Abstract
The UVB component of sunlight, which causes DNA damage and inflammation, is the major cause of nonmelanoma skin cancer (NMSC), the most prevalent of all cancers. Nonsteroidal anti-inflammatory drugs (NSAIDs) and coxibs have been shown to be effective chemoprevention agents in multiple preclinical trials, including NMSC, colon and urinary bladder cancer. NSAIDs, however, cause gastrointestinal irritation, which led to the recent development of nitric oxide (NO) derivatives that may partially ameliorate this toxicity. This study compared the efficacy of several NSAIDs and NO-NSAIDs on UV-induced NMSC in SKH-1 hairless mice and determined whether various short-term biomarkers were predictive of long-term tumor outcome with these agents. Naproxen at 100 (p>.05) and 400 ppm (p<.01) in the diet reduced tumor multiplicity by 26 and 63% respectively. The NO-naproxen at slightly lower molar doses shows similar activities. Aspirin at 60 or 750 ppm in the diet reduced tumor multiplicity by 19 and 50%; while the equivalent doses (108 and 1350 ppm) were slightly less effective. Sulindac at 25 and 150 ppm in the diet doses far below the Human Equivalent Dose, was the most potent NSAID with reductions of 50 and 94% respectively. In testing short-term biomarkers we found that agents that reduce UV-induced prostaglandin E2 synthesis and/or inhibit UV-induced keratinocyte proliferation yielded long-term tumor efficacy.
Keywords: Nonsteroidal anti-inflammatory drugs, prostaglandin, skin, UV, NO-NSAID
INTRODUCTION
Exposure to ultraviolet (UV) light is the major cause of nonmelanoma skin cancer (NMSC) in humans (1). Intense UV exposure is usually marked by an inflammatory response and subsequent hyperplasia. Although the mechanism(s) is not entirely clear, it has been well established that UV elicitation of the prostaglandin (PG) products of arachidonic acid plays a significant role in both UV-induced inflammation and UV-induced skin tumors (2, 3). The enzymes responsible for the production of PGs are referred to as the PGH synthases, of which there are two isoforms, cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2). Both isoforms exhibit similar COX activity and both are expressed in mouse and human epidermis. COX-1 and COX-2, however, are differentially regulated such that COX-1 is usually constitutively expressed in most tissues, including the epidermis, while COX-2, which is generally expressed in unperturbed tissues at low to undetectable levels, is highly inducible by a number of irritating agents, including UV (4).
The relative contribution of each COX isoform to UV-tumorigenesis has recently been elucidated. Using mice deficient in either isoform, we showed that while COX-2 deficiency dramatically reduced tumor development, a reduction in COX-1 had little effect (5). The reason for this is not entirely clear but is likely related to the much greater production of PGE2 from COX-2 due to the high level of COX-2 expression induced by UV (6). The genetic studies were complemented with pharmacological studies using the non-specific COX-1/COX-2 inhibitor indomethacin, one of the nonsteroidal anti-inflammatory drugs (NSAIDs), as well as COX-2 selective inhibitors, referred to as coxibs, e.g., celecoxib. The study with celecoxib showed that it markedly reduced UV-induced skin tumors in mice, as did indomethacin (6). Because pharmacological inhibition of COX-2 with celecoxib mimicked genetic deficiency of COX-2 in the SKH hairless mouse, this model offers an opportunity to identify agents, including other NSAIDs, for their ability to reduce UV-induced PGE2 synthesis and to correlate that with long-term skin tumor development.
The choice of drugs used in the present study was based on several considerations. The coxibs were originally developed to avoid the gastrointestinal irritation caused by the classical NSAIDs, based on the premise that the PGs from COX-1 help maintain the integrity of the mucosa while the PGs from COX-2 are pro-inflammatory. Because of their cardiovascular side effects, however, the use of coxibs, despite clear efficacy, is problematic for long-term chemoprevention (7). The original concerns that NSAIDs cause an increased risk of ulcers and bleeding reduced enthusiasm for considering them as chemopreventive agents. The incidence of death is roughly 1/8,000 for most NSAID users, although the incidence of hospitalization for bleeds may be 1/1,000, suggesting that from a benefit vs risk perspective they may be of great value for the majority of high-risk individuals (8). Recently a new class of NSAIDs was developed in which a nitric oxide (NO) moiety was added to the native NSAID. The rationale was that NO would be released and have a beneficial effect on the gastrointestinal mucosa that counteracts the effects of reduced PG synthesis (9). Both NO-aspirin and NO-naproxen were reported to reduce the macroscopic mucosal damage observed with aspirin and naproxen in rats (10). A comparison of naproxen and NO-naproxen in preventing large urinary tract tumors in rodents showed that they were equally effective, although neither was effective in a model of mammary cancer (11). There thus appears to be tissue specificity, although the basis for the difference is unknown.
As mentioned above, we previously demonstrated the effectiveness of celecoxib and indomethacin in significantly reducing both UV-induced PGE2 levels and skin tumor development (6). In the current study we compared aspirin, NO-aspirin, naproxen, NO-naproxen and sulindac, for their relative abilities to inhibit UV-induced short-term PGE2 synthesis and proliferation, and long-term tumor development in mice. The goal is to determine whether PGE2 or proliferation inhibition are predictive biomarkers for the chemopreventive efficacy of NSAIDs and other agents. We report here that, using human equivalent doses (HED), there is a strong correlation between the extent to which an NSAID or NO-NSAID inhibited acute UV-induced PGE2 synthesis and UV-induced keratinocyte proliferation, and skin tumor development.
MATERIALS AND METHODS
Animals and UV Irradiation
Female SKH-1 hr/hr mice 3-4 wks old were purchased from Charles River Laboratories (Wilmington, MA) and were housed in climate-controlled quarters (22° ± 1°C at 50% humidity) with 12/12 hour light/dark cycle using yellow fluorescent lights. Animals were allowed free access to water and diet and were observed daily. The following NSAIDs were supplied by the Division of Cancer Prevention, NCI: aspirin (lot# TW0592), 3-nitrooxymethylphenyl aspirin (lot#0612105), sodium naproxen (lot# 080204), and NO-naproxen (lot# 0901002), sulindac (lot# UD0534). Indomethacin was purchased from Sigma Chemical Co. (St. Louis, MO) and celecoxib from LKT Laboratories (St. Paul, MN). Powdered AIN-76 diet was purchased from Research Diets (New Brunswick, NJ); the experimental diets were prepared weekly by mixing the NSAID into the diet with an electric mixer. The diets were stored at 4°C and fresh diet was supplied three times weekly in clean glass jars with stainless steel lids. Individual body weights were determined weekly for 20 weeks or more.
The UV apparatus and spectral irradiance used were previously described (6). For tumor studies, groups of 20 (unless noted otherwise) mice were fed their experimental diet starting one week before thrice weekly UV-irradiation, starting with an initial dose of 90 mJ/cm2 for the first week, followed by a weekly 10% increase until a dose of 175 mJ/cm2 was reached. Weekly tumor counts were performed after the appearance of the first tumor and were continued until the termination of the experiment. The tumor data are expressed both as multiplicity (i.e., mean number of tumors per mouse) and incidence (i.e., percent of mice with tumors). At the termination of the experiment, the diameters of the tumors were measured and the tumors were assigned to size categories. All tumors were processed for histological analysis for determination of tumor type. The gastrointestinal tracts of all mice were also removed, washed with PBS and processed for histological staining with hematoxylin and eosin (H&E.) All sections were assessed microscopically for irritation or erosion of the epithelial lining.
