Abstract
High fever and/or rash prior to neutrophil engraftment are frequently observed after umbilical cord blood (UCB) transplantation, and the condition is referred to as pre-engraftment syndrome (PES). Few studies have evaluated the risk factors for and treatment response to PES. Therefore, we retrospectively characterized PES in 57 consecutive engrafted patients (≥12 years old) who received myeloablative dual UCB transplantation. All patients received TBI (≥13.2Gy)-based myeloablative conditioning. Tacrolimus (n=35) or cyclosporine (n=22) combined with mycophenolate mofetil was used as GVHD prophylaxis. PES was defined as the presence of non-infectious fever (≥38.5°C) and/or rash prior to or on the day of neutrophil engraftment. The incidence (95% CI) of PES was 77% (66%–88%). The incidence of PES was significantly higher in patients who received cyclosporine as a GVHD prophylaxis than those who received tacrolimus (P<0.001), and this association was confirmed in the multivariate analysis. The occurrence of PES did not impact overall survival or tumor relapse, although it may have increased non-relapse mortality (P=0.071). The incidence of acute GHVD or treatment-related mortality was not influenced by the choice to use corticosteroids to treat PES. This study suggests that use of cyclosporine for GVHD prophylaxis increases the risk of PES following dual umbilical cord blood transplantation.
Keywords: pre-engraftment syndrome, dual umbilical cord blood transplantation, myeloablative
Introduction
Pre-engraftment syndrome (PES) or pre-engraftment immune reaction (PIR) following umbilical cord blood (UCB) transplantation was first reported by Kishi et al. 1 in a single UCB transplantation series after reduced-intensity conditioning. PES is characterized by high-grade fever, weight gain, and/or skin rash >5 days prior to engraftment. Patel et al. 2 defined PES as unexplained fever and/or unexplained fever occurring before or at the time of neutrophil recovery, and reported PES as a distinct syndrome not associated with graft-versus-host disease (GVHD) after dual UCB transplantation. However, a uniformly accepted definition of PES following UCB transplantation has yet to be established. In addition, the risk factors for PES and its clinical course have been poorly and inconsistently described 1–4. Patel et al. 2 showed that PES responds promptly to short-course corticosteroid therapy and has no association with acute GVHD, whereas Frangoul et al. 5 reported that PES was associated with grade II–IV or III–IV acute GVHD and chronic GVHD.
In the present study, we retrospectively analyzed PES in 57 consecutive engrafted patients who received dual UCB transplantation after total-body irradiation (TBI)-based myeloablative conditioning. We focus particularly on risk factors and treatment response.
Methods
Inclusion and exclusion criteria
We included consecutive patients aged 12 years or older who achieved successful donor engraftment following dual UCB transplantation. All patients received a TBI-based (≥13.2 Gy) myeloablative conditioning regimen for hematological malignancies at the Duke University Medical Center between 2006 and 2011. The 2 UCB grafts were at least 4/6 HLA-matched with the recipient, and with at least 3/6 inter-graft HLA matching (low-resolution for A and B, and high-resolution for DRB1). Patients who had a history of previous allogeneic transplantation or who had received high-dose corticosteroids (1000 mg/day) for pulmonary hemorrhage prior to engraftment were excluded. The study was approved by the institutional review board of the Duke University School of Medicine.
Definition of pre-engraftment syndrome and treatment response
PES was defined as non-infectious fever (≥38.5°C) and/or unexplained rash, occurring prior to or on the day of neutrophil engraftment. Neutrophil engraftment was defined as the first of 3 consecutive days on which a neutrophil count of ≥500/μL is achieved. Non-infectious fever was defined as a febrile episode associated with a negative blood culture. Febrile episodes that quickly responded to antibiotics without corticosteroid treatment were excluded. Drug-associated rash was excluded. The day of PES was defined as the first day of fever or rash, whichever came first. There is no uniform approach to the management of PES at our center. The decision to administer corticosteroid therapy for PES was left to the discretion of the managing physician.
Response was assessed on day 3 and day 7 after starting systemic corticosteroid treatment. Complete response (CR) and no response (NR) of fever in PES after treatment were defined by a temperature of <38.5°C for more than 24 hours and any other status than CR, respectively. CR, partial response (PR), and NR of rash in PES after treatment were defined as complete resolution, any documented improvement, and no response or worsening of rash, respectively. For patients who had both fever and rash, overall response was defined as CR if both symptoms resolved, was defined as PR if either fever resolved OR rash improved or resolved, and was defined as NR if neither fever or rash resolved. Acute GVHD was characterized according to standard criteria 6, 7; however, the date of onset of acute GVHD was not the same as the date of onset of PES. If PES improved with or without corticosteroid treatment, then the next date on which new signs of acute GVHD occurred was the day of onset of acute GVHD. If PES did not show any response (NR) and progressed to acute GVHD, the date of onset of PES was taken as the date of onset of acute GVHD.
