Abstract
Nicotine withdrawal is associated with subtle working memory deficits that predict subsequent relapse. We examined the neural substrates underlying these processes in treatment-seeking smokers, and explored the moderating influence of age on abstinence-induced alterations in brain activity and performance. Sixty-three smokers participated in two blood-oxygen-level dependent (BOLD) functional magnetic resonance imaging (fMRI) scans while performing a visual N-back task on two separate occasions: smoking as usual and after 24 hours of biochemically confirmed abstinence (order counterbalanced). Abstinence (versus smoking) led to reduced accuracy, slower median correct response time, and reduced BOLD signal change in the 3 a priori regions of interest (ROIs): medial frontal/cingulate gyrus and right and left dorsolateral prefrontal cortex. Significant age x session effects were found for BOLD signal change in all three regions, as well as for withdrawal and craving; for all measures, abstinence effects were attenuated in smokers aged >50 years compared to those < 50 years old. These results suggest that abstinence effects on neurocognitive function may be more pronounced for younger smokers, and may indicate a new avenue for research exploring mechanisms underlying age differences in smoking cessation success.
Keywords: Addiction, cognition, fMRI, nicotine, withdrawal, age
Introduction
Withdrawal from nicotine precipitates neurocognitive deficits such as impaired working memory (Mendrek et al, 2006; Patterson et al, 2009). Working memory is a limited-capacity system responsible for active maintenance and manipulation of information, and is a component of a wide range of cognitive processes (Baddeley, 2003). Of relevance to nicotine dependence, working memory facilitates top-down executive control and behavioral inhibition through active maintenance of task-related goals (Mecklinger et al, 2003). Quitting smoking engages these resources by requiring an individual to inhibit habitual, automated behaviors (such as lighting a cigarette) and maintain new goal-oriented behaviors on a regular basis (Sun et al, 2007); therefore, impairments in these systems have the potential to negatively impact smoking cessation attempts. Indeed, subjective cognitive deficits during abstinence as well as objective deficits in working memory, attention and executive function predict relapse among smokers trying to quit (Patterson et al, 2010; Rukstalis et al, 2005). Moreover, effective smoking cessation medications reverse these deficits in rodent models and in human smokers (Patterson et al, 2009; Portugal and Gould, 2008). Elucidating the neurocircuitry underlying abstinence-induced cognitive deficits may therefore aid in understanding the mechanisms that impede successful quitting.
In blood-oxygen-level-dependent functional magnetic resonance imaging (BOLD fMRI) studies of normal volunteers, performance on working memory tasks is accompanied by increased activation in the prefrontal cortex (PFC), with increased activation as task load increases (Owen et al, 2005; Ragland et al, 2002). Nicotinic modulation of dopamine signaling in the PFC may underlie nicotine’s effects on working memory; acute nicotine administration stimulates dopaminergic neurons projecting from the ventral tegmental area to the PFC, and withdrawal from chronic nicotine results in reduced dopaminergic signaling in these areas (Brody et al, 2004). Consistent with these data, variation in genes regulating dopamine neurotransmission is associated with neural response to working memory tasks and behavioral measures of working memory in smokers (Loughead et al, 2009).
Of particular relevance to smoking cessation, initial studies show that nicotine withdrawal alters neural activity in the working memory circuit during an N-back task (Loughead et al, 2010; Xu et al, 2005; Xu et al, 2006). However, there is some debate over the direction and interpretation of these changes in BOLD signal. Some studies report an increase in task-related BOLD signal during abstinence at a comparable level of performance, an effect interpreted by some as a sign of decreased processing efficiency (Jacobsen et al, 2007; Xu et al, 2005; Xu et al, 2006). In contrast, two prior studies using an N-back task that extended to the 3-back level revealed significant abstinence-induced decreases in BOLD signal in working memory-related brain regions at only the highest task loads (Loughead et al, 2010; Loughead et al, 2009). Further, decreased BOLD signal in abstinence correlated with poorer performance in highly dependent smokers (Loughead et al, 2010). Thus, failure to recruit the working memory system sufficiently, rather than inefficient processing, may account for abstinence-induced performance deficits. However, most of the prior studies, including ours, are limited by relatively small sample sizes and use of non-treatment seeking smokers. Investigations of treatment-seeking smokers are more sensitive to effects of efficacious medications on relapse (Perkins et al, 2010), and may therefore afford a more sensitive analysis of the neurocognitive effects of nicotine withdrawal.
Furthermore, none of the prior studies examined age-related differences in the neurocognitive effects of abstinence in chronic smokers. In the general population, increasing age has been associated with alterations in BOLD activation during performance of working memory tasks (Grady 2008; Turner and Spreng, 2012). This may be related to declining efficiency in cholinergic and dopaminergic systems during healthy aging (Störmer et al, 2012). Although there has been some interest in the potential of acute or chronic nicotine to enhance cognition in healthy aging populations (Murray and Abeles, 2002) and those with cognitive impairments (Mehta et al, 2012), no studies have investigated age-related differences in the effects of abstinence on working memory and associated BOLD activation.
