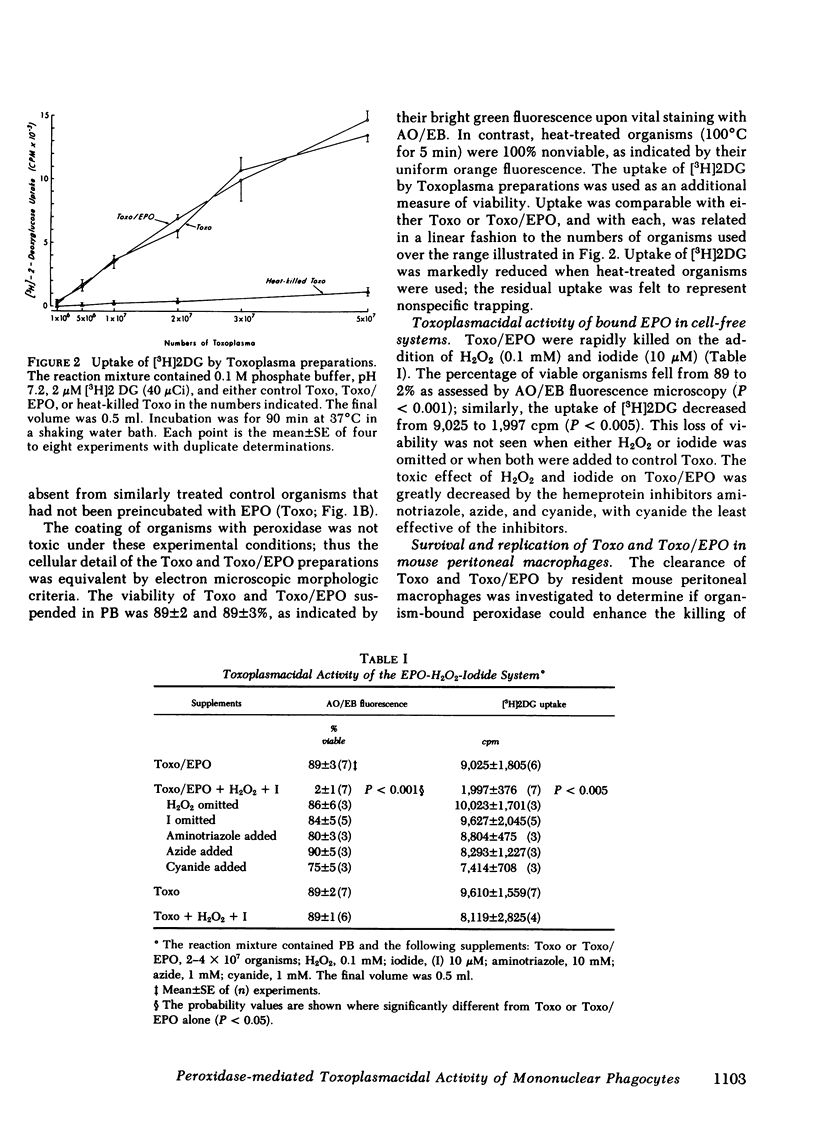
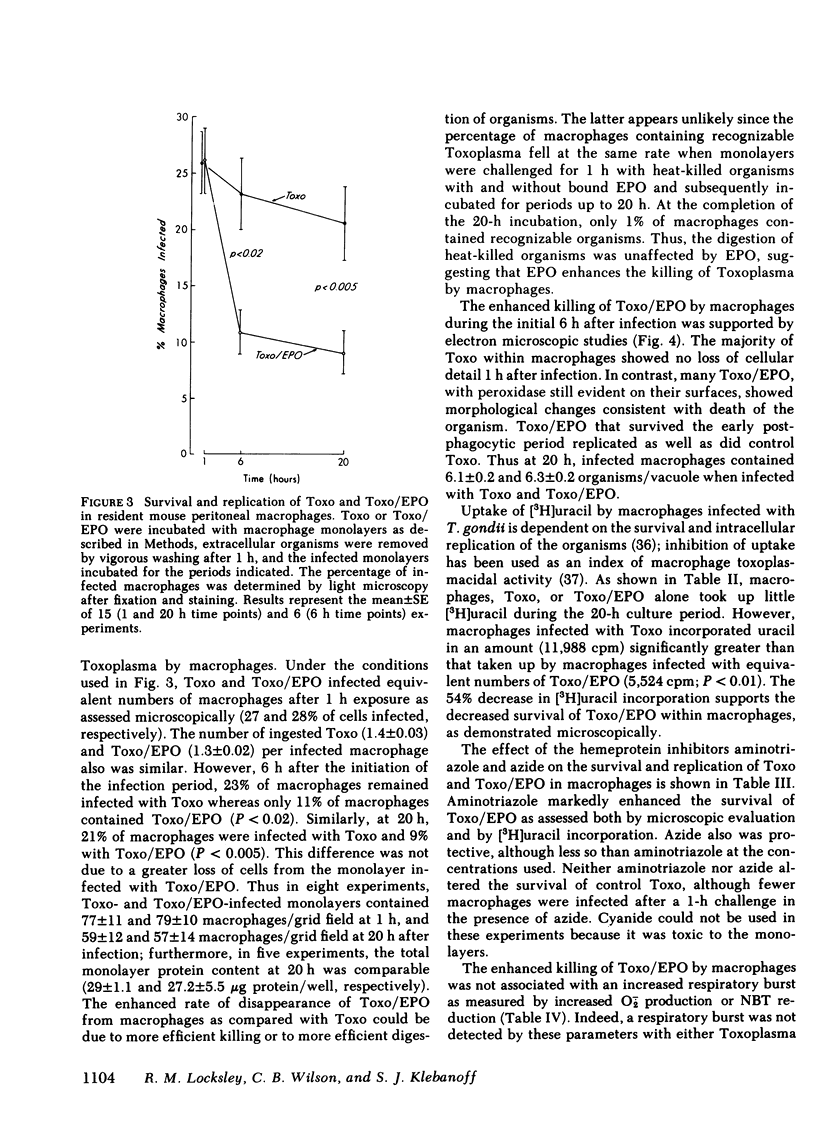
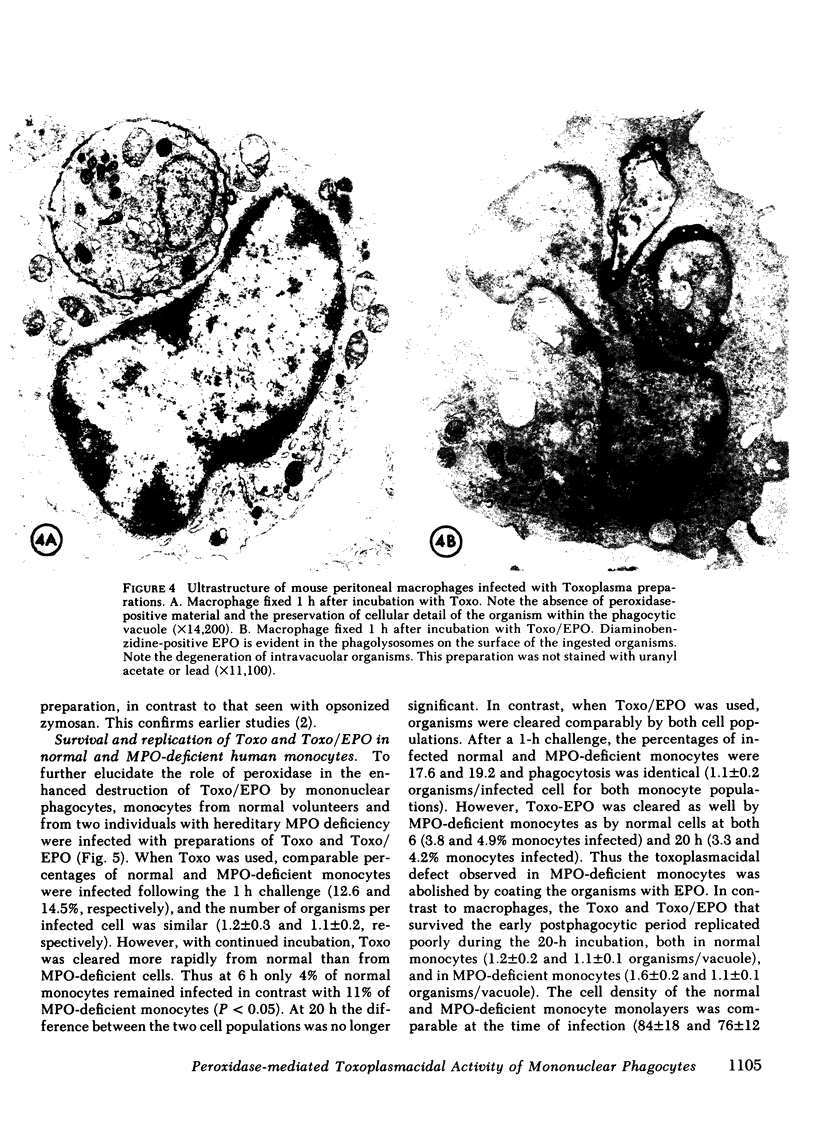
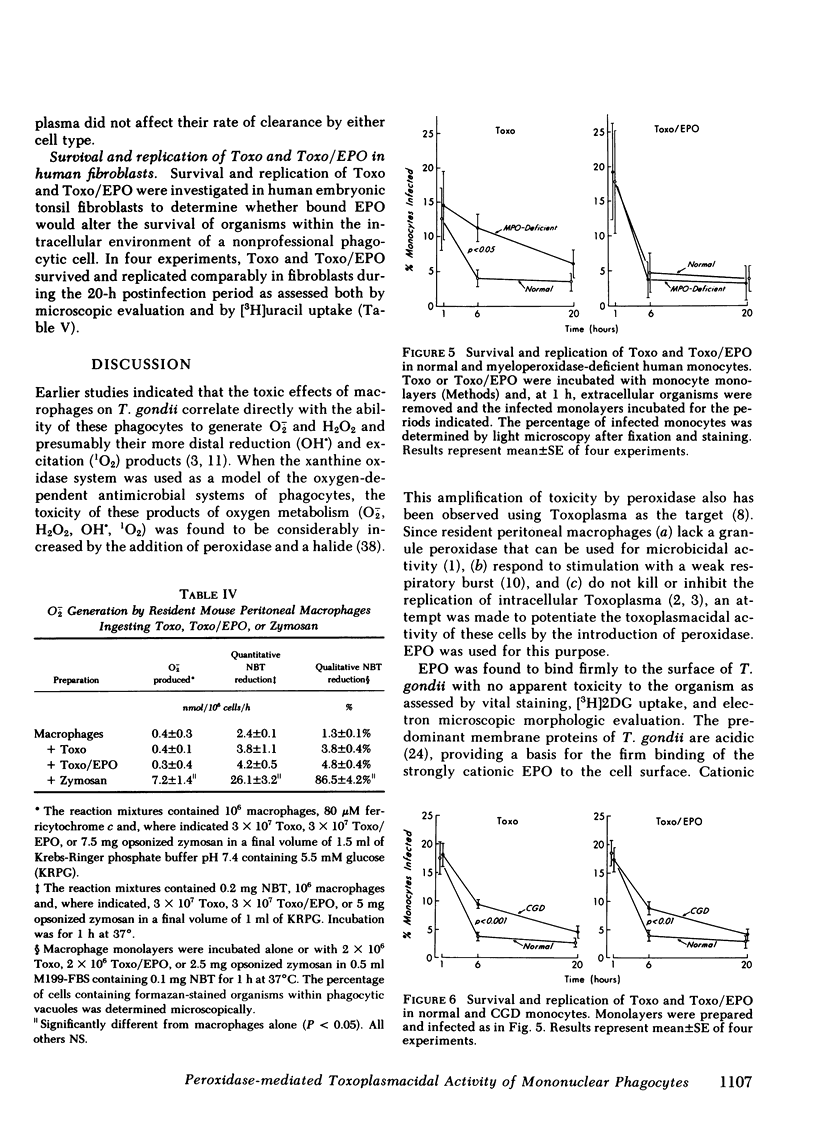
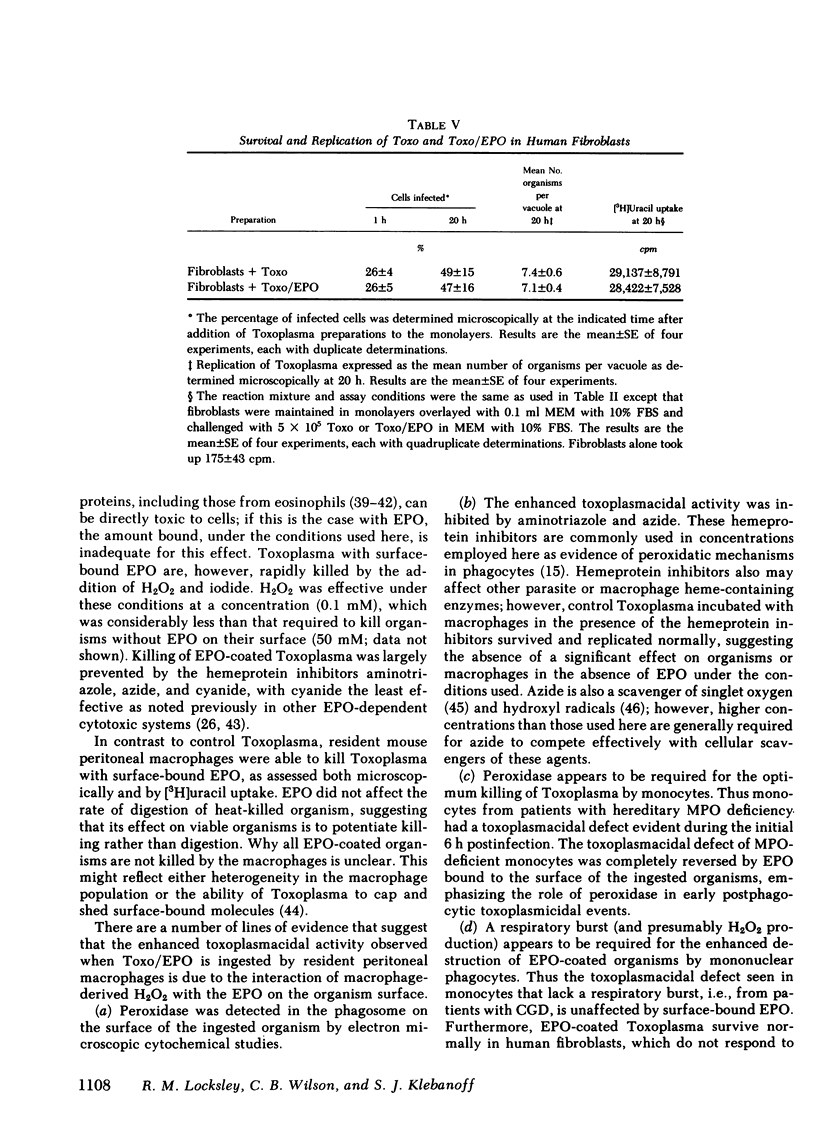
Abstract
Oxygen products generated by the respiratory burst of mononuclear phagocytes are microbicidal to intracellular pathogens including Toxoplasma gondii. The toxicity of one of these products, H2O2, is markedly amplified by the granule peroxidase of circulating phagocytes in the presence of a halide. Eosinophil peroxidase (EPO) binds firmly to the surface of T. gondii and such organisms remain viable as determined by vital staining, uptake of 2-deoxyglucose, and survival and replication in human fibroblasts. They are, however, rapidly killed by the addition of H2O2 and iodide under conditions in which control organisms are unaffected. We have used EPO bound to T. gondii to explore the role of peroxidase in the toxoplasmacidal activity of mononuclear phagocytes. Resident mouse peritoneal macrophages lack a granule peroxidase and have a weak respiratory burst; toxoplasma survive and replicate within these cells. However, these cells acquire significant toxoplasmacidal activity, as assessed microscopically and by the inhibition of uracil uptake, when organisms are coated with EPO before ingestion, an effect which is decreased by the hemeprotein inhibitors, aminotriazole and azide. EPO on the surface of Toxoplasma does not increase their ingestion by macrophages or the associated respiratory burst. Monocytes