PGE2 Analysis
Groups of 6-8 mice were killed 6 hr after a single UV treatment (220 mJ/cm2), their dorsal surfaces were quickly frozen on dry ice and the animals immersed in liquid nitrogen and stored at -70°C. A 1.5 cm2 area of epidermis was chipped from the frozen skin and processed as previously described (12). The PGE2 levels were measured by enzyme immunoassay (Cayman Chemical Co., Ann Arbor, MI) according to the manufacturers instructions.
Histologies, Labeling Index and Apoptosis Detection
For acute treatment studies, groups of 3-4 mice were fed control or NSAID-containing diet for 7 to 10 days before UV irradiation with 220 mJ/cm2 and killed at the 24 and 48 hr. Mice were injected intraperitoneally with a sterile solution of 20 mg/ml 5-bromo-2-deoxyuridine (BrdU; Sigma Chemical Co.) at 0.1 mg/g body weight in phosphate buffered saline 1 hr prior to killing. Three to six 5 mm × 1.5 cm sections of skin were excised and fixed in 10% formalin prior to embedding in paraffin. Tissue sections (4 μm) were stained either with H&E or immunohistochemically stained for BrdU incorporation using a monoclonal rat anti-BrdU antibody, (diluted 1:1; Accurate Chemical and Scientific Corp., Westbury, NY). The bound antibody was visualized with 3,3′-diaminobenzidene (Sigma Chemical Co.) using avidin-biotin horseradish peroxidase (Vectastain Elite ABC kit; Vector Laboratories, Burlingame, CA) linked to an affinity-purified biotin-labeled rabbit anti-rat IgG. The labeling index was calculated as the percentage of basal cells staining positive for BrdU on at least 8 random areas for each of 3 sections from each mouse and the mean percentage and standard error for each treatment group determined. Tissue sections stained with H&E were used to measure the thickness of the epidermis (μm) using Nikon NIS-Elements software (Nikon Instruments Inc., Melville, NY).
For determination of UV-induced apoptosis, 3 mice per experimental group were sacrificed 24 hr after 220 mJ/cm2 UV-irradiation, skin sections fixed in formalin and processed for paraffin embedding. Sections were stained for apoptosis using an antibody against cleaved capsase-3 at a 1:500 dilution, according to the manufacturer's protocol (R&D Systems, Minneapolis, MN). Biotinylated goat anti-rabbit IgG was used at 1:500 dilution as the secondary antibody (Vector Laboratories). The number of positive cells per μm length of basement membrane was counted on 10 to 12 random areas for each section (3/mouse) and the mean and standard error determined.
Western Analysis
Proteins were isolated by scraping the epidermis from removed skin into RIPA buffer (50 mM Tris pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 0.5% Na deoxycholate, 0.2% SDS). Following sonication, protein concentrations were measured using a BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Samples were electrophoresed on 10% or 4-15% gradient SDS-polyacrylamide gels and electroblotted onto nitrocellulose. COX-2 protein was detected with an anti-COX-2 polyclonal antibody (1:1000; Millipore, Bellerica, CA). A horse-radish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (1:10,000; Jackson ImunoResearch Laboratories, West Grove, PA) was used as the secondary antibody. Actin, used as a loading control, was detected with an anti-actin HRP antibody (1:5000; Santa Cruz Biotechnology Inc., Santa Cruz, CA). COX-2 and actin were identified after chemiluminescence detection (Amersham Corp., Arlington Heights, IL) of HRP by comparison to molecular weight markers. Densitometry was performed using ImageQuantTL (GE Healthcare Life Sciences, Pittsburgh, PA); the values for the UV bands were normalized to 1.0 and the NSAID bands expressed as a percentage of the UV only bands. Triplicate experiments were performed, using 3 mice each and the data expressed as a mean ± standard error.
Statistics
Mann-Whitney and unpaired t-tests were done using GraphPad InStat, ver. 3.0 (San Diego, CA) software to determine statistical significance for the proliferation, caspase-3 and PGE2 data. Correlation coefficients were also determined with this software. The tumor multiplicity data was evaluated using Poisson regression analysis; differences in tumor latency were assessed using the Cox proportional hazards model.
RESULTS
Effect of NSAID Consumption on Body Weights
The effect of two weeks of NSAID administration on body weights, as a measure of toxicity, was determined. There were no significant differences in the naproxen, NO-naproxen, aspirin and NO-aspirin groups from the control group fed diet without NSAID (mean weight 24.2 ± 1.34 g). For sulindac, the initial doses given to the mice were 150, 300 and 600 ppm, however, the 300 and 600 ppm doses resulted in the death of several mice (these doses also caused death in FVB mice [data not shown]). For this reason, lower doses (25 ppm and 150 ppm in the diet) of sulindac were used in subsequent experiments. Mice in the tumor experiments were also weighed at the termination of the study, after ~30 weeks on their NSAID diets, with no significant differences between them and the control diet group (mean weight of 30.2 ± 3.6 g). With the exception of high-doses sulindac, it was concluded that the doses of NSAIDS administered in this study were not toxic. Additionally, histological examination of the gastrointestinal tracts of NSAID-fed mice revealed no signs of irritation or ulceration (data not shown).
NSAID Effects on PGE2 Levels
As we have previously demonstrated (6), acute exposure to UV causes high levels of PGE2 to be synthesized in the epidermis. As shown in Table 1, in this study UV caused a nearly 5-fold increase in PGE2 levels. Because indomethacin has previously been shown to prevent PGE2 synthesis after UV, it was included here as a positive control for comparative purposes. Celecoxib, which is a selective COX-2 inhibitor (13), was also previously shown to reduce UV-induced PGE2 by ~60% at the 500 ppm dose level (6); similar decreases were observed in this study (Table 1). The 250 ppm dose of celecoxib, which is roughly equal to the HED, was nearly as effective as the 500 ppm dose while the 125 ppm dose was completely ineffective. The observation that celecoxib is not as effective as indomethacin in reducing PGE2 is likely due the inability of celecoxib to inhibit COX-1 while indomethacin is a dual COX-1/COX-2 inhibitor. All of the other NSAIDs tested also reduced PGE2 synthesis in a dose-responsive manner, although some were more effective than others. Additionally, for naproxen, the addition of a nitroso moiety diminished its COX inhibitory activity. Naproxen was very effective in inhibiting PGE2, with the 200 and 400 ppm doses equivalent to 4 ppm indomethacin. A 400 ppm dose translates to a HED of 320 mg (7). Over-the-counter preparations of naproxen are usually 250 or 550 mg tablets to be consumed four times or twice daily, respectively. The nitroso form of naproxen appears less effective than the parent form, however the higher MW of NO-naproxen would partially correct for these differences, since the MW of NO-naproxen is roughly 55% greater than for naproxen. Interestingly, aspirin appeared significantly less effective. Low dose aspirin, which approximated the HED for the heart dose of aspirin, had limited effects on the induced levels of PGE2. The median dose of aspirin, 250 ppm, reduced levels roughly 60% while the highest dose reduced levels roughly 80%. The nitroso form of aspirin at equimolar doses was very effective at the higher doses in decreasing PGE2 levels almost 90%. The 150 ppm dose of sulindac, which has a HED of 159 mg/day (human doses are usually ≥300 mg/day), was remarkably effective in that it reduced PGE2 almost 95% compared to UV controls.