To clarify the neural mechanisms underlying effects of nicotine withdrawal on working memory, and to explore age-related differences in abstinence effects, we acquired BOLD fMRI in 63 treatment-seeking smokers performing a fractal N-back task during two separate sessions: while smoking as usual (<45 minutes between last cigarette and testing), and after 24 hours of smoking abstinence. We hypothesized that abstinence would be associated with decreased BOLD signal in the medial frontal/cingulate gyrus (MF/CG) and the dorsolateral prefrontal cortex (DLPFC). We also predicted that decreases in activation would predict poorer task performance (i.e., decreased accuracy and slower response times), and as an exploratory analysis tested whether these effects would vary across different age groups of smokers.
Methods and Materials
Participants
All procedures were approved by the University of Pennsylvania Institutional Review Board and carried out in accordance with the Declaration of Helsinki. Treatment-seeking smokers aged 18 to 65 who reported smoking ≥ 10 cigarettes/day for ≥ 6 months were recruited through mass media. All participants provided written informed consent and completed a physical examination including a urine drug screen and breath alcohol test. Female participants completed a urine pregnancy test. Persons with a history of DSM-IV Axis I psychiatric or substance disorders (except nicotine dependence), assessed by self-report and using the Mini International Neuropsychiatric Interview (Sheehan et al, 1998), and those taking psychotropic medications were excluded. Other exclusion criteria included: current use of chewing tobacco, snuff, or smoking cessation products; pregnancy, planned pregnancy or breastfeeding; history of brain injury; left-handedness; fMRI contraindicated material in the body; low or borderline intelligence (<90 score on Shipley’s IQ test); and any impairment that would prevent task performance. Eligible participants completed the Fagerstrom Test for Nicotine Dependence (Heatherton et al, 1991).
Of 118 participants who completed the eligibility phase, 28 voluntarily withdrew prior to completing both scanning sessions; 9 were ineligible at a session due to noncompliance (positive drug screen or carbon monoxide (CO) > 9ppm at abstinent session); 4 were ineligible due to an incidental finding; and 4 were unable to complete the tasks in the time allotted. In total, 73 participants completed all study requirements.
Procedures
The neuroimaging experiment used a within-subject design with two BOLD fMRI sessions in counterbalanced order: (1) smoking as usual (<45 minutes between last cigarette and BOLD imaging) and (2) 24 hours abstinent. The 24-hour time frame was selected because most relapses to smoking occur during this period (Piasecki, 2006). Sessions were scheduled at the same time of day (+/− 3 hours) 1-3 weeks apart; subjects were required to complete both scanning sessions prior to beginning a six-week smoking cessation program involving behavioral counseling. Subjects were instructed to refrain from alcohol or other drugs for at least 24 hours before the session. On the session days, those with a positive drug screen, a breath alcohol test >0.01, or a breath carbon monoxide (CO) test >9ppm (abstinent session only) were excluded. Participants completed the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes et al, 1984) and Questionnaire of Smoking Urges (QSU-Brief; Cox, Tiffany, and Christen, 2001), and completed a practice block at each memory load using a laptop computer. On the smoking as usual day, participants smoked immediately before initiating the scanning protocol to standardize exposure (~20-30 minutes prior to completing the N-back).
Task design
Working memory function was assessed using a visual N-back paradigm (Ragland et al, 2002) used in our prior research (Loughead et al, 2010; Loughead et al, 2009). The N-back task presents complex geometric figures (fractals) for 500 ms, followed by an interstimulus interval of 2500 ms under four conditions: 0-back, 1-back, 2-back and 3-back. In the 0-back condition, participants respond with a button press to a specified target fractal; for the 1-back condition, participants respond if the current fractal was identical to the previous one; for the 2-back condition, if the current fractal was identical to the item presented two trials back; etc. No response was required for nontargets. Each condition was presented three times in 20-trial blocks (33% targets; 60 s). Blocks were presented in order of increasing memory load for one set, after which conditions were presented pseudo-randomly; visual instructions (9 s) preceded each block to indicate the upcoming condition. The task began with a 48 s baseline rest period (fixation point) of which the first 24 s was discarded to ensure the MRI signal reached steady state. Equivalent N-back tasks with unique stimuli were used for the two sessions; version order was counterbalanced.
Image acquisition
All subject imaging sessions were acquired on the same scanner (Siemens Tim Trio 3 Tesla, Erlangen, Germany; 32 channel head coil) using the same imaging sequences. BOLD fMRI was acquired using a whole-brain, single-shot, multi-slice, gradient-echo (GE) echoplanar (EPI) sequence of 308 volumes with the following parameters: TR/TE=3000/30 ms, FOV=448x448 mm, matrix=64X64, flip angle=90°, slices=40, slice thickness/gap=3mm/0mm and effective voxel resolution=3.4 × 3.4 × 3.4. Prior to BOLD fMRI, 5-min magnetization-prepared, rapid acquisition gradient-echo (MPRAGE) T1-weighted image (TR=1810 ms, TE=3.51 ms, FOV =180×240 mm, matrix=256×192, 160 slices, TI=1100 ms, flip angle=9°, effective voxel resolution of 1 × 1 × 1mm) was acquired for anatomic overlays of functional data and to aid spatial normalization to a standard atlas space.