from patients with hereditary myeloperoxidase deficiency have a significant toxoplasmacidal defect that is abolished when EPO-coated organisms are used. In contrast, the toxoplasmacidal defect of monocytes from chronic granulomatous disease patients is unaffected by surface-bound EPO. In these studies, replication of surviving intracellular organisms varied inversely with the magnitude of the respiratory burst: replication was greatest in fibroblasts, slightly less in resident macrophages, and least in monocytes; it was significantly greater in chronic granulomotous disease than in normal or myeloperoxidase-deficient monocytes. These studies support a role for oxygen products and endogenous peroxidase in the optimal killing of T. gondii by monocytes and demonstrate that peroxidase-negative phagocytes can utilize peroxidase on the surface of ingested organisms to augment microbicidal activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwal O. S. Cytoenzymological behavior of peritoneal exudate cells of rat in vivo. I. Histochemical study of enzymatic function of peroxidase. J Reticuloendothel Soc. 1971 Aug;10(2):163–172. [PubMed] [Google Scholar]
- Buchmüller Y., Mauel J. Studies on the mechanisms of macrophage activation: possible involvement of oxygen metabolites in killing of Leishmania enrietti by activated mouse macrophages. J Reticuloendothel Soc. 1981 Mar;29(3):181–192. [PubMed] [Google Scholar]