Table 1.
Effect of NSAIDs on UV-induced PGE2 and Proliferation
| Agent | Dose (ppm) | pg PGE2/μg Protein | % of UV Control | % BrdU positive cells | % of UV control |
|---|---|---|---|---|---|
| no UV | 404 ± 90a | 21.6 | 2.4 ± 0.13a | 15.7 | |
| UV | 1872 ± 391 | 100.0 | 15.3 ± 0.96 | 100.0 | |
| Indo + UV | 4 | 126 ± 37a | 6.7 | 4.8 ± 0.64a | 31.3 |
| Celecoxib + UV | 125 | 1716 ± 84 | 91.7 | nd | nd |
| Celecoxib + UV | 250 | 818 ± 51a | 43.7 | nd | nd |
| Celecoxib + UV | 500 | 681 ± 125a | 36.4 | nd | nd |
| Nap + UV | 100 | 274 ± 84a | 14.6 | 7.2 ± 1.48a | 47.1 |
| Nap + UV | 200 | 168 ± 34a | 9.0 | nd | nd |
| Nap + UV | 400 | 114 ± 54a | 6.1 | 6.7 ±1.36a | 44.1 |
| NO-Nap + UV | 100 | 653 ± 57a | 34.9 | 12.3 ± 2.24 | 80.4 |
| NO-Nap + UV | 200 | 440 ± 87a | 23.5 | nd | nd |
| NO-Nap + UV | 400 | 336 ± 53a | 17.9 | 6.8 ± 2.23a | 44.4 |
| Asp + UV | 60 | 1270 ± 106b | 67.8 | 5.4 ± 2.06a | 35.3 |
| Asp + UV | 250 | 701 ± 53a | 37.4 | nd | nd |
| Asp + UV | 750 | 337 ± 61a | 18.0 | 8.1 ± 1.31a | 52.6 |
| NO-Asp + UV | 108 | 1267 ± 118a | 67.7 | 10.1 ±1.02a | 66.0 |
| NO-Asp + UV | 450 | 232 ± 43a | 12.4 | nd | nd |
| NO-Asp + UV | 1350 | 162 ± 38a | 8.7 | 9.7 ± 1.15a | 63.3 |
| Sul + UV | 25 | 402 ± 196a | 21.5 | 8.3 ± 0.31a | 54.2 |
| Sul + UV | 75 | 174 ± 52a | 9.3 | 8.4 ± 0.68a | 54.9 |
| Sul + UV | 150 | 69 ± 20a | 3.7 | 4.4 ± 0.70a | 29.0 |
For PGE2 analysis, groups of 6-8 mice fed their respective experimental diets for 1 week were exposed to 220 mJ/cm2 UV (except the no UV group) and killed 6 hr later. The epidermis was chipped from frozen skin, PGE2 extracted and subjected to enzyme immunoassay as described under Methods and Materials. For BrdU labeling, groups of 3-4 mice fed their respective experimental diets for 1 week were exposed to 220 mJ/cm2 UV (except the no UV group) and killed 24 hrs later. All mice were injected with BrdU 1 hr prior to killing. Sections of skin were processed for immunohistochemical staining for BrdU. The number of BrdU positive basal cells and total number of basal cells per field were counted. The values represent the mean percentage of positive basal cells ± std. dev. The UV controls were statistically significantly different (p<0.01) from the no UV group. All other groups were compared to the UV only group. The statistical differences are shown by superscripts:
p<0.01
p<0.05.
nd = not determined.
NSAID Effects on Proliferation, Epidermal Thickness and Apoptosis
Similar to previous reports (6) UV irradiation increased the BrdU labeling index by over 6-fold at 24 hrs, as shown in Table 1. All of the NSAIDs studied reduced the labeling index, although there were differences in their efficacies. The highly effective agents indomethacin and sulindac (150 pm) strongly decreased proliferation by roughly 70%. Overall, there is a correlation, although not significant, between the ability of a NSAID to inhibit cyclooxygenases and their ability to inhibit UV-induced DNA synthesis. When modulation of epidermal thickness, which occurs as a result of both proliferation and edema, was used as a potential endpoint we observed maximally a 38%, but this did not correlate with the BrdU labeling index, apoptosis or PGE2 levels.
The apoptotic response of keratinocytes to UV exposure is believed to be at least partly caused by reactive oxygen species (ROS) generated in response to UV. Because a recent study indicated that inhibiting inflammation with thioredoxin significantly reduced apoptosis (14), the ability of NSAIDs to reduce UV-induced apoptosis was tested (Supplemental Table 1). While all of the NSAIDs significantly reduced apoptosis, there was no correlation with the extent of tumor development and is thus not a useful short-term biomarker.
Effect of NSAIDs on COX-2 expression
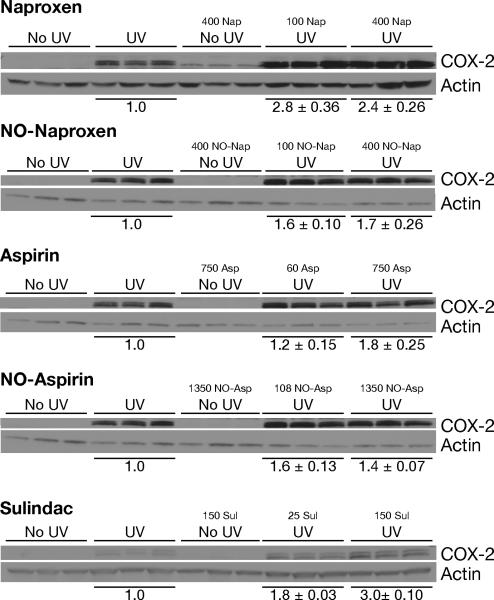
Because we have previously shown that PGE2 can induce the expression of COX-2 in keratinocytes (15), we were interested in determining whether inhibition of PGE2 synthesis would reduce the level of UV-induced COX-2 expression. As shown in Fig. 1, COX-2 was not expressed in untreated mice or in mice fed the high dose NSAIDs without exposure to UV. Naproxen, however, enhanced UV-induced COX-2 by approximately 2-fold; NO-naproxen had less of an enhancing effect. Aspirin and NO-aspirin produced minor enhancement while sulindac at 150 ppm caused a reproducible 3-fold enhancement. Thus there appears to be a negative correlation between the ability of an agent to decrease PGE2 synthesis and the levels of COX-2 expression.
Figure 1. Effect of NSAIDs on UV-induced COX-2 expression.