Quality Control
Two subjects were excluded from analysis for poor task accuracy (>2.5 standard deviations below the mean); one subject’s BOLD data was lost due to a technical error. Image quality assessment procedures compared the mean temporal signal-to-noise ratio (tSNR) in each session for artifacts and poor quality data. To assess excessive head motion, mean relative volume-to-volume displacement in each session was evaluated. Seven subjects were excluded from analysis based on mean tSNR > 2.5 SD and/or mean relative motion > 0.25 in either session.
Image Preprocessing
BOLD time series data were preprocessed and analyzed by standard procedures using fMRI Expert Analysis Tool (FEAT version 5.98) of FSL (FMRIB’s Software Library, Oxford, UK). Single subject preprocessing included nonbrain removal using BET (Smith, 2002), slice time correction, motion correction to the median image using MCFLIRT (Jenkinson et al, 2002), high pass temporal filtering (138 s), spatial smoothing using a Gaussian kernel (6 mm full-width at half-maximum, isotropic) and mean-based intensity normalization of all volumes using the same multiplicative factor. The median functional volume was coregistered to the anatomical T1-weighted structural volume, then transformed into the standard anatomical space (T1 MNI template) using FLIRT (Jenkinson et al, 2002; Jenkinson and Smith, 2001). Transformation parameters were later applied to all statistical contrast maps for group-level analyses.
Image Analysis
Subject-level statistical analyses were carried out voxelwise using FILM (FMRIB’s Improved General Linear Model) with local autocorrelation correction (Woolrich et al, 2001). Four condition events (0-back, 1-back, 2-back, and 3-back) were modeled using a canonical hemodynamic response function. The instruction period and six motion correction parameters were included as nuisance covariates and the three rest periods (fixation point) were treated as the baseline. Image analysis was completed for each individual in subject space, and resulting contrast maps were spatially normalized as described above.
ROI definition
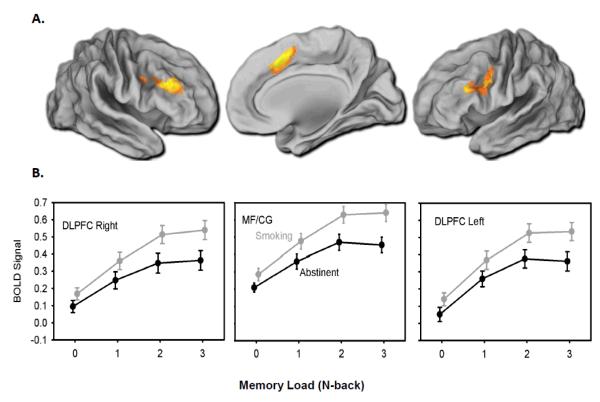
To characterize the session (abstinent versus smoking) by memory load (0back, 1back, 2back, 3back) effects, mean percent signal change was extracted from a priori regions of interest (ROIs) in the dorsal lateral prefrontal cortices (right and left DLPFC) and the dorsal cingulate/medial prefrontal cortex (MF/CG). ROI masks (Figure 1A) were functionally defined using a whole brain voxel-wise session (abstinence, smoking) by memory-load (0-back, 1-back, 2-back, 3-back) ANOVA examining the main effect of working memory load. Type I error was controlled using a whole-brain family-wise error (FWE) correction equivalent to z > 5.30. From this result, right DLPFC (4408 mm3), left DLPFC (5144 mm3), and the MF/CG (6552 mm3) were defined by segmenting out activated voxel clusters using a watershed algorithm implemented in MATLAB (The Mathworks, Inc., Natick, MA). ROI masks were then registered into native subject space using methods described above. Finally, mean percent signal change was calculated per subject for the four load conditions separately for each ROI. These values were exported for further analysis using standard statistical software and procedures described below.
Figure 1. BOLD signal change by session and memory load.
A) Colored regions represent functionally defined ROI masks identified using a whole brain repeated measures ANOVA with a voxel threshold of p<0.0001 and cluster corrected at Z = 6.03. Mean percent signal change was extracted from the ROIs for offline analysis of abstinence effects. B) Mean percent BOLD signal change from baseline extracted at each memory load during abstinent and smoking sessions; means are presented as raw values (unadjusted for covariates) and bars represent standard error (n=63). In the multiple regression models, percent signal change from baseline was significantly reduced during abstinence in the MF/CG and right and left DLPFC (all ps < 0.001). MF/CG = medial frontal/cingulate cortex, DLPFC = dorsolateral prefrontal cortex
To validate the functional localization for the above a priori regions and to address the issue of bias, data were also analyzed using independent ROI analyses, in which the selection of the a priori ROI was made with no information about the data being analyzed. We chose to define the ROIs from an independent sample (n=33) studied under comparable abstinence conditions (Loughead et al, 2009). The results were highly similar across both analyses; the results presented here are based on the functionally defined ROIs from the current population.