- Butterworth A. E., Wassom D. L., Gleich G. J., Loegering D. A., David J. R. Damage to schistosomula of Schistosoma mansoni induced directly by eosinophil major basic protein. J Immunol. 1979 Jan;122(1):221–229. [PubMed] [Google Scholar]
- Cummings N. P., Pabst M. J., Johnston R. B., Jr Activation of macrophages for enhanced release of superoxide anion and greater killing of Candida albicans by injection of muramyl dipeptide. J Exp Med. 1980 Dec 1;152(6):1659–1669. doi: 10.1084/jem.152.6.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Dzbenski T. H., Michalak T., Plonka W. S. Electron microscopic and radioisotopic studies on cap formation in Toxoplasma gondii. Infect Immun. 1976 Nov;14(5):1196–1201. doi: 10.1128/iai.14.5.1196-1201.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigas E., Loegering D. A., Gleich G. J. Cytotoxic effects of the guinea pig eosinophil major basic protein on tracheal epithelium. Lab Invest. 1980 Jan;42(1):35–43. [PubMed] [Google Scholar]
- Gardner I. D., Remington J. S. Aging and the immune response. II. Lymphocyte responsiveness and macrophage activation in Toxoplasma gondii-infected mice. J Immunol. 1978 Mar;120(3):944–949. [PubMed] [Google Scholar]
- Gleich G. J., Frigas E., Loegering D. A., Wassom D. L., Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979 Dec;123(6):2925–2927. [PubMed] [Google Scholar]
- Handman E., Goding J. W., Remington J. S. Detection and characterization of membrane antigens of Toxoplasma gondii. J Immunol. 1980 Jun;124(6):2578–2583. [PubMed] [Google Scholar]
