Abstract
Next generation sequencing offers benefit of improved health through knowledge, but comes with challenges, such as inevitable incidental findings (IFs). The applicability of recommended criteria for disclosure of individual results when applied to disclosure of IFs is not well known. The purpose of this study was to examine how medical genetic specialists, genomic researchers, and Institutional Review Board (IRB) chairs perceive the importance of recommended criteria when applied to genetic/genomic IFs. We conducted telephone interviews with medical genetic specialists (genetic counselors, genetic nurses, medical geneticists, laboratory professionals), genomic researchers, and IRB chairs (N=103). Respondents rated and discussed the importance of nine recommended criteria regarding disclosure of genetic/genomic IFs. Stakeholders agreed the most important criteria for disclosure were: (1) the IF points to a life-threatening condition; (2) there is a treatment; (3) individuals indicate in writing they wanted to be informed of IFs. Criteria rated less important were: analytic validity, high penetrance, association with a young age of onset and relative risk more than 2.0. Respondents indicated that some technical criteria were confusing, and in need of context. Our findings suggest that development of guidelines regarding management of IF include multiple stakeholders' perspectives and be based on a common language.
Keywords: Incidental findings, institutional review board, human genome, genetic research, biomedical ethics, genetic testing
INTRODUCTION
Next generation sequencing (NGS) will transform healthcare with the ability to detect sequences implicated in diseases such as cancer (Reis-Filho, 2009). However, with this knowledge also comes a challenge in what to do with inevitable secondary findings also referred to as incidental findings (IFs) (ten Bosch & Grody, 2008). In the context of genetic and genomic research, an IF is defined as a finding that has “potential health or reproductive importance and is discovered in the course of conducting research but is beyond the aims of the study” (Wolf et al., 2008, p. 219). In the context of clinical practice, IFs or “incidentalomes” are defined as genetic results that the clinician and patient “did not anticipate when the test was ordered” (Kohane, Masys, & Altman, 2006, p. 212). However defined, the discovery of IFs in research and clinical settings requires a decision about whether or not to disclose results to research participants or patients. This presents a quandary for those, such as genetic healthcare providers, whose roles can include interpretation, education, and counseling regarding genetic contributors to disease (Resta et al., 2006).
Recommendations that identify specific criteria for the management and disclosure of individual research results (IRRs) and IFs have been written, discussed, and revised by various experts and working groups for both research and clinical contexts (Berg, Khoury, & Evans, 2011; Beskow et al., 2001; Beskow & Burke, 2010; Bookman et al., 2006; Fabsitz et al., 2010; Green et al, 2012; Henrikson, Burke, & Veenstra, 2008; NBAC, 1999; Netzer, Klein, Kohlhase, & Kubisch, 2009; Van Ness, 2008; Wolf et al., 2008; Wolf et al., 2012). While these recommendations were initially directed at IRRs, many have been extended to include IFs due to the difficulty of distinguishing between IRRs and IFs as in the case of whole-genome and – exome sequencing (Wolf et al., 2012). These recommendations propose various criteria that were the focus of this study, such as disclosure of results that are life threatening, affect quality of life, or pose a reproductive risk to future generations.
There are key areas of agreement among the various published recommendations. For example, there is general agreement that researchers and clinicians should offer to disclose findings that could possibly lead to a substantial risk for life-threatening conditions or, more broadly, have important health implications for individuals (Berg et al., 2011; Beskow & Burke, 2010; Bookman et al., 2006; Fabsitz et al., 2010; Henrikson et al., 2008; NBAC, 1999; Van Ness, 2008; Wolf et al., 2008; Wolf et al., 2012). Experts also concur that results should be actionable, that is, capable of being addressed with clinically available treatments or preventive measures (Berg et al., 2011; Beskow et al., 2001; Beskow & Burke, 2010; Bookman et al., 2006; Fabsitz et al., 2010; Henrikson et al., 2008; NBAC, 1999; Netzer et al., 2009; Van Ness, 2008; Wolf et al., 2008; Wolf et al., 2012).
When genomic testing allows for identification of participants and it is feasible to offer them the option of receiving clinically actionable test results, there is notable agreement that participants should be given the option to receive results through an informed consent or similar process (Berg et al., 2011; Beskow et al., 2001; Bookman et al., 2006; Fabsitz et al., 2010; Netzer et al., 2009; Wolf et al., 2008; Wolf et al., 2012). Experts generally agree that test results should have analytic validity (refers to accuracy and reliability) (CDC, 2010) or emanate from a Clinical Laboratory Improvement Amendments (CLIA) certified laboratory (Beskow et al., 2001; Bookman et al., 2006; Fabsitz et al., 2010; NBAC, 1999; Wolf et al., 2008; Wolf et al., 2012). In general, experts also mention `risk' as a criterion for disclosure although the use of the term 'risk' has changed over time. For example, earlier guidelines suggest the disclosure of genetic IRRs when the relative risk level is greater than 2.0 (Bookman et al., 2006). In newer recommendations, the magnitude of risk has become more broadly defined, using terms such as “established and substantial” health implications (Fabsitz et al., 2010, p.575) or “significant” (Wolf et al., 2008, p.235). Some recent guidelines propose that the balance between risks and benefits be considered in disclosure decisions (Fabsitz et al., 2010; Wolf et al., 2008; Wolf et al., 2012).