Whole Brain Voxelwise Analysis
To characterize age-related differences in the effects of abstinence within the working memory network, percent signal change was also extracted from other significant clusters for the main effect of working memory load. These values along with the a priori ROIS were then exported to further analysis as discussed below.
Whole brain ANOVA results were further examined as part of an exploratory aim to identify additional regions (beyond the a priori ROIs) sensitive to abstinence effects. Group Z statistic image for the main effect of session (collapsed across load) was cluster corrected at Z>3.1 and a corrected cluster significance threshold of p<0.05 using the theory of Gaussian Random Fields (Beckmann and Smith, 2004). A whole brain interaction of working memory load and session did not yield any results that were significant. Anatomic assignment of all clusters was determined by visual inspection and using the FSL atlas tool and pertinent available anatomic templates (MNI atlas, Talairach atlas, Harvard-Oxford cortical and subcortical structural atlases, and Johns Hopkins University DTI-based WM atlas).
Hypothesis Testing
To analyze session effects, mean percent BOLD signal change was modeled using regression with subject-level random effects, and estimated using maximum likelihood techniques (Stata xt-reg; Stata Corporation, College Station, TX, USA) with linear mixed effects models. All BOLD signal models included terms for the main effects of session, memory load, age group, and relevant covariates (sex, FTND score, Shipley IQ score, and session order). Memory load was coded as a categorical variable with the 0-back condition as reference category. Participants were classified as age < 50 years or age ≥ 50 years based on DSM-IV classifications of normal age-related memory impairment beginning at age 50 (Crook et al, 1986; Murray and Abeles, 2002) and prior success in detecting age-related changes in BOLD activation after age 50 (Gunning-Dixon et al, 2003). Session by memory load interactions and FTND by session interactions were tested, but dropped from the models as not significant. Task performance measures (accuracy and response time for correct trials) and subjective withdrawal measures (MNWS score and QSU score) were examined using similar models which replaced BOLD signal change with task performance as the outcomes. BOLD-behavior correlations were examined using models of performance, including percent BOLD signal change as a predictor (controlling for session, memory load, and relevant covariates). We tested hypotheses at a global type I error of α=0.05; based on the high correlation (Pearson’s r≈0.75) between the 3 a priori ROIs, an adjusted alpha of p = 0.04 (Sankoh, Huque, and Dubey, 1997) was applied to the BOLD and BOLD-behavior models. Alpha remained 0.05 for the performance models.
In order to test whether BOLD signal changes mediated the effects of abstinence on performance, separate path models for each ROI were fitted using the Stata v12 SEM routine, and reported standardized coefficients. Variances were adjusted for repeated measures using the cluster-correlated robust estimate (Williams, 2000). Mediation would require that abstinence should predict the BOLD response, and the BOLD response should predict performance. All predictive models controlled for memory load and relevant covariates. In addition to estimating the path model, we estimated the overall strength of the mediating pathway and percent mediation, calculating standard errors using the delta method.
Results
Descriptive Statistics
Sixty-three subjects were included in the final analysis. Of these, 28 (44%) were female. Participants reported an overall mean age of 40.7 years (SD=13.3, range 19-62), had smoked for an average of 23.4 years (SD 13.6, range 1-48), had a mean smoking rate of 16.0 cigarettes per day (SD=5.1, range 10-30), and a mean FTND score of 4.8 (SD=1.8, range 0-8). There were no differences in sex distribution, FTND score, or smoking quantity by age group (Table 1; all ps > 0.1). Mean CO readings were 27.0 ppm (SD=13.8, range 11-84) for the smoking session and 3.9 ppm (SD=2.3, range 1-9) for the abstinence session (p < 0.0001 for session difference). Scores on the MNWS withdrawal discomfort scale (smoking session: mean 3.7, SD 4.3, range 0-23; abstinent session: mean 11.3, SD 8.3, range 0-32) and the QSU-Brief (smoking session: mean 24.3, SD 12.0, range 10-57; abstinent session: mean 43.4, SD 16.0, range 10-70) were also significantly different between the smoking and abstinence sessions (ps < 0.0001), supporting the effectiveness of the abstinence manipulation.
Table 1.
Demographic variables by age group. There were no significant differences between groups for sex, FTND score, or CPD (all ps > 0.1).
| Measure | Age <50 yrs (n = 44) |
Age ≥50 yrs (n = 19) |
|---|---|---|
| Age, mean yrs (SD, range) | 34.3 (10.3, 19-49) | 55.6 (3.9, 50-62) |
| Female, N (%) | 17 (38.6) | 11 (57.8) |
| FTND score, mean (SD) | 4.9 (2.0) | 4.6 (1.4) |
| CPD, mean (SD) | 16.2 (4.8) | 15.5 (5.7) |
Behavioral Performance in Full Sample
There were significant main effects of session on both accuracy (β=−0.516, CI: −0.897 to −0.135, p=0.008) and median correct response time (β=29.59, CI: 11.42 to 47.76, p=0.001); abstinence was associated with poorer performance on both measures (i.e., fewer correct answers and slower response times). There was a marginal session by memory load interaction effect on median correct response time (Wald χ2(3)=7.80, p=0.050) indicating a trend for greater detrimental effect of abstinence at the 3-back level (β=52.4, CI: 1.45 to 103.3, p=0.04).