- Heifets L., Imai K., Goren M. B. Expression of peroxidase-dependent iodination by macrophages ingesting neutrophil debris. J Reticuloendothel Soc. 1980 Oct;28(4):391–404. [PubMed] [Google Scholar]
- Homan-Müller J. W., Weening R. S., Roos D. Production of hydrogen peroxide by phagocytizing human granulocytes. J Lab Clin Med. 1975 Feb;85(2):198–207. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong E. C., Henderson W. R., Klebanoff S. J. Bactericidal activity of eosinophil peroxidase. J Immunol. 1980 Mar;124(3):1378–1382. [PubMed] [Google Scholar]
- Jörg A., Pasquier J. M., Klebanoff S. J. Purification of horse eosinophil peroxidase. Biochim Biophys Acta. 1982 Feb 18;701(2):185–191. doi: 10.1016/0167-4838(82)90112-1. [DOI] [PubMed] [Google Scholar]
- Kazura J. W., Fanning M. M., Blumer J. L., Mahmoud A. A. Role of cell-generated hydrogen peroxide in granulocyte-mediated killing of schistosomula of Schistosoma mansoni in vitro. J Clin Invest. 1981 Jan;67(1):93–102. doi: 10.1172/JCI110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod R., Remington J. S. A method to evaluate the capacity of monocytes and macrophages to inhibit multiplication of an intracellular pathogen. J Immunol Methods. 1979 May 10;27(1):19–29. doi: 10.1016/0022-1759(79)90235-7. [DOI] [PubMed] [Google Scholar]
- McLeod R., Remington J. S. Studies on the specificity of killing of intracellular pathogens by macrophages. Cell Immunol. 1977 Nov;34(1):156–174. doi: 10.1016/0008-8749(77)90238-6. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Juangbhanich C. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Nathan C. F., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. IV. Role of endogenous scavengers of oxygen intermediates. J Exp Med. 1980 Dec 1;152(6):1610–1624. doi: 10.1084/jem.152.6.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Root R. K. Hydrogen peroxide release from mouse peritoneal macrophages: dependence on sequential activation and triggering. J Exp Med. 1977 Dec 1;146(6):1648–1662. doi: 10.1084/jem.146.6.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D. R., Bryan V. M., Oi V. T., Herzenberg L. A. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1962–1966. doi: 10.1073/pnas.76.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferkorn E. R., Pfefferkorn L. C. Specific labeling of intracellular Toxoplasma gondii with uracil. J Protozool. 1977 Aug;24(3):449–453. doi: 10.1111/j.1550-7408.1977.tb04774.x. [DOI] [PubMed] [Google Scholar]
- Ramsey P. G., Martin T., Chi E., Klebanoff S. J. Arming of mononuclear phagocytes by eosinophil peroxidase bound to Staphylococcus aureus. J Immunol. 1982 Jan;128(1):415–420. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Bactericidal activity of a superoxide anion-generating system. A model for the polymorphonuclear leukocyte. J Exp Med. 1979 Jan 1;149(1):27–39. doi: 10.1084/jem.149.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Klebanoff S. J. The eosinophilic leukocyte. Fine structure studies of changes in the uterus during the estrous cycle. J Exp Med. 1966 Oct 1;124(4):653–660. doi: 10.1084/jem.124.