As guidelines and criteria on the return of IRRs and IFs continue to evolve, it is important to consider the perspectives of stakeholders who will be asked to adopt and implement these guidelines and criteria. Studies have examined whether stakeholders see a need to return genetic and genomic IRRs and IFs (Bollinger, Scott, Dvoskin, & Kaufman, 2012; Beskow & Smolek, 2009; Bovenberg, Meulenkamp, Smets, & Gevers, 2009; Downing, Williams, Daack-Hirsch, Driessnack, & Simon, 2012; Dressler et al., 2012; Edwards et al., 2012; Lemke, Trinidad, Edwards, Starks, & Wiesner, 2010; Simon et al., 2011; Williams et al., 2012). However, the importance that various stakeholders place on criteria for guiding decisions on IRR/IF disclosure is not known. These include professional stakeholders such as medical genetic specialists and researchers who may encounter or anticipate encountering IFs in their daily work, as well as chairs of IRBs that review genetic/genomic research studies in which the discovery and return of IFs is a possibility.
The aim of this study was to investigate the perspectives of medical genetic specialists, genomic researchers, and IRB chairs on the relative importance of a range of recommended criteria in guiding decision making on the return of genetic/genomic IFs. By considering the importance that working professionals place on these criteria, the study provides insight into the usefulness of published guidance on the return of genetic/genomic IFs.
METHODS
This study is part of a larger exploratory descriptive study on stakeholder perceptions of genetic/genomic IFs (RC1 HG005786). The methods used in this study are reported in detail elsewhere (Downing et al., 2012; Simon et al., 2011; Simon, Shinkunas, Brandt, & Williams, 2012; Williams et al., 2012). The study was conducted by researchers at the University of Iowa with data collection support from the Center of Social and Behavioral Research (CSBR) at the University of Northern Iowa. IRB approval was obtained at both universities prior to the initiation of the study.
Participants
To access a cross-section of stakeholders, the sample (N=103) was selected using stratified purposive sampling. Medical genetic specialists qualified for the study if they were health professionals who were members of a regional or national professional genetics organization and had the potential of encountering IFs in their work (Downing et al., 2012). IRB Chairs and genomic researchers were recruited from institutions active in genome-wide association studies (Simon et al., 2011; Williams et al., 2012). We are reporting the findings of a subset of data of the larger study, on the importance of nine published criteria aimed at guiding decisions on the disclosure of IFs found in research and clinical practices.
Instrumentation
To develop interview questions, we reviewed published recommended criteria for the return of IRRs and IFs that were available at the time the study was initiated (Beskow et al., 2001; Bookman et al., 2006; Henrikson et al., 2008; NBAC, 1999; Van Ness, 2008; Wolf et al., 2008). We also reviewed relevant literature on the return of IRRs, genetic test results, and IFs in both research (Keane, 2008; Wilfond & Carpenter, 2008) and clinical (Kohane et al., 2006) settings. Based on this review, we selected nine criteria for inclusion in the study (see Table I).
TABLE I.
Interview Questions on Criteria to Consider When Deciding Whether to Disclose Genetic Incidental Findings
| Various guidelines exist to help in decision making as to whether or not patients/research subjects should be contacted with news about a genomic incidental finding. Rather than think about a specific situation, we would like to ask you to think hypothetically about the importance of the following nine factors that may or may not affect your decision to disclose incidental findings back to a patient/research subject. I will read each of these 9 factors to you. Please take your time to think about each factor and then rate it on a scale of 1–5, where 1 is low and 5 is high in terms of importance. Please also feel free to share any thoughts you may have on these factors. |
| The genetic incidental finding is life threatening to the person who had the test |
| The genetic incidental finding has a strong possibility of altering the person's quality of life |
| There is a treatment for the genetic incidental finding |
| The person has indicated in writing that they want to be informed of a genetic incidental finding |
| The test used to generate the genetic incidental finding has established analytic validity |
| The genetic incidental finding presents a relative risk of more than 2.0 |
| The genetic incidental finding has a high penetrance |
| The genetic incidental finding is associated with a young age of onset |
| The genetic incidental finding does not affect the person's health, but involves a reproductive risk for that person's offspring |
| What other characteristics, if any, would you consider in deciding whether or not an incidental finding should be communicated to a patient/research subject? |
Procedures
Prior to administering the interview, informed consent was obtained from each respondent. The interview guide was developed from literature review and clinical experiences of the investigative team. The guide contained a Likert rating scale and an open ended question for each recommended criterion. Participants were first asked to rate the importance of each criterion, and then to provide comments about each criterion. Interviews were conducted by telephone by one of two trained staff members of the University of Northern Iowa Center for Social and Behavioral Research. Participant responses were audio recorded and transcribed for data analysis. Transcripts were validated with the audiotapes. Respondents were asked to provide a quantitative rating for each criterion according to their perceived importance in deciding whether or not to disclose an IF to a research participant or patient. Respondents were also asked to provide a qualitative explanation of their rating for each criterion. Additionally, respondents were asked if there were any other characteristics they might consider when deciding whether or not to disclose IFs.
Data Analysis
Narrative data were managed using Excel and analyzed with SAS 9.3 (Cary, NC). For each question, means and 95% confidence intervals were computed for each of the three stakeholder groups. To summarize the ratings for each question we report the unweighted average of the three stakeholder group means, which we refer to as the overall mean, and its corresponding 95% confidence interval. Confidence intervals were computed treating the means as normally distributed. Although the ratings are not normally distributed, the sample sizes are large enough such that, by the central limit theorem, the distribution of each mean is approximately normal; hence the confidence intervals should have approximately 95% coverage. Additionally, for each criterion we conducted a global test of the null hypothesis of equality of the three group rating means using a nonparametric Kruskal-Wallis test. Significant global tests were followed by pairwise comparisons using a Wilcoxon rank-sum test. All tests were performed using alpha = .05.