Three groups of 3 mice each were fed their respective experimental diets for one week, exposed to 220 mJ/cm2 UV (except the no UV group) and killed 6 hr later. The epidermis was chipped from frozen skin into RIPA buffer and subjected to Western blot analysis as described under Methods and Materials. A representative experiment is shown; densitometry was performed on each blot, the UV values set to 1.0 and the NSAID data expressed as the percentage of the UV control ± SE.
Effect of NSAIDs on Tumor Development
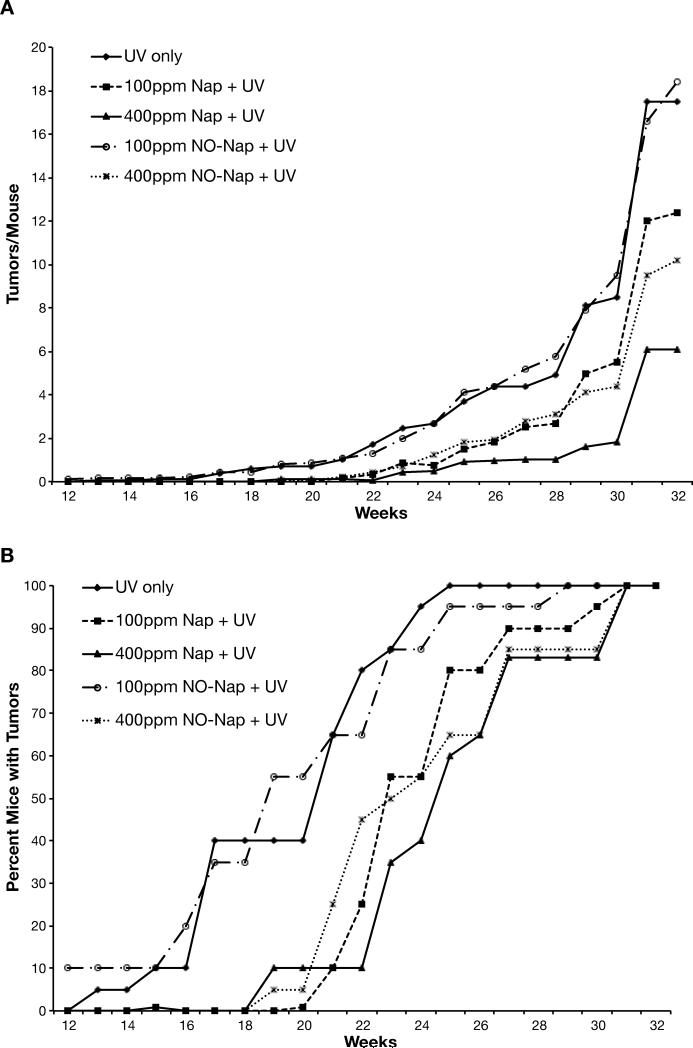
We have previously shown that indomethacin and celecoxib were very effective in preventing UV-induced skin tumors. Indomethacin at 4 ppm reduced tumor yield by 78% while mice fed celecoxib at 150 or 500 ppm showed a dose-dependent reduction of 60% and 80%, respectively (6). As shown in Fig. 2, in a UV tumor experiment testing the efficacy of naproxen and NO-naproxen, tumors were first observed between 10 and 11 weeks, which is consistent with our previous study on indomethacin and celecoxib (6). Naproxen at 100 ppm significantly reduced tumor yield by ~26% and at 400 ppm very significantly reduced it by 63%. NO-naproxen at 400 ppm, which is roughly 2/3 of the molar dose of naproxen, was relatively effective in reducing tumor yield by 52% (Fig. 2A). The doses of naproxen and NO-naproxen were effective in reducing tumor number also very significantly increased latency and the time to 100% tumor incidence.
Figure 2. Effect of naproxen or NO-naproxen on UV-induced skin tumorigenesis.
Groups of 20 female SKH-1 mice were placed on their experimental diets and exposed to UV irradiation thrice weekly. Tumors were counted weekly and the data calculated as the average number of tumors per mouse (panel A). Tumor multiplicity for 100 ppm naproxen was significantly (p= 0.05) different from the UV control group; 400 ppm naproxen and NO-naproxen were very significantly (p<0.001) different from the UV control. Tumor incidence (panel B) was calculated as the percentage of mice bearing tumors. Mice in the 400 ppm naproxen, 100 ppm and 400 ppm NO-naproxen groups developed tumors significantly (p<0.001 for all 3 groups) slower than the UV control.
Differences in the size distribution of the tumors were also observed, as shown in Supplemental Table 2. The groups having the largest percentage of tumors with diameters greater than 7 mm were also the groups with the most tumors, i.e., the control and 100 ppm NO-naproxen groups. Both naproxen groups had the smallest number of large (>7 mm) and medium (3-7 mm) size tumors and thus the greatest percentage of very small (<3 mm) tumors. There is thus a correlation between latency, tumor size and tumor yield, suggesting that naproxen and NO-naproxen inhibit proliferation within the tumors.
Histological analysis of the tumors showed that most of them were papillomas, although several squamous cell carcinomas (SCCs) were found. No differences in histological appearance were noted among the different treatment groups. An assessment of the tumors showed that the UV control group had the highest number of SCCs and the highest conversion rate of papillomas to SCCs. The naproxen and NO-naproxen groups had both fewer SCCs and reduced rates of conversion, indicating that their anti-tumorigenic activity was not restricted to papillomas (Supplemental Table 2).
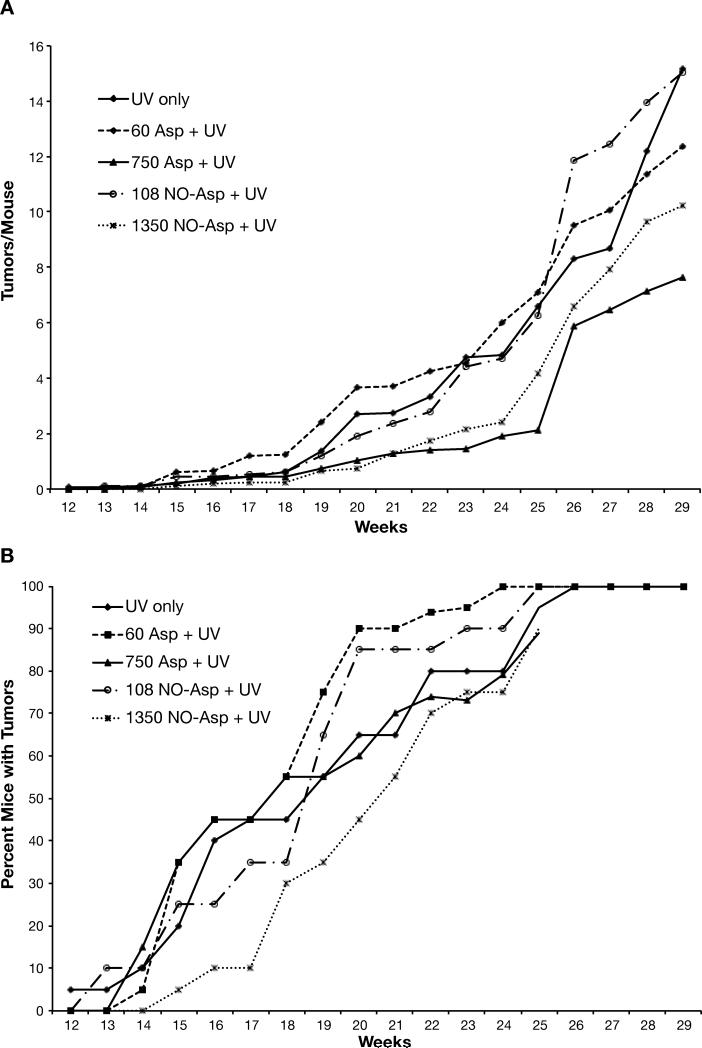
The effectiveness of aspirin and NO-aspirin on tumor development was also determined (Fig. 3). Aspirin at 60 ppm and 750 ppm in the diet significantly reduced tumor yield by 19% and 50%, respectively. Low dose NO-aspirin was ineffective in reducing tumor yield while the high dose (1350 ppm) NO-aspirin that is equimolar to 750 ppm of aspirin reduced tumor numbers by a significant 33% (Fig. 3A). There was, however, no suggestion of a dose-response reduction in tumor size (Supplemental Table 2) with either aspirin or NO-aspirin, although the number of SCCs and the conversion rates were about half of that of the UV control group.