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J., de Souza W. A morphological study of the interaction between Trypanosoma cruzi and rat eosinophils, neutrophils and macrophages in vitro. J Cell Sci. 1979 Jun;37:275–286. doi: 10.1242/jcs.37.1.275. [DOI] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R. J., Zgliczynski J. M., Paul B. B., Sbarra A. J. Enhanced killing of myeloperoxidase-coated bacteria in the myeloperoxidase-H2O2-Cl- system. J Infect Dis. 1978 Apr;137(4):481–485. doi: 10.1093/infdis/137.4.481. [DOI] [PubMed] [Google Scholar]
- Simmons S. R., Karnovsky M. L. Iodinating ability of various leukocytes and their bactericidal activity. J Exp Med. 1973 Jul 1;138(1):44–63. doi: 10.1084/jem.138.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassom D. L., Gleich G. J. Damage to Trichinella spiralis newborn larvae by eosinophil major basic protein. Am J Trop Med Hyg. 1979 Sep;28(5):860–863. [PubMed] [Google Scholar]
- Welch D. F., Sword C. P., Brehm S., Dusanic D. Relationship between superoxide dismutase and pathogenic mechanisms of Listeria monocytogenes. Infect Immun. 1979 Mar;23(3):863–872. doi: 10.1128/iai.23.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Remington J. S. Activity of human blood leukocytes against Toxoplasma gondii. J Infect Dis. 1979 Dec;140(6):890–895. doi: 10.1093/infdis/140.6.890. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Remington J. S. Effects of monocytes from human neonates on lymphocyte transformation. Clin Exp Immunol. 1979 Jun;36(3):511–520. [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]