Qualitative data were managed using NVivo v.8.0 (QSR International Pty Ltd, 2008) and analyzed using conventional content analysis (Hsieh & Shannon, 2005). Two members of the research team independently read and coded the qualitative data. Coders met to reconcile their coding and reach consensus. Findings were organized thematically for each criterion and reviewed by the research team.
RESULTS
Sample
A total of 103 respondents participated in the study, including 50 medical genetic specialists (13 medical geneticists, 13 genetic counselors, four genetic nurses, and 20 laboratory professionals), 19 genomic researchers, and 34 IRB chairs. Respondents were drawn from 85 institutions in the United States. Most respondents were White and non-Hispanic, with slightly more males participating. Over half of the medical genetic specialists reported some experience returning and discussing IFs with patients. A minority of the researchers also reported experience returning and discussing IFs with research participants. A majority of IRB chairs reported the topic of IFs in research had been addressed at IRB meetings. The time to complete the entire study data collection ranged from 13–52 minutes, with this component being one set of questions in the interviews. Table II provides complete demographic and descriptive information about the sample.
TABLE II.
Demographics
| Medical Genetic Specialist | Researcher | IRB chair | Overall | |||||
|---|---|---|---|---|---|---|---|---|
| 50 | (%) | 19 | (%) | 34 | (%) | 103 | (%) | |
| Race | ||||||||
| Asian | 9 | (18.0) | 0 | 1 | (2.9) | 10 | (9.7) | |
| Black or African American | 2 | (4.0) | 0 | 0 | 2 | (1.9) | ||
| White | 39 | (78.0) | 19 | (100) | 31 | (91.2) | 89 | (86.4) |
| Missing data | 0 | 0 | 2 | (5.9) | 2 | (1.9) | ||
|
| ||||||||
| Ethnicity | ||||||||
| Hispanic | 1 | (2.0) | 1 | (5.3) | 0 | 2 | (1.9) | |
| Not Hispanic | 49 | (98.0) | 18 | (94.7) | 34 | (100) | 101 | (98.1) |
|
| ||||||||
| Gender | ||||||||
| Female | 28 | (56.0) | 10 | (52.6) | 7 | (20.6) | 45 | (43.7) |
| Male | 22 | (44.0) | 9 | (47.4) | 27 | (79.4) | 58 | (56.3) |
|
| ||||||||
| Degree | ||||||||
| Bachelor's degree | 0 | 0 | 1 | (2.9) | 1 | (1.0) | ||
| Master's degree | 16 | (32.0) | 2 | (10.5) | 4 | (11.8) | 22 | (21.4) |
| MD | 13 | (26.0) | 4 | (21.1) | 10 | (29.4) | 27 | (26.2) |
| PharmD | 0 | 1 | (5.3) | 1 | (2.9) | 2 | (1.9) | |
| PhD | 15 | (30.0) | 10 | (52.6) | 17 | (50.0) | 42 | (40.8) |
| MD/PhD | 4 | (8.0) | 1 | (5.3) | 1 | (2.9) | 6 | (5.8) |
| Missing data | 2 | (4.0) | 1 | (5.3) | 0 | 0 | 3 | (2.9) |
|
| ||||||||
| Experience (years) | ||||||||
| ≤ 5 | 7 | (14.0) | 0 | 23 | (67.7) | 30 | (29.1) | |
| 6–10 | 12 | (24.0) | 3 | (15.8) | 7 | (20.6) | 22 | (21.4) |
| 11–20 | 14 | (28.0) | 10 | (52.6) | 4 | (11.8) | 28 | (27.2) |
| ≥ 21 | 17 | (34.0) | 6 | (31.6) | 0 | 23 | (22.3) | |
|
| ||||||||
| Discussion of IF with patient/research subject | ||||||||
| Yes | 31 | (62.0) | 3 | (15.8) | -- | 34 | (49.3) | |
| No | 17 | (34.0) | 15 | (78.9) | -- | 32 | (46.4) | |
| Missing data | 2 | (4.0) | 1 | (5.3) | -- | 3 | (4.3) | |
|
| ||||||||
| Discussion of IF at IRB meeting | ||||||||
| Yes | -- | -- | 25 | (73.5) | 25 | (73.5) | ||
| No | -- | -- | 8 | (23.5) | 8 | (23.5) | ||
| Missing data | -- | -- | 1 | (2.9) | 1 | (2.9) | ||
Main Findings
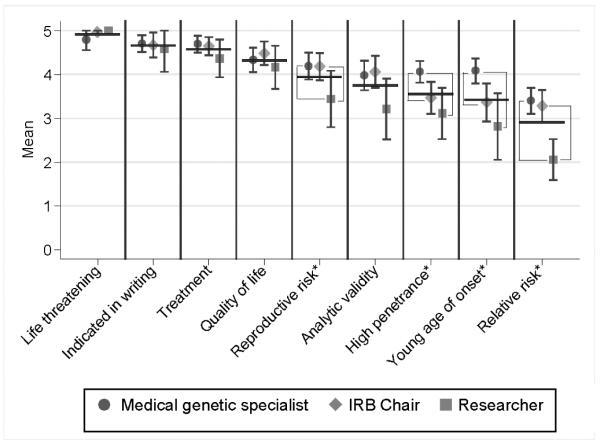
The nine criteria are presented in order of the overall mean of the stakeholder groups on perceived importance. Narrative data are presented throughout to illustrate typical respondent explanations for ratings. The section concludes with a summary of other considerations that respondents raised with respect to decisions about genetic/genomic IF disclosure. Figure 1 presents stakeholder and overall rating means for individual factors and displays the pair-wise comparisons among stakeholder groups.