Figure 3. Effect of aspirin or NO-aspirin on UV-induced skin tumorigenesis.
Groups of 20 female SKH-1 mice were placed on their experimental diets and exposed to UV irradiation thrice weekly. Tumors were counted weekly and the data calculated as the average number of tumors per mouse (panel A). Tumor multiplicity for 60 ppm aspirin and 1350 ppm NO-aspirin were significantly (p<0.05) different from the UV control group; 750 ppm aspirin was very significantly (p<0.001) different from the UV control. Tumor incidence (panel B) was calculated as the percentage of mice bearing tumors. Although mice in the 1350 ppm aspirin group developed tumors more slowly than the other groups, this was not statistically different.
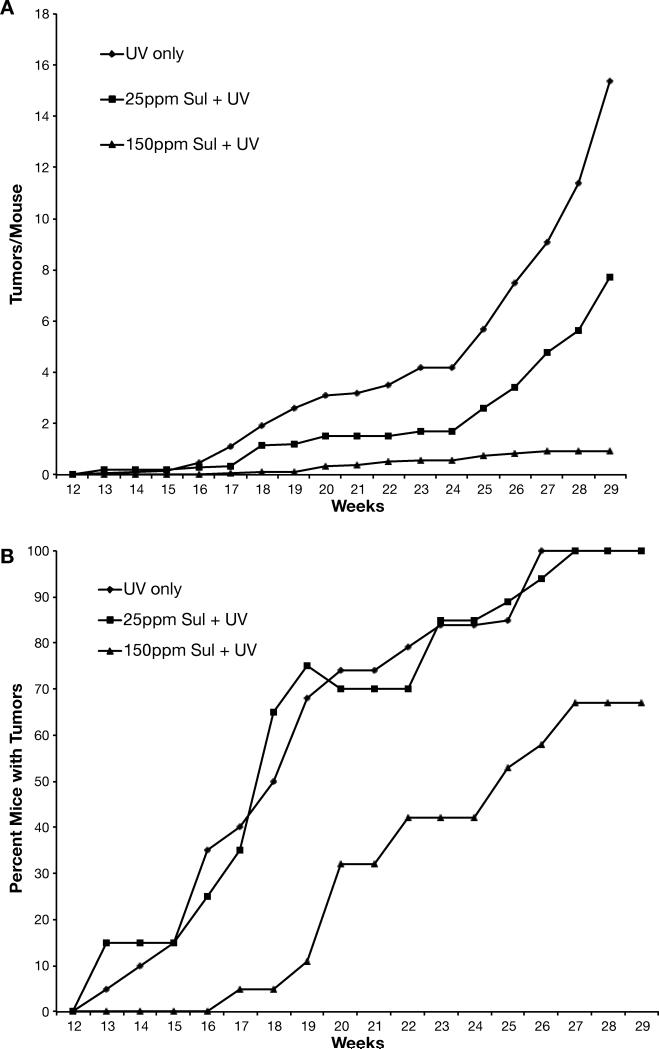
The third study examined whether oral sulindac was an effective inhibitor of UV-induced skin tumorigenesis. As shown in Fig. 4A, low dose (25 ppm) sulindac reduced the number of tumors by 50%, while the high dose (150 ppm) inhibited by a very significant ~94%. The high dose also significantly delayed the appearance of tumors (Fig. 4B). Both doses also had a marked effect on the size of the tumors in that neither sulinac group had tumors larger than 7 mm in diameter (Supplemental Table 2). Histological analysis of the tumors showed that only 3 tumors in the 25 ppm group were SCCs, while no SCCs were found in the 150 ppm group. Thus of all the NSAIDs tested here, sulindac had the greatest chemopreventive efficacy by far with regard to tumor multiplicity, incidence, size and progression to SCC.
Figure 4. Effect of sulindac on UV-induced skin tumorigenesis.
Groups of 20 female SKH-1 mice were placed on their experimental diets and exposed to UV irradiation thrice weekly. Tumors were counted weekly and the data calculated as the average number of tumors per mouse (panel A). Tumor multiplicity for 25 ppm and 150 ppm sulindac were very significantly (p<0.001) different from the UV control. Tumor incidence (panel B) was calculated as the percentage of mice bearing tumors. Mice in the 150 ppm sulindac group developed tumors significantly (p<0.001) slower than the UV control group.
The data in Table 1 and Fig. 2-4 were used to calculate percent reduction from the UV control for PGE2 levels, BrdU labeling index and tumor multiplicity (Table 2). These values were then subjected to calculations for coefficients of correlation. Although it is recognized that the relationship between the early biomarkers, PGE2 and proliferation, are not likely to be linear, significant correlations were found for both PGE2 and BrdU labeling with tumor outcome. These correlation coefficients indicate that either PGE2 or BrdU labeling are excellent short-term predictors for tumor outcome (multiplicity), with PGE2 a slightly better predictor. Multiple regression analysis gave an r2 value of 84.80% (p=0.0014) suggesting that use of the two short-term biomarkers together are even better predictors of tumor outcome.
Table 2.
Correlating PGE2 levels or proliferation with tumor development
| % reduction from UV control | |||||
|---|---|---|---|---|---|
| experiment | treatment | Dose (ppm) | PGE2 | BrdU | Tumors/mouse |
| #1 | nap | 100 | 85 | 53 | 26 |
| nap | 400 | 94 | 56 | 63 | |
| NO-nap | 100 | 65 | 20 | 0 | |
| NO-nap | 400 | 82 | 56 | 52 | |
| #2 | asp | 60 | 31 | 65 | 19 |
| asp | 750 | 82 | 47 | 50 | |
| NO-asp | 108 | 32 | 34 | 0 | |
| NO-asp | 1350 | 91 | 37 | 33 | |
| #3 | Sul | 25 | 79 | 46 | 50 |
| Sul | 150 | 96 | 71 | 94 | |
| r = 0.7236a | |||||
| r = 0.7096b | |||||
Values represent the % reduction from UV controls for each experiment (the number of tumors/mouse was set at 100%). The data were used to calculate correlation coefficients. The statistical differences are shown by superscript:
p=0.0180
p=0.0215.