Statistical analysis
The primary objective of this study was to assess for associations between PES and patient transplanted-related characteristics. The primary endpoint was the incidence of PES. The secondary endpoints were incidence of acute GVHD, non-relapse mortality, relapse, and overall survival. Because we included the patients who achieved successful donor engraftment, death was not a competing risk in the calculation of PES incidence. We compared the median day of PES onset based on the Wilcoxon rank sum test. We used 2-tailed Fisher’s exact test to assess if there were nonrandom associations between the incidences of PES and patient characteristics. We also fitted logistic regression models to assess if patient characteristics predicted PES. Univariate analysis was performed, and predictors were identified separately for PES. All variables with a P value <0.5 in the initial univariate analysis were considered potential predictors of PES in the multivariate logistic regression model. We further evaluated possible association between PES and other symptoms by 2-tailed Fisher’s exact test. We estimated overall survival with Kaplan-Meier method, and estimated the incidences of acute GVHD, non-relapse mortality, and relapse with the cumulative incidence method 8. The difference in survival rate based on PES classification was assessed by log-rank test, and the difference in the cumulative incidence curves were determined by Gray test.
The patient characteristics that were assessed for an association with PES included the following: recipient age, recipient gender, disease risk at transplant (standard or high risk), disease (myeloid disease or others), HLA mismatching (4/6 + 4/6 match or others), total nucleated cell (TNC) doses of engrafted cord blood unit (<2.5 or ≥ 2.5 × 107 /kg) or, combined TNC doses of 2 cord blood units (<5.0 or ≥ 5.0 × 107 /kg), GVHD prophylaxis (cyclosporine or tacrolimus), and conditioning regimen (fludarabine + TBI vs. others). Standard-risk diseases were defined as acute myelogenous leukemia in the first or second complete remission; acute lymphoblastic leukemia in the first or second complete remission; myelodysplastic syndromes; refractory anemia or refractory anemia with excess of blasts-1; Hodgkin or non-Hodgkin lymphoma in any chemotherapy-sensitive remission; and chronic myelogenous leukemia in the first or second chronic phase. High-risk diseases were those other than standard-risk diseases.
We collected the following information of other symptoms than PES: weight gain (>10% increase over body weight just before the beginning of conditioning regimen); non-infectious diarrhea; increase in serum total bilirubin (>2 mg/dL), serum AST/ALT (>2 times higher than the normal upper limit), and serum creatinine levels (>2 times higher than baseline just before the beginning of conditioning regimen); pulmonary edema; and requirement for oxygen or pulse oximeter-measured oxygen saturation less than 92, until or at the neutrophil engraftment.
Results
Patients and transplant characteristics
Of the 59 consecutive patients who met inclusion criteria for this study, 2 patients were excluded due to corticosteroid treatment for diffuse alveolar hemorrhage before engraftment (days 8 and 22); both died from diffuse alveolar hemorrhage at day 48 and 85. Table 1 shows patient and transplant characteristics of 57 patients. Median patient age was 28 (range, 12–58) years. Disease status at transplant was standard risk in 41 patients (72%) and high risk in 16 patients (28%). Primary disease for transplant was acute myelogenous leukemia in half of the patients. Tacrolimus (n = 35) or cyclosporine (n = 22) combined with mycophenolate mofetil (MMF) was used as GVHD prophylaxis. The choice of GVHD prophylaxis was determined by the active local and national UCB transplantation protocols that were active during the study period. All patients received TBI (≥13.2 Gy)-based myeloablative conditioning, and half of them received a reduced toxicity myeloablative conditioning regimen consisting of fludarabine (160 mg/m2) and TBI (13.5 Gy) with the use of tacrolimus as GVHD prophylaxis. The median combined TNC dose was 4.8 (range, 2.8–10.1) × 107/kg. The median TNC dose of engrafted and failed cord blood was 2.5 (1.4–5.5) and 2.4 (1.3–5.7), respectively. Median time to neutrophil engraftment was day 23 (range, 15–43). The median follow-up period of survivors was 662 (range, 101–1817) days.
Table 1.