FIGURE 1.
Stakeholder and overall rating means for individual criteria.
Overall means, computed as the unweighted averages of the three stakeholder means, are denoted by horizontal lines. Factors having a significant (P < .05) difference between stakeholder groups are indicated by an asterisk; for these factors, significantly different pairs (P < .05) are indicated by brackets. To maintain an overall alpha = .05 per question, follow-up pairwise comparisons were performed only if the global test (HO: equal means for groups) was significant. P-values were computed using exact Kruskal-Wallis test for global test and the exact Wilcoxon rank-sum test for pairwise comparisons.
Nine rated criteria on the disclosure of genetic/genomic IFs
1) The IF is life threatening (overall mean=4.9, 95% CI [4.8–5.0])
Respondents gave this criterion the highest aggregate rating. Some respondents indicated that they considered this criterion so important, it affected their responses to other criteria: “quality of life is certainly an important issue…it's maybe not quite as important as if the issue were life threatening” (IRB Chair). Although there was no statistical difference among medical genetic specialists, researchers, and IRB chairs in how they rated `life threatening,' medical genetic specialists specifically emphasized the personal utility of learning about a life threatening IF, as illustrated in this quote: “that may be information they need in order to make life changing decisions” (Medical Genetic Specialist).
2) Preference to receive IF is indicated in writing (overall mean = 4.7, 95% CI [4.5– 4.8])
This was the second-highest rated criterion. When asked to explain their ratings, respondents described an obligation to respect the wishes of the participant/patient to receive results, for example: “If the person wishes to be informed of incidental findings then we have an obligation to communicate that to them” (Researcher). Some stakeholders qualified their ratings with concerns about individuals' genetic literacy, for example: “I don't think that people understand enough about genetics to…understand what they're asking for” (IRB Chair).
3) Availability of treatment (overall mean =4.6 (95% CI [4.4–4.7])
Explanations for this rating, particularly from medical genetic specialists, were typically focused on a perceived obligation to treat: “I don't think it's ethical to withhold information from a patient [when] you can do something beneficial for the patient” (Medical Genetic Specialist). Some respondents qualified this response by pointing out that the decision making process would also need to consider the cost and impact of available treatments because, “not all treatments are essential. Some treatments are very expensive, some treatments are harmful” (Researcher).
4) The IF has a strong possibility of altering quality of life (overall mean=4.3, 95% CI [4.1 – 4.5])
Rated marginally below `availability of treatment,' this criterion was considered important because of the potential for people to modify their lifestyle as a result of learning about an IF; for example: “if there are ways that people could alter their lifestyle to modify the risk [of] the genetic incidental finding, then I think it [is] something that should be a high priority for reporting” (Researcher). Yet, some respondents were concerned that `quality of life' was a vague criterion: “[When] it's just about quality of life, not a health finding, [then] I think it becomes much murkier, in terms of whether such findings should be released” (Researcher). IRB chairs were notable in their tendency to evaluate the importance of the `quality of life' criterion with reference to familiar ethical concepts in research, for example: “Both [the quality of life and life threatening criteria] revolve for me very heavily around the respect for person's issue, and the autonomy issue. I think that it's their information; they have not only a right to know, but a need to know” (IRB Chair).
5) The IF involves a reproductive risk for the individual's offspring (overall mean =3.9, 95% CI [3.7 – 4.2])
Explanations for the ratings of this criterion revealed the complexity of decision making beyond the scope of the research participant/patient. For example: “I just think it's a complicated issue to talk about beyond the individual who donated the sample” (Researcher). Another person said: “I think the implications for the offspring are about as important as the implication[s] for the participant” (Researcher). Conversely, “If it's not going to impact [the patient], and they didn't ask about it, I probably wouldn't share it” (Medical Genetic Specialist).
Researchers gave lower numeric ratings to `reproductive risk' than medical genetic specialists (p= 0.019) or IRB chairs (p= 0.044). Researchers who gave `reproductive risk' lower scores focused on the context of the IF: “I'd have to know if the disease was devastating or not or whether it was more of a trait” (Researcher).
Some respondents were unclear as to whether reproductive risk pertained to the person being tested or to their offspring, for example: “Does the reproductive risk mean that danger could happen to the person or that they could have a kid that has a problem?” (IRB Chair). Five respondents opted not to assign a numeric rating to this criterion. Some of these respondents mentioned that assigning a numeric rating would require more information regarding the diagnosis of the IF or individual information.
6) The IF has established analytic validity (overall mean =3.8, 95% CI [3.5 – 4.0])
In commenting on the importance of analytic validity, respondents turned to the issues associated with a lack of analytic validity. For example: “if it's not a valid test the information isn't going to do anybody any good or may lead them down the wrong path” (IRB Chair). Moreover, some respondents also suggested that analytic validity was implied: “I'd made sort of an assumption that I trusted that the laboratory had analyzed [the finding] correctly and that there were some known implications….I think that I made the assumption that there was analytic validity” (Medical Genetic Specialist).
Analytic validity was given a lower rating by researchers when compared to medical genetic specialists (p= 0.038) or IRB chairs (p= 0.034). As in `reproductive risk', researchers who gave analytic validity lower scores suggested the need for more context of the IF.
7) The IF has high penetrance (overall mean =3.5 (95% CI [3.3– 3.8])
Respondents generally assessed the importance of high penetrance in relation to the health significance of the finding, for example: “If it's like brown eyes or blue eyes, that probably [is] not very important but again if it's something that's crucial to the health of the person it would [be] important to report that back” (IRB Chair).