Multiple regression analysis gave r2=84.80% with a p value of 0.0014.
DISCUSSION
NMSC is most commonly associated with excessive exposure to the UVB component of sunlight and is the most common form of cancer in the world (16, 17). The use of mouse models, most notably those employing the SKH-1 hairless mouse, have been instrumental in identifying the critical molecular and biological changes that are required for skin tumor development (5). Thus the SKH mouse model employs both the same carcinogenic insult and results in the same driving p53 mutations, as in human squamous cell skin cancer. Exposure to UVB results in both DNA damage and inflammation (16, 17), with the latter characterized by an infiltration of inflammatory cells into the dermis and the upregulation of genes and proteins producing soluble mediators of inflammation, including cytokines and PGs. The upregulation of COX-2, with consequent elevation of PGs, is crucial to the development of skin tumors (5). This is based on a number of observations, including pharmacological studies showing that mice fed celecoxib or indomethacin had a significantly reduced tumor multiplicity (6). Because many drugs, including the NSAIDs and coxibs, have off-target effects (7), genetic approaches were also used. Although the loss of one allele of COX-1 had no effect on UV-induced skin tumor development, the loss of only one allele of COX-2 significantly reduced (50-65%) tumor multiplicity (18). COX-2 transgenic SKH-1 mice were shown to respond to UV with a decreased tumor latency and increased tumor multiplicity (12). These genetic studies clearly demonstrated that susceptibility to UV-induced skin cancer is correlated with COX-2 gene copy number and expression levels.
The goals of the study presented here were two-fold. The first goal was to compare NO-NSAIDs with their parent NSAID counterpart for efficacy in reducing short-term biomarkers, particularly inhibition of PGE2 synthesis. The second goal was to determine whether efficacy against one or more short-term biomarkers induced by UV correlated with long-term efficacy against skin tumor development. Establishing the strength of such a correlation could be useful in designing long-term prevention studies for the human population in which such short-term biomarker and long-term tumor endpoint studies are not feasible.
The NSAIDs used in this study were chosen for several reasons. They are among the most commonly consumed NSAIDs and have been shown to have cancer preventive activity in a number of rodent tissues and are associated with reduced incidence of several human cancers (reviewed in 7). Naproxen is a member of the 2-arylproprionic family of NSAIDs and inhibits COX-1 and COX-2 at comparable IC50 levels. The molecular basis for Naproxen inhibition of COX, which is reversible, has recently been described (19). Naproxen also appears to have the best CV profile of any NSAID other than aspirin (20). Aspirin is a salicylate drug that inhibits COX-1 to a greater extent than COX-2 and does so through irreversible acetylation of the enzyme (21). Sulindac belongs to the arylalkanoic acid class of NSAIDs. Following absorption, it undergoes two biotransformations, to a reversible reduction of the sulfide metabolite and an irreversible oxidation to the sulfone metabolite. The sulfide form is the active metabolite with regard to inhibiting COX, although the pro-apoptotic effect of the sulfone metabolite may also contribute to its anti-tumor activity (22). This agent in conjunction with the ornithine decarboxylase inhibitor difluoromethylornithine was profoundly effective in inhibiting colon adenomas in a human trial (23). Although we performed studies with NO-naproxen and NO-aspirin, we were unable to carry out studies with NO-sulindac. The NO-sulindac was not soluble or readily dispersed in a variety of vehicles (R.A. Lubet and C.J. Grubbs, unpublished data) and we were therefore unable to test it properly.
One of the major limitations to the use of NSAIDs is their damaging effect on the gastrointestinal mucosa and exacerbation of pre-existing gastric lesions, which occurs as a result of the loss of the cytoprotective PGs. Like PGs, NO is cytoprotective to the gastric mucosa. Thus nitric oxide-releasing NSAIDs were developed based on the beneficial effects of NO on the gastric mucosa (9), although in human trials the decrease in gastric toxicity appears imperfect.
The NO-NSAIDs have been reported to produce lower gastric toxicity while still inhibiting PGE2 synthesis to a level comparable to the parent NSAID (24). NO-aspirin and NO-naproxen in particular were reported to inhibit PGE2 synthesis to essentially the same level as aspirin and naproxen in rat gastric mucosa (10). In the SKH-1 skin model used here, we found that naproxen and its NO derivative had similar effects given that the NO was administered at roughly 2/3 of the molar dose of the parent compound. Low dose aspirin and NO-aspirin gave comparable, albeit weak, inhibition. In contrast, the higher doses of aspirin or equimolar NO-aspirin (750 or 1350 ppm) were relatively effective. However, the dose of aspirin which was effective is 4-5 times higher than the cardiovascular dose of aspirin (<100 mg) based on standard FDA scaling factors (7). The high dose of all the NSAIDs tested inhibited PGE2 synthesis by more than 80%, which is a significant reduction. The relative lack of efficacy of aspirin is consistent with data in rat bladder, rat colon, and the Min mouse (7). The apparent discrepancy with regards to the human, where aspirin is effective is somewhat puzzling. It is presumably not totally a species-dependent result since this low activity is seen both in mice and rats. Furthermore the data with low dose aspirin or NO-aspirin is certainly in line with the probability COX-1-mediated PGE2 production is inhibited.
Increased proliferation is one of the hallmarks of tumor promotion. Several mechanisms have been proposed for this increased proliferation of keratinocytes in skin, including dysregulated growth factor signaling and over-production of PGs (25-27). Thus the inhibition of PG synthesis by NSAIDs would be expected to reduce UV-induced proliferation. This was observed for all the NSAIDs tested here and in a previous study (6). What was not clear was whether the degree to which inhibition of proliferation after acute UV would correlate with tumor outcome, due in part to the unlikely linear relationship between the two and possible (probable) off-target effects of specific NSAIDs that affect proliferation and/or cell death. Given these caveats, the observation that there is a significant correlation suggests that this short-term biomarker should be of value in screening for the most efficacious chemopreventive drugs.
The effect of the NSAIDS studied here on COX-2 expression levels was also investigated. Although we previously showed that neither celecoxib nor indomethacin reduced UV-induced COX-2 expression (6), another type of anti-inflammatory drug, 5-aminosalicylic acid, suppresses COX-2 expression (28). In a study on UV phototoxicity Athar et al (29) found that sulindac markedly enhanced UV-induced COX-2 expression even though PGE2 synthesis was suppressed. We observed a similar enhancement in that the higher doses of sulindac and naproxen, both of which strikingly decreased PGE2 levels (>90%), increased protein levels of COX-2.