Patient and transplant characteristics
| Characteristics | n = 57 |
|---|---|
| Age (years), median (range) | 28 (12–58) |
| Sex | |
| Male | 28 (49%) |
| Female | 29 (51%) |
| Disease risk at transplant | |
| Standard-risk | 41 (72%) |
| High-risk | 16 (28%) |
| Disease | |
| Acute myelogenous leukemia | 31 (54%) |
| Acute lymphoblastic leukemia | 12 (21%) |
| Mixed lineage acute leukemia | 1 (1%) |
| Chronic myelogenous leukemia | 2 (4%) |
| Myelodysplastic syndrome | 6 (11%) |
| Malignant lymphoma | 5 (9%) |
| Conditioning regimen | |
| FLU/TBI | 26 (46%) |
| FLU/TBI/CY | 16 (28%) |
| FLU/TBI/THIO | 7 (12%) |
| FLU/TBI/MEL | 2 (4%) |
| TBI/CY/ATG | 3 (5%) |
| TBI/MEL/ATG | 3 (5%) |
| GVHD prophylaxis | |
| Tacrolimus + MMF | 35 (61%) |
| Cyclosporine + MMF | 22 (39%) |
| HLA matching | |
| 4/6 + 4/6 | 25 (44%) |
| 4/6 + 5/6 | 17 (29%) |
| 4/6 + 6/6 | 2 (4%) |
| 5/6 + 5/6 | 7 (12%) |
| 5/6 + 6/6 | 4 (7%) |
| 6/6 + 6/6 | 2 (4%) |
| Combined dose of cryopreserved TNC (×107/kg) | |
| Median (range) | 4.8 (2.8–10.1) |
Abbreviations: GVHD, graft-versus-host disease; TNC, total nucleated cells; FLU, fludarabine; TBI, total-body irradiation; CY, cyclophosphamide; THIO, thiotepa; MEL, melphalan; ATG, antithymocyte globulin; MMF, mycophenolate mofetil
Pre-engraftment syndrome
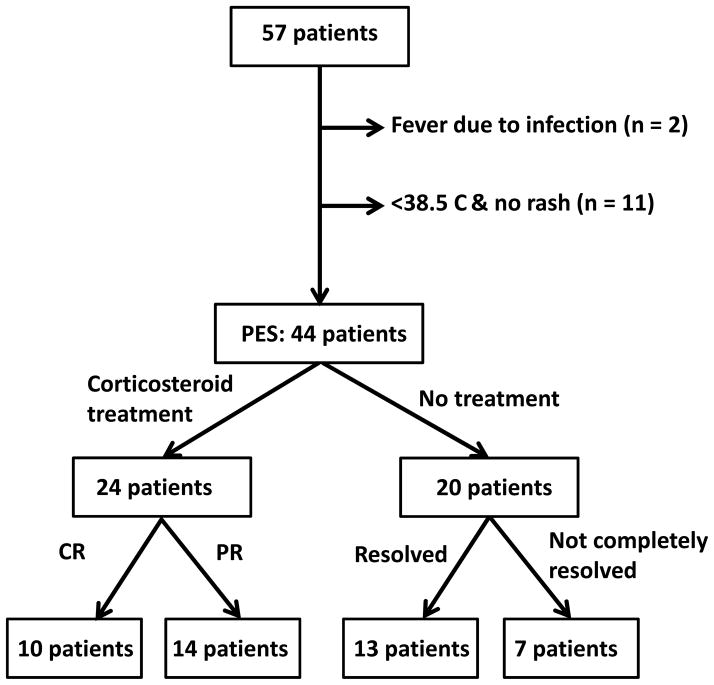
Forty-four of 57 patients met the criteria of PES (Figure 1). Median PES onset was at day 12 (range, 4–22), and the incidence (95% confidence interval [CI]) of PES was 77% (66%–88%). Median interval between PES and neutrophil engraftment was 11 (range, 0–26) days. Median PES onset was significantly earlier in the cyclosporine group compared with in the tacrolimus group (day 10 vs. day 14, P = 0.015), and the incidence of PES was significantly higher in the cyclosporine group (incidence rate of 100%) compared with in the tacrolimus group (incidence rate of 63% with 95% CI, 47%–79%), whereas the incidence of PES was similar in the other stratified group (Table 2). The univariate logistic regression confirmed that only the use of cyclosporine was significantly associated with the occurrence of PES; the odds ratio of PES for the cyclosporine group versus tacrolimus group was 27.0 (95% CI, 1.4–513.3, P = 0.03). We obtained the same finding even after adjusting for the possible confounding variables of age and conditioning regimen. Multivariate logistic regression analysis determined that the odds ratio of PES for the cyclosporine group versus tacrolimus group was 59.6 (95% CI,1.9–>999.9, P = 0.02).
Figure 1.
Flow chart of diagnosis of and treatment response for PES
Table 2.