Medical genetic specialists gave higher ratings to this criterion than researchers (p= 0.003) or IRB chairs (p= 0.007). Medical genetic specialists associated high penetrance with the heritability of the IF and the need to inform family members of highly penetrant IFs: “because they would need to know. It would be not just important for them, it would be important for other members of their family” (Medical Genetic Specialist).
8) The IF is associated with a young age of onset (overall mean =3.4, 95% CI [3.2 – 3.7])
With respect to IFs, young age of onset was interpreted in two ways: 1) as disorders that affect children or 2) disorders that typically affect middle-aged to older adults, but that are associated with younger ages of onset. Thus respondents considered the age of the individual at the time of the finding: “if it's a young age of onset and your participants are older, then that's not [a] terribly important finding. If you're looking at children however and it's a young age of onset, then that's a quite important finding because it will have a potentially greater effect over the course of their life” (IRB Chair).
Some respondents considered it a right of parents to know about findings when the onset of the associated disease is childhood: “[if] it was young age of onset you probably found [it] in a child, and I think parents have a right to know what the possibly is for their child” (Medical Genetic Specialist).
Medical genetic specialists gave higher ratings to `young age of onset' than IRB chairs (p= 0.005) or researchers (p= 0.0014). Medical genetic specialists emphasized the personal and clinical utility of learning about variants associated with a younger age of onset: “I do think that's important especially for patients' quality of life, life planning, and any treatment or modifications that could be made” (Medical Genetic Specialist).
9) The IF has a relative risk more than 2.0 (overall mean =2.9, 95% CI [2.7–3.1])
This criterion was rated the lowest of all criteria, with eight respondents declining to give it a rating for reasons such as: “unless I find out what the baseline risk is, the relative risk doesn't tell me anything…I can't answer that in the abstract” (IRB Chair). Lower ratings of this criterion were also tied to questions regarding the meaning of the numeric cut-off, “2.0”, for example: “A 2.0 what? I'm not sure what you mean” (IRB Chair).
Researchers gave this criterion lower ratings than medical genetic specialists (p <0.001) or IRB chairs (p<0.001). One researcher explained the need for a lower rating of this criterion as follows: “it's double the risk, but it's 1) not that high and 2) it doesn't tell you what the chance of the person getting the condition would be” (Researcher).
Other considerations
In addition to rating and discussing the nine criteria above, respondents shared other considerations for deciding whether or not to disclose genetic/genomic IFs. For example, respondents in all stakeholder groups said that it was important to consider more than one criterion when deciding whether or not to disclose: “we look at all these things in combination with one another. It's more than just each of these things in isolation” (Researcher). One respondent proposed this systematic approach: “first and foremost is how convinced am I that the results are valid. The second is, what do I think the implications are for the subject. The third is, to some extent whether it's treatable or not, and would it make a difference. The fourth is, is this gonna affect the person in the long term in terms of autonomy…reproductive rights…other kinds of things. Therefore, they need to have the information” (IRB Chair). Respondents also reported the need for a risk-benefit assessment: “[it's] the balance of benefits and harms to the patient to constitute a test that can be considered of high value or be considered of any value to either the patient or the clinician-patient dyad” (Medical Genetic Specialist).
Respondents in all stakeholder groups felt there was a need to consider the nature of the IF, for example: “The psychology of families or individuals who carry the Huntington's gene is entirely different, and you would probably deal with such an incidental finding more circumspectly…than [if] you would tell somebody `Hey, you're carrying a dominant gene for Alport syndrome'” (Medical Genetic Specialist).
Respondents also considered the perspective of the individual receiving the results. Some raised the issue of the individual's preparedness and their capacity to understand information on IFs, for example: “I think [it depends on] what the patient is ready to hear…if you've got eight things to discuss you don't have to throw all eight at a patient or a family immediately in the first ten minutes of a counseling session and then clean up the mess. I think you can deal with it over time….To prioritize…in a manner that allows the patient or the family to adapt…gain knowledge and then be able to handle something new” (Medical Genetic Specialist). Respondents also reported they might factor into their disclosure decisions the amount of undue stress or anxiety that disclosure of an IF with uncertain significance might cause the individual: “There's uncertainty about the significance of something in a research setting. It may be prudent not to communicate it because it might raise anxieties” (Medical Genetic Specialist).
IRB chairs and researchers raised the need to consider research participants' expectations for receiving their results, for example: “I would consider the overall study design and if it's the kind of study where people would expect to receive incidental findings, or if it's not the kind of study where they would expect to receive incidental findings…I would throw in there just the more broader context of the research. Those two factors I think could affect whether people really would expect to receive incidental findings or not” (IRB Chair).
DISCUSSION
This study examined professionals' perspectives on nine selected criteria proposed in the literature with respect to the importance of IF disclosure. As such, it represents a novel step in assessing the unique perspectives and concerns of stakeholders with respect to the establishment and adoption of a workable set of criteria for guiding decisions on the disclosure of IFs. Findings from the study suggest that, in a sample of medical genetic specialists, researchers, and IRB chairs, there is a shared sense of the importance of some disclosure criteria, concerns about other criteria, and some differences with respect to how clinically and research oriented professionals see the importance of certain criteria. These findings are significant given that effective workplace adoption of IF disclosure guidelines will likely be impacted by the degree of importance that professionals place on the criteria that reside at the core of published guidelines.
