Abstract
Using resting state (RS) functional magnetic resonance imaging (fMRI) and diffusion tensor imaging (DTI), we identified the predictors of clinical improvement following constraint-induced movement therapy (CIMT) in pediatric patients with chronic hemiplegia.From 14 children with congenital or acquired brain injury and 10 sex- and age-matched healthy controls, brain dual-echo, DTI and RS fMRI sequences were acquired before CIMT. The Quality of Upper Extremities Skills Test and the Gross Motor Function Measure (GMFM) were administered at baseline, at the end of CIMT (10 weeks), and after 6 months. Mean diffusivity and fractional anisotropy (FA) were measured in the lesion responsible for the clinical symptomatology, the affected and unaffected corticospinal tract (CST), motor transcallosal fibers, and uncinate fasciculus (as an internal control). Independent component analysis was used to identify the sensorimotor RS network. The ability of baseline MRI variables to predict clinical changes over time was assessed using multivariate linear models. At baseline, patients had increased mean diffusivity in the symptomatic lesion and decreased FA in the symptomatic lesion, affected corticospinal tract, and motor transcallosal fibers. A reduced RS functional connectivity was found in the bilateral cerebellum, left precentral gyrus, and right secondary sensorimotor cortex. At follow up, Quality of Upper Extremities Skills Test and GMFM scales improved significantly. Baseline average lesion FA predicted clinical improvement at week 10, and baseline functional connectivity of the right secondary sensorimotor cortex and cerebellum predicted GMFM improvement at month 6. DTI and RS fMRI offer promising and objective markers to predict clinical outcomes following CIMT in pediatric patients with congenital or acquired hemiplegia.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0189-2) contains supplementary material, which is available to authorized users.
Keywords: MRI, DTI, Tractography, Resting-state functional connectivity, Brain injury, Constraint-induced movement therapy
Introduction
In children affected by congenital or acquired hemiplegia, several treatments have been developed to improve recovery of function of the upper limb. The majority of these treatments is based on learning of compensatory skills and prevent deformities. Constraint-induced movement therapy (CIMT), a combination of a restraint in the use of the unimpaired limb and an intensive training of the skills of the affected one [1], has been proposed as a strategy to improve upper limb function of these patients [2]. Considering the economic burden of CIMT and its effect on daily activities of patients’ families, there is an urgent need to identify those patients with the highest chances of benefiting from this treatment.
Magnetic resonance imaging (MRI) offers several metrics to quantify the structural and functional abnormalities of the central nervous system in patients with neurologic disorders. Diffusion tensor imaging (DTI) exploits the diffusion properties of water and allows the assessment of the microstructural integrity of white matter (WM) tracts in the brain. Resting-state (RS) functional MRI (fMRI) allows mapping of the function of the brain’s large-scale neuronal networks. Different from active fMRI, this task-free approach allows us to draw inferences related to differences between healthy and diseased patients, which are not influenced by task performance.
Previous DTI studies in pediatric patients with brain injury have shown that measures of microstructural damage to the sensorimotor system correlate with the severity of motor dysfunction [3–5]. Studies that have applied functional imaging techniques, such as fMRI, transcranial magnetic stimulation, and positron emission tomography, have detected an increased activity of the motor areas of the affected hemisphere following CIMT [2, 6]. However, whether these measures are useful in predicting the recovery of motor function or response to rehabilitation has not yet been explored.
In this prospective study, we combined DTI and RS functional connectivity (FC) analysis to quantify abnormalities of the sensorimotor network in pediatric patients with brain injury who underwent CIMT to identify baseline MRI parameters predictive of clinical improvement at the end of treatment and 6 months later.
Methods
Patients
Children with congenital or acquired hemiplegia were recruited from the Scientific Institute “Eugenio Medea”, Bosisio Parini, Italy. Patients were eligible for CIMT if they 1) had a diagnosis of acquired brain injuries (ABI) or cerebral palsy (CP) with chronic hemiplegia based on anamnestic, clinical, and neuroimaging data; 2) were aged between 5 and 18 years; 3) had the ability to understand and follow test instructions (total Intelligence Quotient ≥ 60); 4) had the ability to tolerate wearing a removable bivalve cast; and 5) had the ability to initiate movement of the impaired upper limb, regardless of the active range of motion or the degree of synergy and volitional grasp and release.
The eligible children were screened by an experienced investigator who confirmed the diagnosis of hemiplegia, examined active movements and asymmetry of hand function, and assessed interest in study participation. The exclusion criteria were: 1) a diagnosis of severe learning disabilities; 2) behavioral abnormalities; 3) visual or hearing difficulties that would affect function and participation; and 4) a previous restraint therapy, serial casting, treatment with antispastic drugs, or orthopedic surgery of the impaired upper limb within 6 months of study initiation.
Ten right-handed healthy pediatric volunteers with no previous history of neurologic dysfunction and who had normal neurologic examinations were recruited as controls and had a MRI scan performed on them (4 boys, 6 girls; mean age: 11.0 years; range: 8.5–13.7 years).