PES and patient characteristics
| Category | PES (−) | PES (+) | P value |
|---|---|---|---|
| Age (years) | |||
| <18 | 1 | 9 | |
| ≥18 | 12 | 35 | 0.426 |
| Sex | |||
| Male | 7 | 21 | |
| Female | 6 | 23 | 0.759 |
| Disease risk | |||
| Standard | 10 | 31 | |
| High | 3 | 13 | 0.739 |
| Disease | |||
| Myeloid disease | 10 | 29 | |
| Others | 3 | 15 | 0.519 |
| HLA matching | |||
| 4/6 + 4/6 | 8 | 24 | |
| Others | 5 | 20 | 0.756 |
| TNC of engrafted cord unit* | |||
| <2.5 × 107/kg | 5 | 21 | |
| ≥2.5 × 107/kg | 8 | 22 | 0.545 |
| Combined TNC* | |||
| <5.0 × 107/kg | 7 | 25 | |
| ≥ 5.0 × 107/kg | 6 | 18 | 1.000 |
| GVHD prophylaxis | |||
| Tacrolimus + MMF | 13 | 22 | |
| Cyclosporine + MMF | 0 | 22 | <0.001 |
| Conditioning regimen | |||
| FLU/TBI | 8 | 18 | |
| Others | 5 | 26 | 0.219 |
Abbreviations: TNC, total nucleated cells, GVHD, graft-versus-host disease; MMF, mycophenolate mofetil; FLU/TBI, fludarabine/total-body irradiation.
P values were from Fisher’s Exact test.
Data not available for one patient.
Body weight gain (>10%), diarrhea, and hyperbilirubinemia (>2 mg/dL) were more frequently observed in patients with PES than in those without PES (body weight gain, 23% vs. 8%; diarrhea, 32% vs. 15%; hyperbilirubinemia, 14% vs. 0%), though none of these differences were statistically significant (Table 3). Rates of transaminitis and hypoxia were low. Among the 44 patients with PES, 3 had fever only, 13 had rash only and 28 had both fever and rash; 60–70% of patients had other systemic symptoms in three categories (67%, 62%, and 68%, respectively). Non-infectious diarrhea was not observed in 3 patients who had fever, while 5 of 13 (39%) and 9 of 28 (32%) had symptoms of non-infectious diarrhea.
Table 3.
The association of post-transplantation complications with and without PES
| Category | PES (−) | PES (+) | P value |
|---|---|---|---|
| Body weight gain (>10%) | 1 (8%) | 10 (23%) | 0.426 |
| Non-infectious diarrhea | 2 (15%) | 14 (32%) | 0.313 |
| Increase in T-bil (>2 mg/dL) | 0 (0%) | 6 (14%) | 0.319 |
| Increase in AST/ALT (>2 times) | 0 | 2 (5%) | 1.000 |
| Increase in Cre (>2 times) | 0 | 5 (11%) | 0.579 |
| Pulmonary edema | 3 (23%) | 11 (25%) | 1.000 |
| Hypoxia/ oxygen requirement | 0 | 1 (2%) | 1.000 |
Abbreviations: T-bil, total bilirubin; AST, aspartate amino transferase; ALT, alanine amino transferase; Cre, creatinine
P values were from Fisher’s Exact test.
Acute graft-versus-host disease
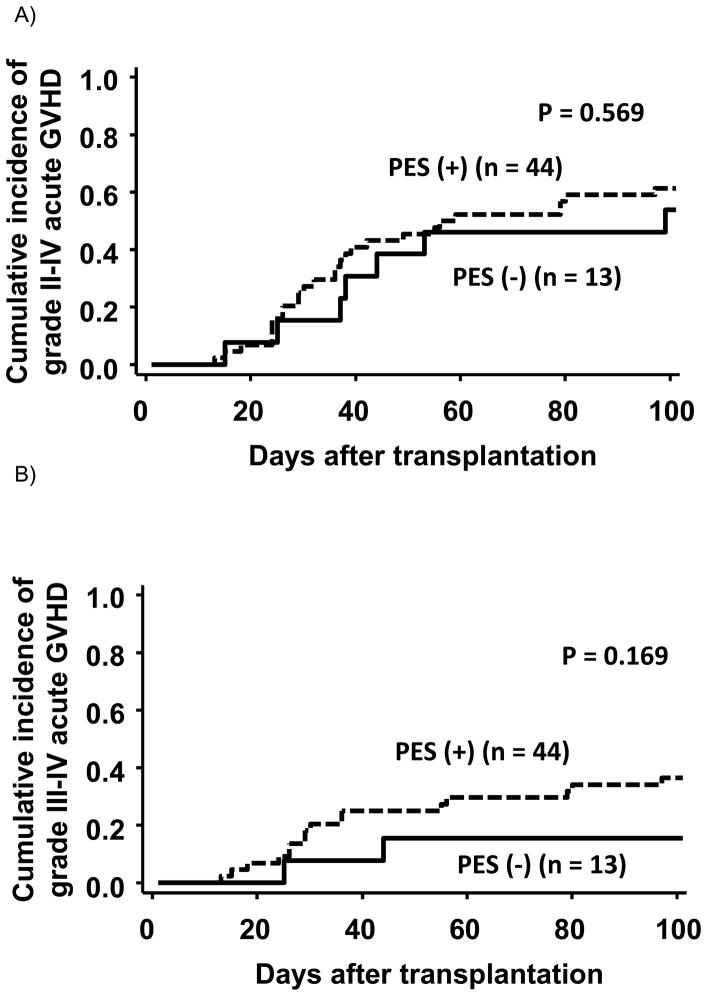