ROI Analysis of BOLD Signal Change in Full Sample
There was a significant effect of session (abstinence versus smoking) for all three a priori ROIs: MF/CG (β=−0.135, CI:-0.179 to −0.090, p<0.001), left DLPFC (β=−0.130, CI:-0.182 to −0.078, p<0.001), and right DLPFC (β=−0.132, CI:0.187 to −0.078, p<0.001) (Figure 1B). There were no significant session by memory load interactions.
Whole Brain Analyses
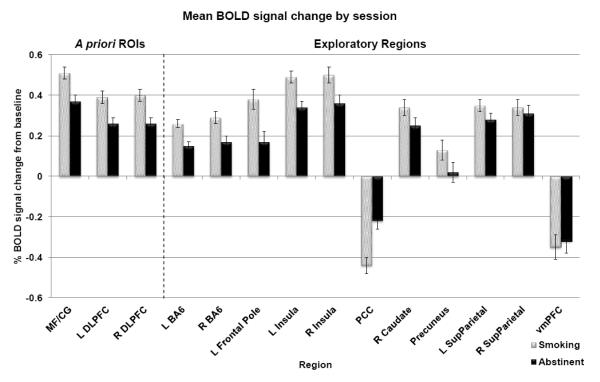
Whole brain ANOVA revealed a significant main effect of working memory load in the MF/CG, caudate nucleus, precuneus, posterior cingulate cortex (PCC), frontal pole, ventromedial prefrontal cortex (VMPFC) and bilaterally in DLPFC, Brodmann area 6 (BA6), insula and superior parietal cortex. Nine of these 11 additional regions also displayed a significant effect of session in offline analysis (Figure 2). Eight regions showed less activation during abstinence compared to smoking (left BA6, p<0.001; right BA6, p<0.001; left frontal pole, p<0.001; left insula, p<0.001; right insula, p<0.001; left superior parietal cortex, p=0.010; right caudate nucleus, p=0.001; and precuneus, p=0.002); one region (the PCC, a “default mode network” region that is normally deactivated during task performance) showed less deactivation during abstinence compared to smoking (p<0.001). There were no significant session by memory load interactions after correcting for multiple comparisons.
Figure 2. Whole brain results.
Mean percent BOLD signal change from baseline across task levels during abstinent and smoking sessions; bars represent standard error (n=63). In the multiple regression models, percent signal change from baseline was significantly reduced during abstinence in all three a priori ROIs (MF/CG, L DLPFC, and R DLPFC), and in 8 of the 11 additional regions identified by main effect of task (bilateral BA6, left frontal pole, bilateral insula, right caudate, precuneus, and left superior parietal cortex). One region (the PCC) displayed significantly less deactivation below baseline during abstinence. MF/CG = medial frontal/cingulate cortex, DLPFC = dorsolateral prefrontal cortex, BA6 = Brodmann Area 6, PCC = posterior cingulate cortex, SupParietal = superior parietal cortex, vmPFC = ventromedial prefrontal cortex
BOLD-Behavior Correlations
We examined associations between BOLD signal, accuracy, and correct response times, controlling for session and memory load. BOLD signal in the MF/CG and right and left DLPFC was positively associated with accuracy (MF/CG: β =1.17, 95% CI =0.47 to 1.87, p = 0.001; right DLPFC: β =1.06, 95% CI =0.47 to 1.65, p< 0.001; left DLPFC: β = 1.17 95% CI =0.57 to 1.77, p< 0.001). Furthermore, when percent BOLD signal change was included as a predictor in the model of accuracy, the prior effect of session (p< 0.001) was reduced, becoming nonsignificant (p > 0.05). Similar relationships were found in all but two of the exploratory ROIs; there was no relationship between BOLD signal in the PCC or vmPFC and task accuracy. BOLD signal in the MF/CG was marginally associated with correct response time (β =38.1, 95% CI =2.13 to 74.1, p = 0.04); in this model the main effect of session was not reduced. In the exploratory regions, BOLD signal in the left and right superior parietal cortex was significantly associated with correct response time (p = 0.03 and 0.02 respectively) with no reductions in the main effect of session.
We then tested the hypothesis that BOLD signal in the a priori ROIs mediates the effect of abstinence on accuracy using path analysis. The overall mediation pathway was significant for the MF/CG model (p = 0.024); BOLD signal accounted for ~30% of the effect of abstinence on task accuracy (95% CI: 0.002 to 0.597).