Of the three NSAIDs tested here, sulindac was by far the most potent, with the 150 ppm dose resulting in a 94% reduction in tumor number. One of the most striking effects was the complete prevention of squamous cell carcinoma development. Although we are unaware of epidemiological studies in humans to support this finding, sulindac has previously been reported to inhibit experimentally-induced skin cancer. Kim et al (30) found that a 320 ppm dose reduced chemically-induced skin tumors in mice by ~50%. Oral sulindac (160 ppm in drinking water) was also shown to significantly reduce markers of photodamage, including edema, hyperplasia and inflammatory cell infiltration, in SKH-1 hairless mice exposed to UV (29). Using a xenograft model with human A451 carcinoma cells, Cheng et al (31) found that oral dosing (150 mg/kg/d) with a novel phospho-derivative of sulindac reduced tumor growth by 43%. Collectively, these findings suggest that sulindac has significant potential as a NMSC prevention agent.
In this study oral naproxen was found to significantly reduce skin tumor development (56% reduction at the 400 ppm dose). Using topical treatment, naproxen was reported to suppress UV-induced skin tumors by 63% (32). In comparing naproxen to NO-naproxen, we found that the NO derivative was less effective than naproxen. In the azoxymethane-induced rat colon aberrant crypt foci assay, oral naproxen was also found to be more effective than its NO counterpart, while in a rat urinary bladder model both forms were similarly very effective (11).
Aspirin has been previously tested for efficacy against UVB-induced skin cancer in mice. Using topical applications of 10 or 40 micromole doses before each UV exposure, Bair et al (33) reported that the 40 micromole dose reduced tumor multiplicity by ~25% but had a greater effect on tumor size. Topical treatment with aspirin was also shown to markedly block UVB-induced AP-1 transactivation in murine epidermis, an event required for skin tumor development (34). With regard to comparing the efficacy of aspirin with its NO derivative, we found that NO-aspirin was less effective, which differs from observations by others in other organ models of cancer. In the hamster model of pancreatic cancer, NO-aspirin was found to significantly reduce cancer development while aspirin had no chemopreventive effect (35). NO-aspirin also significantly inhibited colon carcinomas in azoxymethane-treated rats (36), however, no side-by-side comparison was made with the parent NSAID. The reason for the difference between skin and other tissues is not known. There is also conflicting evidence in the human population on the efficacy of aspirin in preventing NMSC. A recent large prospective study found that aspirin and other NSAIDs did not reduce the risk of NMSC or melanomas (37), although two recent population based case-control studies found that NSAID use, including aspirin, reduced risk of squamous cell carcinoma but not basal cell carcinoma (38, 39). Furthermore, there was a clinical trial of the COX-2 inhibitor celecoxib which caused a roughly 60% decrease in SCCs (40). Although this was not the primary endpoint of the trial, the placebo-controlled design and the consistency of the COX-2 inhibitor, with regard to both dose and frequency of administration, is a major strength when compared to most epidemiologic studies. Additionally, the fact that topical treatment with NSAIDS is effective in blocking SCC formation also makes these results particularly promising.
In summary, this study showed that different classes of NSAIDs, at human equivalent doses, have chemopreventive activity against NMSC induced by exposure to UV light, with sulindac being the most potent. The addition of a NO moiety did not significantly alter the efficacy. These data suggest that daily consumption of NSAIDs by the human population could reduce the incidence, and possibly severity, of developing NMSC. An important facet of this study was the determination that the short-term biomarkers, PGE2 and proliferation levels, are excellent predictors for the long-term tumor response. This suggests that these biomarkers should have utility in screening agents for their chemopreventive activity. This would be both a cost and time effective approach to identifying the best drugs available.
Supplementary Material
Acknowledgements
The authors thank Joyce Rundhaug for her statistical analyses of the data.
This work was supported by funds (N01-CN-53300) from The National Cancer Institute, The University of Texas MD Anderson Cancer Center Support Grant CA16672, and the National Institute of Environmental Health Science Center Grant ES07784.
Footnotes
Potential Conflicts of Interest: none of the authors have financial interests or other relationships of a commercial nature that would be conflicts of interest
References
- 1.Ananthaswamy HN. Ultraviolet light as a carcinogen. In: Bowden GT, Fischer SM, editors. Chemical Carcinogens and Anticarcinogens. Cambridge University Press; Cambridge, UK: 1997. pp. 255–79. [Google Scholar]
- 2.Dlugosz AA, Yuspa SH. Staurosporine induces protein kinase C agonist effects and maturation of normal and neoplastic mouse keratinocytes in vitro. Cancer Res. 1991;51:4677–84. [PubMed] [Google Scholar]
- 3.Hruza LL, Pentland AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993;100:35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- 4.Muller-Decker K. Cyclooxygenase-dependent signaling is causally linked to nonmelanoma skin carcinogenesis: pharmacological, genetic, and clinical evidence. Cancer Metastasis Rev. 2011;30:343–61. doi: 10.1007/s10555-011-9306-z. [DOI] [PubMed] [Google Scholar]
- 5.Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol. 2008;84:322–9. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 6.Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25:231–40. [PubMed] [Google Scholar]
- 7.Fischer SM, Hawk ET, Lubet RA. Coxibs and other nonsteroidal anti-inflammatory drugs in animal models of cancer chemoprevention. Cancer Prev Res (Phila) 2011;4:1728–35. doi: 10.1158/1940-6207.CAPR-11-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanas A, Perez-Aisa MA, Feu F, Ponce J, Saperas E, Santolaria S, et al. A nationwide study of mortality associated with hospital admission due to severe gastrointestinal events and those associated with nonsteroidal antiinflammatory drug use. The American journal of gastroenterology. 2005;100:1685–93. doi: 10.1111/j.1572-0241.2005.41833.x. [DOI] [PubMed] [Google Scholar]
- 9.Rigas B, Williams JL. NO-donating NSAIDs and cancer: an overview with a note on whether NO is required for their action. Nitric Oxide. 2008;19:199–204. doi: 10.1016/j.niox.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Drozdowicz D, Kwiecien S, et al. Gastroprotective and ulcer healing effects of nitric oxide-releasing nonsteroidal anti-inflammatory drugs. Dig Liver Dis. 2000;32:583–94. doi: 10.1016/s1590-8658(00)80840-3. [DOI] [PubMed] [Google Scholar]