Median onset of acute grade II–IV aGVHD in patients with and without PES was 35 (range, 12–96) and 37 (range, 14–98), respectively, and there was no statistical difference between the 2 groups (P = 0.609). The incidence (95% CI) of grades II–IV acute GVHD (aGVHD) at day 100 in patients with and without PES was 61% (45%–74%) and 54% (25%–76%), respectively, and that of grades III–IV aGVHD at day 100 in patients with and without PES was 36% (23%–50%) and 15% (2%–39%), respectively (Figure 2). There was no difference in the incidences of grades II–IV (P = 0.569) or grades III–IV (P = 0.169) between the 2 groups. We also analyzed ratio of steroid refractory acute GVHD in patients who developed grades II–IV acute GVHD. Interestingly, we found that steroid refractory acute GVHD occurred only among patients who also developed PES (non-PES vs. PES group; 0% vs. 40%, P = 0.040).
Figure 2.
Cumulative incidence of grade II–IV or grade III–IV acute GVHD stratified by the presence of pre-engraftment syndrome
Response to treatment
Twenty-four of 44 patients with PES were treated with ≥1 mg/kg/day of corticosteroids (Table 4 and Figure 1). Fever resolved quickly after the treatment, whereas rash improved but remained present in most patients at day 3 of treatment. Overall, corticosteroid treatment resulted in CR in 10 patients and PR in 14 patients, at day 7. In 2 patients, PES progressed to or overlapped with GVHD after partial response to initial corticosteroid therapy. Among the 22 PES patients who could be evaluated, grade II and grade III–IV aGVHD developed in 7 and 8 patients, respectively.
Table 4.
Response to systemic corticosteroid therapy for pre-engraftment syndrome
| Treatment response | Fever (n) | Rash (n) |
|---|---|---|
| day 3 after treatment | ||
| CR | 21 | 2 |
| PR | - | 16 |
| NR | 1 | 3 |
| No fever or rash* | 2 | 3 |
| day 7 after treatment | ||
| CR | 22 | 7 |
| PR | 0 | 14 |
| NR | 0 | 0 |
| No fever or rash* | 2 | 3 |
Abbreviations: CR, complete response; PR, partial response; NR, no response
Treatment response for fever or rash was not evaluable because patients had either fever or rash as clinical manifestation of PES.
Twenty of 44 patients with PES were not treated with corticosteroids; therefore, they were not evaluable for treatment response. Among the 20 patients, PES resolved without systemic corticosteroid treatment in 13 patients. Among 18 of 20 patients who could be evaluated for acute GVHD, 12 developed grade II–IV aGVHD (grade II in 4 and grades III–IV aGVHD in 8). The occurrence of grade II–IV aGVHD was not influenced by treatment of PES with corticosteroids (P = 0.328).
The only difference in PES characteristics between patients who were treated with ≥1 mg/kg/day of corticosteroids or not was an increase in serum creatinine level >2 times above baseline (0% vs. 20.8%, P = 0.030).