Exploratory Analysis of Moderating Effects of Age
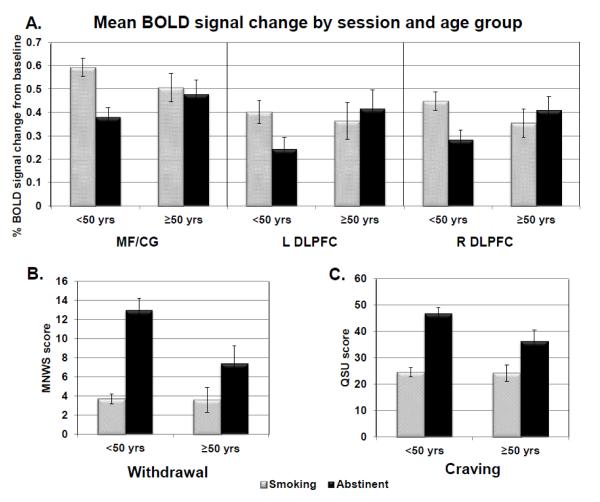
There were significant age by session interaction effects indicating that the effects of abstinence on withdrawal and craving were reduced in the >50 group compared to the <50 group (MNWS Wald χ2(1)=7.31, p=0.007; QSU Wald χ2(1)=7.02, p=0.008) (Figure 3B-C). There were no age x session interaction effects on task performance (either accuracy or response time); however, there was a significant age x memory load interaction effect indicating poorer performance at higher memory loads in the >50 group (β=[0 −0.62 −1.96 −1.62], Wald χ2(3)=14.02, p=0.003).
Figure 3. Age by Session Interactions.
A) Significant session and age group interaction on BOLD signal change (MF/CG Wald χ2(1)=14.52, p=0.0001; left DLPFC Wald χ2(1)=16.08, p=0.0001; right DLPFC Wald χ2(1)=17.45, p<0.0001). BOLD signal was significantly reduced in all three a priori ROIs during the abstinent session for the <50 age group but not for those ≥50 yrs. (p values of 0.0001 for MF/CG and R DLPFC in the > 50 age group and p<0.001 for the L DLPFC; corresponding p values in the <50 age group: 0.90 for MF/CG, 0.44 for R DLPFC, and 0.58 for L DLPFC). B) Significant session by age interaction on withdrawal (Wald χ2(1)=7.31, p=0.007). Session effects were significant in both age groups (ps < 0.0001) but attenuated in the >50 age group. C) Significant session by age interaction on craving (Wald χ2(1)=7.02, p=0.008). Again, session effects were significant in both age groups (ps < 0.0001) but attenuated in the >50 age group.
There were significant age by session interaction effects in all three a priori ROIs (MF/CG Wald χ2(1)=14.52, p=0.0001; left DLPFC Wald χ2(1)=16.08, p=0.0001; right DLPFC Wald χ2(1)=17.45, p<0.0001). In all of these regions, post-hoc t-tests revealed that the effect of session was significant in the <50 group (ps < 0.001), whereas the session effects were attenuated or nonsignificant in the >50 group (Figure 3A). In the additional regions identified by the whole brain ANOVA, there were significant age by session interaction effects in the right BA6 (p = 0.005), left frontal pole (p = 0.018), bilateral insula (ps < 0.01), and bilateral superior parietal cortex (ps < 0.01). In all of these regions, the effect of abstinence on BOLD signal change was smaller in the >50 group compared to the <50 group.
Discussion
In the largest sample of adult smokers studied to date for nicotine abstinence effects on working memory in an fMRI study, we report poorer performance and decreased brain activation during abstinence across regions comprising a task-related working memory circuit (Owen et al, 2005). The extent of reduction in BOLD signal in the MF/CG mediated the effect of abstinence on accuracy but not response time. Seed-based connectivity between the MF/CG and other regions in the task network was also reduced during abstinence compared to smoking. The effects of abstinence on BOLD signal in these regions, as well as withdrawal and craving, were reduced in adults aged 50 years and older compared to younger smokers.
The effects of abstinence were most pronounced in task-activated regions, suggesting that an inability to recruit sufficient resources to meet cognitive demands during abstinence may underlie working memory deficits. This effect is consistent with prior studies investigating the effects of abstinence on working memory in smokers (Loughead et al, 2009; Loughead et al, 2010). These task-activated regions are components of two distinct but related networks: the fronto-parietal or executive control network, including the DLPFC, which activates during working memory task performance; and a cingulo-opercular or salience network, including the MF/CG and insula, which plays a role in shifting attention between the executive control network and default mode network (Dosenbach et al, 2008; Sridharan et al, 2008). It is possible that the abstinence-induced working memory deficits and reduced activation in task-positive regions, and the role these regions play in executive function, is the link between cognitive impairment and smoking relapse. Notably, working memory training can decrease measures of impulsive behavior in addicts (Bickel et al, 2011), suggesting a possible avenue of exploration for future smoking cessation research.