Our study suggests that there may be two tiers of disclosure criteria when these are organized according to the importance and concerns that stakeholders associate with them. The first and least problematic tier includes the criteria `life threatening', `indicated in writing', `availability of treatment', `quality of life', and `reproductive risks'. Interestingly, the most highly rated criterion in this tier and in our study, `life threatening,' has been replaced in more recent guidelines with adjectives such as `significant” or `substantial' health findings (e.g., Fabsitz et al., 2010). How workable this vocabulary may be is not known, however a recent study suggests there is concern for the different interpretations that individuals (i.e. professionals and lay public) can bestow on terms such as `relevance' or `seriousness' (Townsend et al., 2012, p. 3). Policy makers may wish to consider that the professional stakeholders sampled in our study, among them those who have encountered IFs, perceive concrete terms such as `life threatening' as a highly important characteristic in decision making on disclosure of IFs.
The second, more problematic, tier includes the criteria `analytic validity', `high penetrance', `young age of onset' and `relative risk.' Lower respondent enthusiasm for this second tier may be the result of several factors. First, as stakeholders noted, the importance of these more technical criteria in IF decision making is challenging to conceptualize given no concrete context, that is, information on a patient or research participant's age, diagnosis, personal circumstances, and other relevant details. The importance of context has been noted in recent work on the disclosure of genetic results, particularly with regard to individualized decision making based on the researcher's knowledge of the participant's preferences (Beskow & Burke, 2010). The second tier also included criteria that respondents may not have understood on technical or terminological grounds, or with respect to how they ought to be applied to IF decision making. There may have been limited familiarity with or confusion over terms used in the guidelines (i.e. analytical validity, penetrance, and relative risk). Such confusion about terminology is not new to the disclosure of IFs. A recent systematic review of ethical reflection on IFs concludes that lack of standardized language regarding IFs continues to limit understanding of what is meant by `incidental findings' in guidelines, scientific articles, and informed consent documents (Christenhusz, Devriendt, & Dierickx, 2012). We should also note, however, that even criteria that were highly rated in our study and considered relatively unproblematic at face value, such as the need to obtain permission in writing before disclosing IFs, are potentially challenging to implement effectively, as pointed out by Wolf et al. (2012) in the context of genomic research involving biobanks and archived data sets.
Our study suggests that there is agreement among medical genetics experts, genomic researchers, and IRB chairs with respect to certain criteria for guiding decisions on the disclosure of incidental findings. Our study also confirms, however, that medical genetics specialists, genomic researchers, and IRB chairs diverge with respect to the relative importance of other criteria when asked to rate these individually. The discordance between the relative importance of some criteria is similar to findings from Green et al. (2012) who reported that medical genetics specialists held differing opinions on the relative value of specific criteria.
Study Limitations
This study had limitations. We used a stratified purposive sample; therefore, the results may not be generalizable. However, the sampling plan for this study allowed for systematic and consistent data collection from those who volunteered to share their perspectives. The study was conducted during a time when newer guidelines were being discussed and issued, and it is not possible to ascertain if respondents were familiar with the newer guidelines. Questions asked for consideration of a hypothetical circumstance, the possible disclosure of an IF, which cannot be viewed as reflecting actual decisions in actual cases. While many studies in this area have assessed stakeholder opinion hypothetically in the same manner, a strength of the current study is that over half of the participants could approach these nine criteria from a position of an experience with IFs. We were not able to analyze the data from questions on individual criteria into logical clusters. In some cases, responses for individual criteria items addressed multiple criteria. Respondents also specifically noted that criteria could not be rated in isolation from each other. Primary care providers were not included as subjects in this study. However, in some cases, community based health care providers may become part of the communication and application process (Biesecker et al., 2012). Finally, it is possible that ratings may have been affected by the sequence of the questions regarding the nine criteria.
Practice Implications
Findings from this study have implications for genetic counselors. As pointed out by Hawkins (2010) in regards to biobanking, there are multiple genetic counselor roles in managing disclosure of IFs both in the development of policies and procedures for disclosure, as well as implementing and managing the impact of implementation on individuals and families. Moreover, as NGS becomes more readily used, genetic healthcare providers, including genetic counselors, will be required to allocate more time to dealing with IFs (Downing et al., 2012) Findings from this study confirm the need for a common language and shared perspectives with respect to the management of IFs. Our findings suggest that some published criteria for guiding the disclosure of IF are considered more important than others. These criteria should be the starting point for discussion among stakeholders for purposes of developing recommendations for disclosure of IFs that can be broadly agreed upon. While some criteria have traditionally considered research and clinical practice as separate entities, as pointed out by Wolf et al. (2012), the debate about disclosure of IRRs and IFs may force us to reconsider the shrinking divide between research and clinical care.
Finally, if published, national-level guidelines on the disclosure of IRR and IF are to be consistently followed, they will need to have credibility among professionals who are expected to implement them. How important stakeholders consider individual criteria for guiding decisions on IRR and IF is one indication of how much credibility these criteria are likely to have. Findings from our study suggest that our upper tier of criteria likely have significant credibility, while the second tier may not. If this is generally true, policy makers may need to consider recommending only the first tier of published criteria, or else seek ways of refining the remaining criteria, if the full complement of criteria examined in this study are to have real-world applicability.
Research Recommendations
Further recommendations for research include the continued empirical investigation of the usefulness of recommendations for the disclosure of IFs generated by NGS. Issues influencing the impact of genetic counseling for patients and research subjects, as well as the costs to participants and providers have yet to be examined. Continued investigation should include other stakeholder groups, such as the primary care providers', and the public's perspectives; and factors influencing effective implementation of genetic counseling for IFs in clinical or research settings.
Conclusion
Some of the recommended criteria for disclosure of genetic results are given more importance than others by medical genetic specialists, researchers, and IRB chairs when applied to genetic/genomic incidental findings. Second-tier criteria that were considered less important raise questions about their usefulness in efforts to determine whether or not IFs should be disclosed. From a professional standpoint, criteria and their accompanying guidelines may be more workable and reflective of rapid changes in genomics if they are based on a common language and understanding of stakeholder perspectives for management and disclosure.