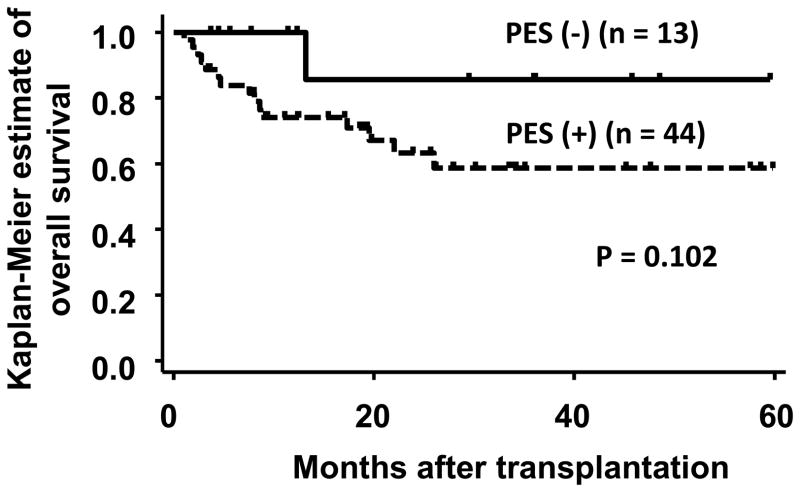
Overall survival, relapse, and non-relapse mortality
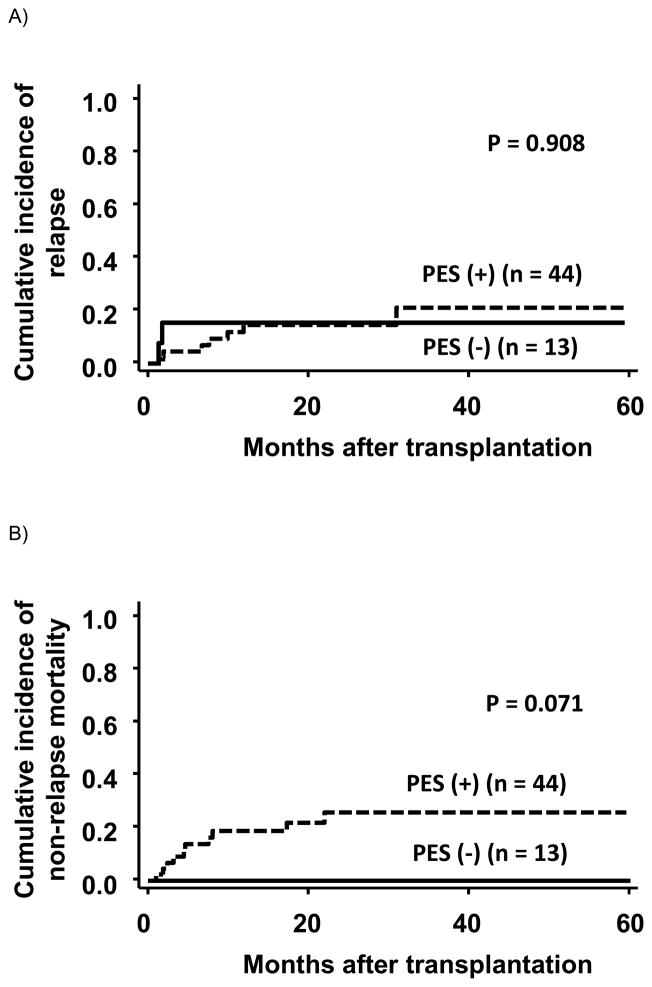
The overall survival (95% CI) at 2 years was 86% (33%–98%) and 63% (45%–77%) in patients with and without PES, respectively. This difference was not statistically significant (P = 0.102) (Figure 3). The cumulative incidence of relapse was comparable between patients with and without PES (relapse incidence at 2 years, 15% (95% CI, 2%–39%) and 15% (95% CI, 6%–27%), respectively) (Figure 4). The incidence of non-relapse mortality at 2 years in patients with PES was 26% (95% CI, 13%–41%). In contrast, none of the 13 patients without PES died of treatment-related complications (Figure 4). Among 44 patients with PES, 10 patients died of treatment-related complications without relapse: 7 died of infection, 1 of GVHD, 1 of diffuse alveolar hemorrhage, and 1 of unknown cause. The incidence of treatment-related mortality was not influenced by treatment of PES with corticosteroids (P = 0.346), although 5 of the 7 patients who died from infection received systemic corticosteroid treatment for PES (parainfluenza virus 1, adenovirus 1, adenovirus + aspergillus 1, vancomycin-resistant enterococcus 1, and multiple bacterial organisms 1), and 2 of the 5 patients died within 100 days after transplantation.
Figure 3.
Overall survival stratified by the presence of pre-engraftment syndrome
Figure 4.
Relapse and non-relapse mortality stratified by the presence of pre-engraftment syndrome
Discussion
In this study, we confirmed the high incidence of PES following UCB transplantation. In addition, we found that the use of cyclosporine + MMF vs. tacrolimus + MMF was a risk factor for PES. Although PES did not have a statistically significant impact on overall survival, patients with PES died more often because of transplant-related complications; half of the mortality was associated with infection. PES resolved in most of the patients treated with ≥1 mg/kg corticosteroids. However, in 13 of 20 patients, it also resolved in the absence of systemic corticosteroid treatment.