In addition to the task-positive regions, we also noted significant effects of abstinence in the PCC. This region is considered part of the “default mode network” (DMN) that is typically deactivated during task performance (Raichle et al, 2001). It has been suggested that the DMN is involved in internally-focused or self-referential functions (i.e., mind wandering); and that deactivation of this network is important for successful performance of externally-focused tasks (Aticevic et al, 2012). In our sample, abstinence was associated with less deactivation in DMN regions, suggesting an impaired ability to suppress goal-irrelevant cognitive functions during the task. These findings are consistent with other work which has shown that nicotine may enhance cognitive function by deactivating areas of the DMN (Hahn et al, 2007), and that nicotine withdrawal symptoms are associated with decreased negative coupling between executive control and default networks (Cole et al, 2010; Sutherland et al, 2012).
This study is the first to report that effects of abstinence on working memory related BOLD signal change and withdrawal symptoms are more pronounced in younger smokers. In general, older adults have been shown to exhibit different patterns of BOLD response to working memory tasks than younger adults (Grady 2008; Turner and Spreng, 2012). In addition, population-based surveys suggest that older smokers are more likely to quit smoking (Hymowitz et al, 1997) and nicotine dependence may decrease after age 50 (Park et al, 2012). It is possible that age-related reductions in nicotinic receptor availability (Mitsis et al, 2009) and decreased cholinergic signaling efficiency (Störmer et al, 2012) contribute to blunted withdrawal symptoms when nicotine is eliminated. In addition, nicotine withdrawal alters dopaminergic tone (DeBiasi and Dani 2011); thus, age-associated declines in dopamine D1 and D2 receptors and the dopamine transporter (Bäckman et al, 2006) may attenuate withdrawal effects on dopamine transmission. Although additional research is necessary to replicate our findings, our results may suggest an underlying neurocognitive mechanism for reduced nicotine dependence and greater smoking cessation success among older adults.
Strengths of our study include the large number of participants as well as the use of treatment-seeking smokers, which may increase the relevance of the results for smoking cessation (Perkins et al, 2010). One potential limitation of our study is the brief delay between the last cigarette smoked and the beginning of BOLD imaging with the N-back task during the smoking scan. Withdrawal symptoms can begin within a few minutes of smoking and it is possible that smokers were experiencing mild withdrawal even at the smoking scan. However, breath CO readings at each session and cravings ratings obtained prior to BOLD imaging were significantly different between the smoking and abstinent sessions, indicating effectiveness of the abstinence manipulation. Also, because BOLD fMRI measures changes in the relative ratio of oxygenated to deoxygenated hemoglobin, it may be sensitive to nicotine-induced changes in global cortical vasodilation (Mathew and Wilson, 1991). However, perfusion MRI has shown that nicotine does not alter cerebral perfusion in a regionally non-specific manner (Hahn et al, 2007); and indeed, the effects of abstinence on BOLD signal in our study were in opposite directions in task-activated regions compared to regions in the default network. Another potential limitation is our exclusion of individuals with comorbid Axis I psychiatric disorders. Individuals with mental disorders present unique considerations for imaging studies due to alterations in brain structure and signaling associated with many psychiatric conditions; we therefore chose to exclude them from this imaging study (as is the convention in many nicotine dependence imaging studies). However, psychiatric disorders occur at a higher rate among smokers than among non-smokers, and smokers with comorbid psychopathologies represent an important and substantial subset of treatment-seeking smokers; care should be taken when extrapolating our findings to the general smoking population. Finally, it is important to note that a potential confound in the analysis of age-related differences is the length of smoking history. Age and number of years of smoking were highly correlated in our sample (Pearson’s r ≈ 0.9), and it is not possible to fully distinguish the effects of one factor over the other.
In conclusion, we demonstrate a robust effect of 24 hours of abstinence from nicotine on working memory performance and related brain activation, which was attenuated in smokers over the age of 50. Given the association between abstinence-induced cognitive deficits and smoking relapse, further investigation into this pattern of brain and behavioral response may provide support for a potential imaging biomarker for screening smoking cessation medications and identifying the most at-risk treatment-seeking smokers. Furthermore, this is the first study to demonstrate age-related differences in abstinence effects on neurocognitive deficits and withdrawal symptoms. Future research into other age-related differences in the effects of smoking abstinence may aid in targeting the most appropriate smoking cessation treatments for older adults.
Acknowledgments
This research was supported by NIH grants P50 CA143187 and R01 DA026849 to C.L. M.F. is supported by NIH grant T32 GM008076.
Footnotes
Author contributions M. Falcone contributed to data collection, analysis, and manuscript writing; E.P. Wileyto contributed to data analysis and manuscript preparation; K. Ruparel and R. Gerraty contributed to data analysis and manuscript preparation; L. LaPrate assisted in data collection and manuscript preparation; R. Gur contributed to interpretation of data and manuscript preparation; J. Detre, J. Loughead and C. Lerman were responsible for study design, data analysis and manuscript writing. All authors have approved the final version of the manuscript.
Conflict of Interest Dr. Lerman has served as a consultant and/or has received research funding from GlaxoSmithKline, AstraZeneca, and Pfizer. Dr. Wileyto has served as a consultant for Pfizer. Dr. Loughead and Dr. Gur have received investigator-initiated grant support from Astra Zeneca and Pfizer. The current study was not supported by industry funds. The other authors report having received no relevant lecture fees, consulting fees, or other financial interests and have no potential conflicts of interest.