ACKNOWLEDGMENTS
The study was supported by an (ARRA) grant from the National Human Genome Institute of the National Institutes of Health (NIH) (RC1HG005786). Support was also provided by Grant Number TR000443-06 (training support for DB) from the National Center for Advancing Translational Sciences and the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors thank the American College of Medical Genetics, the National Society of Genetic Counselors, the International Society of Nurses in Genetics, and the Heartland Regional Genetics & Newborn Screening Collaborative for assistance in recruitment. The authors would also like to thank the University of Northern Iowa Center for Social and Behavioral Research for collaboration on interview guide development, and data collection.
Footnotes
DISCLOSURE OF INTERESTS None of the authors has a conflict of interest. We have full control of all primary data, and we agree to allow the journal to review the data if requested.
REFERENCES
- Berg JS, Khoury MJ, Evans JP. Deploying whole genome sequencing in clinical practice and public health: meeting the challenge one bin at a time. Genetics in Medicine. 2011;13(6):499–504. doi: 10.1097/GIM.0b013e318220aaba. doi: 10.1097/GIM.0b013e318220aaba. [DOI] [PubMed] [Google Scholar]
- Beskow LM, Burke W. Offering individual genetic research results: context matters. Science Translational Medicine. 2010;2(38):38cm20. doi: 10.1126/scitranslmed.3000952. doi: 10.1126/scitranslmed.3000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskow LM, Burke W, Merz JF, Barr PA, Terry S, Penchaszadeh VB, et al. Informed consent for population-based research involving genetics. JAMA: The Journal of the American Medical Association. 2001;286(18):2315–2321. doi: 10.1001/jama.286.18.2315. [DOI] [PubMed] [Google Scholar]
- Beskow LM, Smolek SJ. Prospective biorepository participants' perspectives on access to research results. Journal of Empirical Research on Human Research Ethics. 2009;4(3):99–111. doi: 10.1525/jer.2009.4.3.99. doi: 10.1525/jer.2009.4.3.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker LG, Burke W, Kohane I, Plon SE, Zimmern R. Next-generation sequencing in the clinic: Are we ready? Nature. 2012;15:818–824. doi: 10.1038/nrg3357. doi:10.1038/nrg3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollinger JM, Scott J, Dvoskin R, Kaufman D. Public preferences regarding the return of individual genetic research results: findings from a qualitative focus group study. Genetics in Medicine. 2012;14(4):451–457. doi: 10.1038/gim.2011.66. doi: 10.1038/gim.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookman EB, Langehorne AA, Eckfeldt JH, Glass KC, Jarvik GP, Klag M, et al. Reporting genetic results in research studies: summary and recommendations of an NHLBI working group. American Journal of Medical Genetics, Part A. 2006;140(10):1033–1040. doi: 10.1002/ajmg.a.31195. doi: 10.1002/ajmg.a.31195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovenberg J, Meulenkamp T, Smets E, Gevers S. Biobank research: reporting results to individual participants. European Journal of Health Law. 2009;16(3):229–247. doi: 10.1163/157180909x453062. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (CDC) [Accessed 22 August 2012];Genomic testing. 2010 http://www.cdc.gov/genomics/gtesting/ACCE/index.htm.
- Christenhusz GM, Devriendt K, Dierickx K. To tell or not to tell? A systematic review of ethical reflections on incidental findings arising in genetics contexts. European Journal of Human Genetics. 2012 doi: 10.1038/ejhg.2012.130. Retrieved from http://www.nature.com/ejhg/journal/vaop/ncurrent/pdf/ejhg2012130a.pdf doi:10.1038/ejhg.2012.130. [DOI] [PMC free article] [PubMed]
- Downing NR, Williams JK, Daack-Hirsch S, Driessnack M, Simon C. Managing genomic incidental findings in the clinical setting. Patient Education and Counseling. 2012 Oct; doi: 10.1016/j.pec.2012.09.010. 2012. doi: 10.1016/j.pec.2012.09.010 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler LG, Smolek S, Ponsaran R, Markey JM, Starks H, Gerson N, et al. IRB perspectives on the return of individual results from genomic research. Genetics in Medicine. 2012;14(2):215–222. doi: 10.1038/gim.2011.10. doi: 10.1038/gim.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KL, Lemke AA, Trinidad SB, Lewis SM, Starks H, Snapinn KW, et al. Genetics researchers' and IRB professionals' attitudes toward genetic research review: a comparative analysis. Genetics in Medicine. 2012;14(2):236–242. doi: 10.1038/gim.2011.57. doi: 10.1038/gim.2011.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabsitz RR, McGuire A, Sharp RR, Puggal M, Beskow LM, Biesecker LG, et al. Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung, and Blood Institute working group. Circulation. Cardiovascular Genetics. 2010;3(6):574–580. doi: 10.1161/CIRCGENETICS.110.958827. doi: 10.1161/CIRCGENETICS.110.958827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RC, Berg JW, Berry GT, Biesecker LG, Dimmock DP, Evans JP, Grody WW, Hegde MR, Kalia S, Korf BR, Krantz I, McGuire AL, Miller DT, Murray MF, Nussbaum RL, Plon SE, Rehm HL, Jacob HJ. Exploring concordance and discordance for return of incidental findings from clinical sequencing. Genetics in Medicine. 2012;14(4):405–410. doi: 10.1038/gim.2012.21. doi: 10.1038/gim.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins AK. Biobanks: Importance, implications and opportunities for genetic counselors. Journal of Genetic Counseling. 2010;19(5):423–429. doi: 10.1007/s10897-010-9305-1. doi: 10.1007/s10897-010-9305-1. [DOI] [PubMed] [Google Scholar]
