1):64–70. doi: 10.1001/archneurol.2009.295. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2008;23(5):631–40. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- 29.Delorme TL, Watkins AL. Technics of progressive resistance exercise. Arch Phys Med Rehabil. 1948;29(5):263–73. [PubMed] [Google Scholar]
- 30.Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol. 2007;98(2):821–34. doi: 10.1152/jn.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spraker MB, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE. Basal ganglia hypoactivity during grip force in drug naive Parkinson’s disease. Hum Brain Mapp. 2010;31(12):1928–41. doi: 10.1002/hbm.20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valls-Sole J, Pascual-Leone A, Brasil-Neto JP, Cammarota A, McShane L, Hallett M. Abnormal facilitation of the response to transcranial magnetic stimulation in patients with Parkinson’s disease. Neurology. 1994;44:735–41. doi: 10.1212/wnl.44.4.735. [DOI] [PubMed] [Google Scholar]
- 33.Kidgell DJ, Stokes MA, Castricum TJ, Pearce AJ. Neurophysiological responses after short-term strength training of the biceps brachii muscle. J Strength Cond Res. 2010;24(11):3123–32. doi: 10.1519/JSC.0b013e3181f56794. [DOI] [PubMed] [Google Scholar]
- 34.LeWitt PA, Rezai AR, Leehey MA, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10(4):309–19. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 35.USDHHS [accessed 20 August 2012];Physical activity guidelines for Americans. 2008 http://www.health.gov/paguidelines/pdf/paguide.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.