References
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev. 2006;30:791–807. doi: 10.1016/j.neubiorev.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69:260–265. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. Smoking-induced ventral striatum dopamine release. American J Psychiat. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage. 2010;52:590–599. doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Crook T, Bartus R, Ferris S, Whitehouse P, Cohen G, Gershon S. Age-associated memory impairment: proposed diagnostic criteria and measures of clinical change. Report of a National Institute of Mental Health Work Group. Dev Neuropsychol. 1986;2:261–276. [Google Scholar]
- De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Ann Rev Neurosci. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals NY Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, Chan RM, Loughead JW, Alsop DC, Maldjian J, Gur RE. Age-related differences in brain activation during emotional face processing. Neurobiol Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA. Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Pickens RW, Krahn D, Malin S, Luknic A. Effect of nicotine on the tobacco withdrawal syndrome. Psychopharmacology. 1984;83:82–87. doi: 10.1007/BF00427428. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, Lynn WR, Pechacek TF, Hartwell TD. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(Suppl 2):S57–62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology. 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Loughead J, Ray R, Wileyto EP, Ruparel K, Sanborn P, Siegel S, Gur RC, Lerman C. Effects of the alpha4beta2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol Psychiatry. 2010;67:715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Valdez JN, Sanborn P, Tang K, Strasser AA, Ruparel K, Ray R, Gur RC, Lerman C. Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol Psychiatr. 2009;14:820–826. doi: 10.1038/mp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. Substance abuse and cerebral blood flow. Am J Psychiatr. 1991;148:292–305. doi: 10.1176/ajp.148.3.292. [DOI] [PubMed] [Google Scholar]
- Mehta M, Adem A, Kahlon MS, Sabbagh MN. The nicotinic acetylcholine receptor: smoking and Alzheimer’s disease revisited. Frontiers Biosci. 2012;4:169–180. doi: 10.2741/367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecklinger A, Weber K, Gunter TC, Engle RW. Dissociable brain mechanisms for inhibitory control: effects of interference content and working memory capacity. Cognitive Brain Res. 2003;18:26–38. doi: 10.1016/j.cogbrainres.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsis EM, Cosgrove KP, Staley JK, Bois F, Frohlich EB, Tamagnan GD, Estok KM, Seibyl JP, van Dyck CH. Age-related decline in nicotinic receptor availability with [(123)I]5-IA-85380 SPECT. Neurobiol Aging. 2009;30:1490–1497. doi: 10.1016/j.neurobiolaging.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KN, Abeles N. Nicotine’s effect on neural and cognitive functioning in an aging population. Aging Ment Health. 2002;6:129–138. doi: 10.1080/13607860220126808. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee JY, Song TM, Cho SI. Age-associated changes in nicotine dependence. Public Health. 2012;126:482–489. doi: 10.1016/j.puhe.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depen. 2010;106:61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Fonte CA, Mercincavage M, Stitzer ML, Chengappa KN, Jain A. Cross-validation of a new procedure for early screening of smoking cessation medications in humans. Clin Pharmacol Ther. 2010;88:109–114. doi: 10.1038/clpt.2010.65. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Genetic variability in nicotinic acetylcholine receptors and nicotine addiction: converging evidence from human and animal research. Behav Brain Res. 2008;193:1–16. doi: 10.1016/j.bbr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, Chan R, Gur RE. Working memory for complex figures: an fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–379. [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. PNAS. 2001;98:676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis M, Jepson C, Patterson F, Lerman C. Increases in hyperactive-impulsive symptoms predict relapse among smokers in nicotine replacement therapy. J Subst Abuse Treat. 2005;28:297–304. doi: 10.1016/j.jsat.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Cinical Psychiat. 1998;59(Suppl 20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. PNAS. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Störmer VS, Passow S, Biesenack J, Li SC. Dopaminergic and cholinergic modulations of visual-spatial attention and working memory: Insights from molecular genetic research and implications for adult cognitive development. Dev Psychol. 2012;48:875–889. doi: 10.1037/a0026198. [DOI] [PubMed] [Google Scholar]
- Sun X, Prochaska JO, Velicer WF, Laforge RG. Transtheoretical principles and processes for quitting smoking: a 24-month comparison of a representative sample of quitters, relapsers, and non-quitters. Addict Behav. 2007;32:2707–2726. doi: 10.1016/j.addbeh.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. NeuroImage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. Executive functions and neurocognitive aging: dissociable patterns of brain activity. Neurobiol Aging. 2012;33:826, e821–813. doi: 10.1016/j.neurobiolaging.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Williams RL. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, Ernst M, London ED. Brain activity in cigarette smokers performing a working memory task: effect of smoking abstinence. Biol Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Simon S, Brody AL, Jarvik M, Rodriguez P, Ernst M, London ED. Effects of acute smoking on brain activity vary with abstinence in smokers performing the N-Back task: a preliminary study. Psychiatr Res. 2006;148:103–109. doi: 10.1016/j.pscychresns.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]