The incidence of PES in our cohort (77%) was comparable to that reported by Kishi et al. (78%) 1, but higher than that reported by Patel et al. (31%) 2 or Frangoul et al. (20%) 5. The definition of PES used in our study was similar to that used in the study by Patel et al., but the median onset of PES (12 [range, 4–22] days) in our study was later (9 [range, 5–12] days), and we report more PES occurrence close to neutrophil engraftment. Therefore, we recalculated the incidence of PES by excluding PES that occurred within 5 days from the neutrophil engraftment. The incidence of PES based on this definition was 63% (95% CI, 51%–75%), and was still higher than their incidence. The rate of PES by this definition was also significantly higher in the cyclosporine group compared with the tacrolimus group. To decrease the collection bias inherent to retrospective studies and clarify the incidence of PES in UCB transplantation, PES-related information should be prospectively collected using uniform criteria, ensuring homogeneous patient background. The lower incidence of PES reported by Frangoul et al. might be partly explained by the routine use of anti-thymocyte globulin and methylprednisolone (1 mg/kg day+1 – day+4, 2 mg/kg day+5 – day+19 or the day of neutrophil engraftment) for GVHD prophylaxis.
We found that the use of cyclosporine + MMF vs. tacrolimus + MMF was a risk factor for PES after myeloablative dual UCB transplantation. This may reflect the fact that the immunosuppressive effect of tacrolimus + MMF may be stronger than that of cyclosporine + MMF 9–12 in the early phase after transplantation. Clearly, the differential activity of tacrolimus vs. cyclosporine remains controversial, as demonstrated by the lack of difference in acute or chronic GVHD with use of either agent following mobilized peripheral blood stem cell transplantation 13. Uchida et al. 3 recently showed that the addition of MMF to tacrolimus for GVHD prophylaxis in single UCB transplantation was associated with a lower incidence of PES and acute GVHD than that by the use of tacrolimus alone. In addition, the incidence of PES was only 20% in the study by Frangoul et al., where anti-thymocyte globulin and high dose methylprednisolone was used for GVHD prophylaxis. Collectively, these findings suggest that PES as well as GVHD can be prevented by intensifying immune suppression following transplantation.
The next question that arises is how to differentiate between PES and GVHD. Engraftment syndrome characterized by pre-engraftment fever, rash, and noncardiogenic pulmonary edema has been well described in autologous transplantation 14, 15. Hyperacute GVHD characterized by a median onset of 8 days was first described in matched sibling transplantation without GVHD prophylaxis 16, and later it was shown that this type of GVHD was common in patients receiving transplants from a mismatched related or matched unrelated donor 17. Therefore, there may be 2 mechanisms in PES: one related to the cytokine storm associated with the expansion of stem cells as seen in autologous transplantation and the other related to the graft-versus-host reaction against major or minor mismatched antigen by donor T cells. Thus, in order to diagnose PES and elucidate its mechanism, the potential biomarkers specific to PES are worthy of investigation 18.
The appropriateness of using stronger immunosuppression to prevent PES is a clinically relevant question. We did not find a significant difference in overall mortality between patients with and without PES. However, patients with PES were more likely to die because of transplant-related complications, mainly due to infection. Although treatment-related mortality was not different according to systemic corticosteroid treatment, 5 of 7 patients who died from infection received corticosteroid treatment for PES. This raises the consideration that the use of corticosteroids is perhaps better avoided unless strictly necessary. PES resolved without systemic corticosteroid treatment in 13 of 20 patients, suggesting the need to identify patients who require treatment for PES. However, the decision to start the corticosteroid treatment was determined by physician preference. Therefore, it is possible that our cohort contained patients who needed corticosteroids for a long period to treat resistant PES, leading to a profoundly immunocompromised state. These questions could be answered by implementing a uniform treatment strategy for PES in future studies.
Limitations of this study involving heterogeneity of the conditioning regimens and linkage of the conditioning regimen to specific GVHD prophylaxis strategies should be noted. Although myeloablative doses of TBI (≥13.2 Gy) were uniformly used in our cohort, regimen intensity was slightly different. Particularly, the reduced toxicity myeloablative combination of fludarabine and TBI was linked to tacrolimus and MMF as GVHD prophylaxis. While multivariate analysis would suggest otherwise, there remains the possibility that use of this reduced toxicity regimen, instead of the use of tacrolimus, contributed to the lower incidence of PES. While we were unable to detect a difference in treatment-related mortality in patients treated with or without corticosteroids, it is conceivable that this finding was confounded by differences in severity of PES between the two treatment groups. As a result of the small sample size, observations drawn from the multivariate logistic regression model must be interpreted with caution. The findings described in this study should be considered hypothesis generating, and provide a foundation for further investigation on a much larger scale, using a standardized definition of PES.
In conclusion, GVHD prophylaxis with cyclosporine was a risk factor for PES after myeloablative dual UCB transplantation in our cohort. PES did not have an impact on overall survival but may affect treatment-related complications partly due to complications of systemic corticosteroid therapy. The impact of GVHD prophylaxis (cyclosporine vs. tacrolimus) in UCB transplantation would need to be tested in a prospective trial with a uniform conditioning regimen and a common PES definition and treatment strategy.