- Henrikson NB, Burke W, Veenstra DL. Ancillary risk information and pharmacogenetic tests: social and policy implications. The Pharmacogenomics Journal. 2008;8(2):85–89. doi: 10.1038/sj.tpj.6500457. doi: 10.1038/sj.tpj.6500457. [DOI] [PubMed] [Google Scholar]
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qualitative Health Research. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- Keane MA. Institutional review board approaches to the incidental findings problem. The Journal of Law, Medicine, and Ethics. 2008;36(2):352–355. 213. doi: 10.1111/j.1748-720X.2008.00279.x. doi: 10.1111/j.1748-720X.2008.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohane IS, Masys DR, Altman RB. The incidentalome: a threat to genomic medicine. JAMA: The Journal of the American Medical Association. 2006;296(2):212–215. doi: 10.1001/jama.296.2.212. doi: 10.1001/jama.296.2.212. [DOI] [PubMed] [Google Scholar]
- Lemke AA, Trinidad SB, Edwards KL, Starks H, Wiesner GL, GRRIP Consortium Attitudes toward genetic research review: results from a national survey of professionals involved in human subjects protection. Journal of Empirical Research on Human Research Ethics. 2010;5(1):83–91. doi: 10.1525/jer.2010.5.1.83. doi: 10.1525/jer.2010.5.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Bioethics Advisory Comission (NBAC) [Accessed 30 May 2012];Research involving human biological materials: ethical issues and policy guidance. 1999 1 http://hdl.handle.net/1805/22. [Google Scholar]
- Netzer C, Klein C, Kohlhase J, Kubisch C. New challenges for informed consent through whole genome array testing. Journal of Medical Genetics. 2009;46(7):495–496. doi: 10.1136/jmg.2009.068015. doi: 10.1136/jmg.2009.068015. [DOI] [PubMed] [Google Scholar]
- Resta R, Biesecker BB, Bennett RL, Blum S, Hahn SE, Strecker MN, et al. A new definition of genetic counseling: National Society of Genetic Counselors' Task Force Report. Journal of Genetic Counseling. 2006;15(2):77–83. doi: 10.1007/s10897-005-9014-3. doi: 10.1007/s10897-005-9014-3. [DOI] [PubMed] [Google Scholar]
- Reis-Filho JS. Next-generation sequencing. Breast Cancer Research. 2009;11(Suppl 3):S12. doi: 10.1186/bcr2431. doi: 10.1186/bcr2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Shinkunas LA, Brandt D, Williams JK. Individual genetic and genomic research results and the tradition of informed consent: exploring US review board guidance. Journal of Medical Ethics. 2012;38(7):417–422. doi: 10.1136/medethics-2011-100273. doi: 10.1136/medethics-2011-100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon CM, Williams JK, Shinkunas L, Brandt D, Daack-Hirsch S, Driessnack M. Informed consent and genomic incidental findings: IRB chair perspectives. Journal of Empirical Research on Human Research Ethics. 2011;6(4):53–67. doi: 10.1525/jer.2011.6.4.53. doi: 10.1525/jer.2011.6.4.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Bosch JR, Grody WW. Keeping up with the next generation: massively parallel sequencing in clinical diagnostics. The Journal of Molecular Diagnostics. 2008;10(6):484–492. doi: 10.2353/jmoldx.2008.080027. doi: 10.2353/jmoldx.2008.080027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A, Adam S, Birch PH, Lohn Z, Rousseau F, Friedman JM. “I want to know what's in Pandora's box”: comparing stakeholder perspectives on incidental findings in clinical whole genomic sequencing. American Journal of Medical Genetics, Part A. 2012;158A(10):2519–2525. doi: 10.1002/ajmg.a.35554. doi: 10.1002/ajmg.a.35554. [DOI] [PubMed] [Google Scholar]
- Van Ness B. Genomic research and incidental findings. The Journal of Law, Medicine, and Ethics. 2008;36(2):292–297. doi: 10.1111/j.1748-720X.2008.00272.x. doi: 10.1111/j.1748-720X.2008.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfond BS, Carpenter KJ. Incidental findings in pediatric research. The Journal of Law, Medicine, and Ethics. 2008;36(2):332–340. 213. doi: 10.1111/j.1748-720X.2008.00277.x. doi: 10.1111/j.1748-720X.2008.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JK, Daack-Hirsch S, Driessnack M, Downing N, Shinkunas L, Brandt D, et al. Researcher and institutional review board chair perspectives on incidental findings in genomic research. Genetic Testing and Molecular Biomarkers. 2012;16(6):508–513. doi: 10.1089/gtmb.2011.0248. doi: 10.1089/gtmb.2011.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM. The past, present, and future of the debate over return of research results and incidental findings. Genetics in Medicine. 2012;14(4):355–357. doi: 10.1038/gim.2012.26. doi: 10.1038/gim.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Crock BN, Van Ness B, Lawrenz F, Kahn JP, Beskow LM, et al. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genetics in Medicine. 2012;14(4):361–384. doi: 10.1038/gim.2012.23. doi: 10.1038/gim.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, Clayton EW, et al. Managing incidental findings in human subjects research: Analysis and recommendations. The Journal of Law, Medicine, and Ethics. 2008;36(2):219–248. 211. doi: 10.1111/j.1748-720X.2008.00266.x. doi: 10.1111/j.1748-720X.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]