- 11.Steele VE, Rao CV, Zhang Y, Patlolla J, Boring D, Kopelovich L, et al. Chemopreventive efficacy of naproxen and nitric oxide-naproxen in rodent models of colon, urinary bladder, and mammary cancers. Cancer Prev Res (Phila) 2009;2:951–6. doi: 10.1158/1940-6207.CAPR-09-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer SM, Pavone A, Mikulec C, Langenbach R, Rundhaug JE. Cyclooxygenase-2 expression is critical for chronic UV-induced murine skin carcinogenesis. Mol Carcinog. 2007;46:363–71. doi: 10.1002/mc.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, et al. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA. 1994;91:3228–32. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono R, Masaki T, Dien S, Yu X, Fukunaga A, Yodoi J, et al. Suppressive effect of recombinant human thioredoxin on ultraviolet light-induced inflammation and apoptosis in murine skin. J Dermatol. 2012;39:843–51. doi: 10.1111/j.1346-8138.2012.01566.x. [DOI] [PubMed] [Google Scholar]
- 15.Maldve RE, Kim Y, Muga SJ, Fischer SM. Prostaglandin E(2) regulation of cyclooxygenase expression in keratinocytes is mediated via cyclic nucleotide-linked prostaglandin receptors. J Lipid Res. 2000;41:873–81. [PubMed] [Google Scholar]
- 16.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166:1069–80. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 17.Firnhaber JM. Diagnosis and treatment of Basal cell and squamous cell carcinoma. Am Fam Physician. 2012;86:161–8. [PubMed] [Google Scholar]
- 18.Rundhaug JE, Mikulec C, Pavone A, Fischer SM. A role for cyclooxygenase-2 in ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 2007;46:692–8. doi: 10.1002/mc.20329. [DOI] [PubMed] [Google Scholar]
- 19.Duggan KC, Walters MJ, Musee J, Harp JM, Kiefer JR, Oates JA, et al. Molecular basis for cyclooxygenase inhibition by the nonsteroidal anti-inflammatory drug naproxen. J Biol Chem. 2010;285:34950–9. doi: 10.1074/jbc.M110.162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fosbol EL, Kober L, Torp-Pedersen C, Gislason GH. Cardiovascular safety of nonsteroidal anti-inflammatory drugs among healthy individuals. Expert Opin Drug Saf. 2010;9:893–903. doi: 10.1517/14740338.2010.501331. [DOI] [PubMed] [Google Scholar]
- 21.Capone ML, Tacconelli S, Di Francesco L, Sacchetti A, Sciulli MG, Patrignani P. Pharmacodynamic of cyclooxygenase inhibitors in humans. Prostaglandins Other Lipid Mediat. 2007;82:85–94. doi: 10.1016/j.prostaglandins.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Curiel-Lewandrowski C, Swetter SM, Einspahr JG, Hsu CH, Nagle R, Sagerman P, et al. Randomized, double-blind, placebo-controlled trial of sulindac in individuals at risk for melanoma: Evaluation of potential chemopreventive activity. Cancer. 2012 doi: 10.1002/cncr.27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyskens FL, Jr., McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi K, Suzuki K, Yamamoto H, Araki H, Mizoguchi H, Ukawa H. Cyclooxygenase-2 selective and nitric oxide-releasing nonsteroidal anti-inflammatory drugs and gastric mucosal responses. J Physiol Pharmacol. 1998;49:501–13. [PubMed] [Google Scholar]
- 25.Abel EL, Angel JM, Kiguchi K, DiGiovanni J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat Protoc. 2009;4:1350–62. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marks F, Furstenberger G, Muller-Decker K. Tumor promotion as a target of cancer prevention. Recent Results Cancer Res Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2007;174:37–47. doi: 10.1007/978-3-540-37696-5_3. [DOI] [PubMed] [Google Scholar]
- 27.Ansari KM, Rundhaug JE, Fischer SM. Multiple signaling pathways are responsible for prostaglandin E2-induced murine keratinocyte proliferation. Mol Cancer Res. 2008;6:1003–16. doi: 10.1158/1541-7786.MCR-07-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyoshi J, Yajima T, Shimamura K, Matsuoka K, Okamoto S, Higuchi H, et al. 5-aminosalicylic acid mediates expression of cyclooxygenase-2 and 15-hydroxyprostaglandin dehydrogenase to suppress colorectal tumorigenesis. Anticancer Res. 2012;32:1193–202. [PubMed] [Google Scholar]
- 29.Athar M, An KP, Tang X, Morel KD, Kim AL, Kopelovich L, et al. Photoprotective effects of sulindac against ultraviolet B-induced phototoxicity in the skin of SKH-1 hairless mice. Toxicol Appl Pharmacol. 2004;195:370–8. doi: 10.1016/j.taap.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 30.Kim DJ, Prabhu KS, Gonzalez FJ, Peters JM. Inhibition of chemically induced skin carcinogenesis by sulindac is independent of peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta). Carcinogenesis. 2006;27:1105–12. doi: 10.1093/carcin/bgi346. [DOI] [PubMed] [Google Scholar]
- 31.Cheng KW, Mattheolabakis G, Wong CC, Ouyang N, Huang L, Constantinides PP, et al. Topical phospho-sulindac (OXT-328) is effective in the treatment of nonmelanoma skin cancer. Int J Oncol. 2012 doi: 10.3892/ijo.2012.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez Maglio DH, Paz ML, Ferrari A, Weill FS, Nieto J, Leoni J. Alterations in skin immune response throughout chronic UVB irradiation-skin cancer development and prevention by naproxen. Photochem Photobiol. 2010;86:146–52. doi: 10.1111/j.1751-1097.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 33.Bair WB, 3rd, Hart N, Einspahr J, Liu G, Dong Z, Alberts D, et al. Inhibitory effects of sodium salicylate and acetylsalicylic acid on UVB-induced mouse skin carcinogenesis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2002;11:1645–52. [PubMed] [Google Scholar]
- 34.Huang C, Ma WY, Hanenberger D, Cleary MP, Bowden GT, Dong Z. Inhibition of ultraviolet B-induced activator protein-1 (AP-1) activity by aspirin in AP-1-luciferase transgenic mice. J Biol Chem. 1997;272:26325–31. doi: 10.1074/jbc.272.42.26325. [DOI] [PubMed] [Google Scholar]
- 35.Ouyang N, Williams JL, Tsioulias GJ, Gao J, Iatropoulos MJ, Kopelovich L, et al. Nitric oxide-donating aspirin prevents pancreatic cancer in a hamster tumor model. Cancer Res. 2006;66:4503–11. doi: 10.1158/0008-5472.CAN-05-3118. [DOI] [PubMed] [Google Scholar]
- 36.Rao CV, Reddy BS, Steele VE, Wang CX, Liu X, Ouyang N, et al. Nitric oxide-releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats: effects on molecular targets. Mol Cancer Ther. 2006;5:1530–8. doi: 10.1158/1535-7163.MCT-06-0061. [DOI] [PubMed] [Google Scholar]
- 37.Jeter JM, Han J, Martinez ME, Alberts DS, Qureshi AA, Feskanich D. Nonsteroidal anti-inflammatory drugs, acetaminophen, and risk of skin cancer in the Nurses’ Health Study. Cancer Causes Control. 2012;23:1451–61. doi: 10.1007/s10552-012-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johannesdottir SA, Chang ET, Mehnert F, Schmidt M, Olesen AB, Sorensen HT. Nonsteroidal anti-inflammatory drugs and the risk of skin cancer: a population-based case-control study. Cancer. 2012;118:4768–76. doi: 10.1002/cncr.27406. [DOI] [PubMed] [Google Scholar]
- 39.Torti DC, Christensen BC, Storm CA, Fortuny J, Perry AE, Zens MS, et al. Analgesic and nonsteroidal anti-inflammatory use in relation to nonmelanoma skin cancer: a population-based case-control study. J Am Acad Dermatol. 2011;65:304–12. doi: 10.1016/j.jaad.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elmets CA, Viner JL, Pentland AP, Cantrell W, Lin HY, Bailey H, et al. Chemoprevention of nonmelanoma skin cancer with celecoxib: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2010;102:1835–44. doi: 10.1093/jnci/djq442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.