Acknowledgments
J.K. is a Research Fellow of the Japan Society for the Promotion of Science.
Funding: This work was supported in part by National Cancer Institute (NIH) 5P01-CA047741-18 (M.H., N.C.) and a Grant-in-Aid for JSPS Fellows (J.K.).
Footnotes
Conflict-of-interest disclosure: Authors have no relevant financial relationship to disclose.
References
- 1.Kishi Y, Kami M, Miyakoshi S, Kanda Y, Murashige N, Teshima T, et al. Early immune reaction after reduced-intensity cord-blood transplantation for adult patients. Transplantation. 2005;80:34–40. doi: 10.1097/01.tp.0000163289.20406.86. [DOI] [PubMed] [Google Scholar]
- 2.Patel KJ, Rice RD, Hawke R, Abboud M, Heller G, Scaradavou A, et al. Pre-engraftment syndrome after double-unit cord blood transplantation: a distinct syndrome not associated with acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:435–40. doi: 10.1016/j.bbmt.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchida N, Wake A, Nakano N, Ishiwata K, Takagi S, Tsuji M, et al. Mycophenolate and tacrolimus for graft-versus-host disease prophylaxis for elderly after cord blood transplantation: a matched pair comparison with tacrolimus alone. Transplantation. 2011;92:366–71. doi: 10.1097/TP.0b013e318223d7ac. [DOI] [PubMed] [Google Scholar]
- 4.Narimatsu H, Terakura S, Matsuo K, Oba T, Uchida T, Iida H, et al. Short-term methotrexate could reduce early immune reactions and improve outcomes in umbilical cord blood transplantation for adults. Bone Marrow Transplant. 2007;39:31–9. doi: 10.1038/sj.bmt.1705539. [DOI] [PubMed] [Google Scholar]
- 5.Frangoul H, Wang L, Harrell FE, Jr, Ho R, Domm J. Preengraftment syndrome after unrelated cord blood transplant is a strong predictor of acute and chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:1485–8. doi: 10.1016/j.bbmt.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 7.Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–9. [PubMed] [Google Scholar]
- 8.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–8. [PubMed] [Google Scholar]
- 10.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–14. [PubMed] [Google Scholar]
- 11.Hiraoka A, Ohashi Y, Okamoto S, Moriyama Y, Nagao T, Kodera Y, et al. Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2001;28:181–5. doi: 10.1038/sj.bmt.1703097. [DOI] [PubMed] [Google Scholar]
- 12.Yanada M, Emi N, Naoe T, Sakamaki H, Takahashi S, Hirabayashi N, et al. Tacrolimus instead of cyclosporine used for prophylaxis against graft-versus-host disease improves outcome after hematopoietic stem cell transplantation from unrelated donors, but not from HLA-identical sibling donors: a nationwide survey conducted in Japan. Bone Marrow Transplant. 2004;34:331–7. doi: 10.1038/sj.bmt.1704596. [DOI] [PubMed] [Google Scholar]
- 13.Inamoto Y, Flowers ME, Appelbaum FR, Carpenter PA, Deeg HJ, Furlong T, et al. A retrospective comparison of tacrolimus versus cyclosporine with methotrexate for immunosuppression after allogeneic hematopoietic cell transplantation with mobilized blood cells. Biol Blood Marrow Transplant. 2011;17:1088–92. doi: 10.1016/j.bbmt.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893–8. doi: 10.1038/sj.bmt.1703015. [DOI] [PubMed] [Google Scholar]
- 15.Maiolino A, Biasoli I, Lima J, Portugal AC, Pulcheri W, Nucci M. Engraftment syndrome following autologous hematopoietic stem cell transplantation: definition of diagnostic criteria. Bone Marrow Transplant. 2003;31:393–7. doi: 10.1038/sj.bmt.1703855. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan KM, Deeg HJ, Sanders J, Klosterman A, Amos D, Shulman H, et al. Hyperacute graft-v-host disease in patients not given immunosuppression after allogeneic marrow transplantation. Blood. 1986;67:1172–5. [PubMed] [Google Scholar]
- 17.Saliba RM, de Lima M, Giralt S, Andersson B, Khouri IF, Hosing C, et al. Hyperacute GVHD: risk factors, outcomes, and clinical implications. Blood. 2007;109:2751–8. doi: 10.1182/blood-2006-07-034348. [DOI] [PubMed] [Google Scholar]
- 18.Morita-Hoshi Y, Mori SI, Soeda A, Wakeda T, Ohsaki Y, Shiwa M, et al. Identification of molecular markers for pre-engraftment immune reactions after cord blood transplantation by SELDI-TOF MS. Bone Marrow Transplant. 2010;45:1594–601. doi: 10.1038/bmt.2010.18. [DOI] [PubMed] [Google Scholar]