Ethics Committee Approval
Approval was received from the local ethical standards committee on human experimentation, and written informed consent was obtained from all volunteers participating in the study.
Treatment
CIMT was conducted according to the protocol proposed by Facchin et al. [7]. Children had to wear a restraining, but fairly comfortable, fabric glove with a built-in, stiff plastic volar splint on the unaffected hand, which prevented them from flexing their fingers and from grasping. The thumb was kept in a fixed position, tight against the index finger. The children could, however, use their hand for support or for breaking a fall. The intervention lasted for 10 weeks, 7 days a week. The children were expected to wear the glove for 3 consecutive h/day and, at this time, they were administered the therapeutic training under the supervision of a therapist and/or the parents, without removing the glove. During the treatment period, children also underwent an intensive rehabilitation programme based on unimanual activities involving play sessions and a daily-life activity (e.g., memory card, puzzles, playing with bowls and cards, use of spoon or fork, dust a surface, etc.). These sessions were held 3 times a week at the rehabilitation centre: a trained therapist encouraged patients to perform tasks requiring the unilateral use of the paretic hand. Task goals involved the performance of 1) perceptual motor activities; 2) reaching, grasping, holding, and manipulating; 3) postural and balance activities; and 4) self-care and daily-life activities. These sessions lasted 3 h: during the first part (1.5 h), the therapist interacted with children and prompted them to perform unimanual activities with an appropriate level of difficulty; in the second part, parents were instructed to interact with their own children who were asked to perform unilateral tasks based on playing and daily-life activities. Parents were trained to carry out similar 3-h sessions at home on the other 4 days of the week [8].
Study Design
All patients underwent neurologic and MRI assessment at baseline (within 3 days of CIMT initiation). Neurologic assessment was repeated at the end of CIMT (week 10, + 3 days maximum) and 6 months after the cessation of CIMT (+ 3 days maximum).
Clinical examination included the Quality of Upper Extremities Skills Test (QUEST) [9] and the Gross Motor Function Measure (GMFM) [10]. QUEST is an internationally-validated scale designed to measure treatment outcome in children with upper extremity movement disorders [9]. It explores four domains: dissociated movement, grasp, weight-bearing, and protective extension. The domain score is a summed-item score converted into a standardized percentage, and the total score is the average of domain scores, with higher scores representing a better quality of movement [11]. The GMFM measures the child’s overall functional abilities and consists of 88 items, divided into the following sections: 1) lying and rolling; 2) sitting; 3) crawling and kneeling; 4) standing; 5) walking, running, and jumping. Each section contributes to the total GMFM score [10].
MRI Acquisition
Using a 3.0 Tesla Intera scanner (Philips Medical Systems, Best, the Netherlands), the following sequences of the brain were obtained from each participant at baseline: 1) T2*-weighted single-shot echo-planar imaging (EPI) sequence during RS [repetition time (TR) /echo time (TE) = 3000/35 ms, flip angle = 90°, field of view (FOV) = 240 mm2; matrix = 128 × 128, slice thickness = 4 mm; 200 sets of 30 contiguous axial slices]; 2) dual-echo turbo spin echo (TR/TE = 2200/24–120 ms, echo train length = 6; flip angle = 90º, matrix size = 256 × 256, FOV = 240 × 180 mm, 44 axial 3-mm-thick slices); and 3) pulsed-gradient spin echo (SE) echo-planar (TE/TR = 58/8775 ms, acquisition matrix size = 112 × 88, FOV = 240 × 231 mm, 55 contiguous, 2.3-mm-thick axial slices) with sensitivity encoding (SENSE) (acceleration factor = 2) and diffusion gradients applied in 35 noncollinear directions. Two optimized b factors were used for acquiring diffusion-weighted images (b1 = 0, b2 = 900 s/mm2).
The total acquisition time of RS fMRI was about 10 mins. During scanning, participants were instructed to remain motionless, to close their eyes, and not to think about anything in particular.
Image Analysis
All MRI analyses were performed by a single, experienced observer, unaware to whom the scans belonged. Whenever possible, lesion volumes were measured on dual echo (DE) scans using a local thresholding segmentation technique (Jim 5.0; Xinapse Systems, Aldwincle, UK). Diffusion-weighted images were corrected for distortions caused by eddy currents and for movements; they were then inspected visually, and all volumes corrupted by the presence of excessive motion artifacts were removed from the analysis. After transformation to the Montreal Neurological Institute space the diffusion tensor was estimated, maps of fractional anisotropy (FA) and mean diffusivity (MD) derived, and tractography run for all voxels with an FA value of more than 0.17 (Diffusion Toolkit, www.trackvis.org/dtk). Trackvis software (www.trackvis.org) [12] was used to segment the corticospinal tracts (CST) and the motor transcallosal fibers (mTC) by placing seed and target masks on axial FA and color-coded maps [13] on the basis of a priori anatomic knowledge and previous tractography studies [3, 5, 14–16]. Using the same approach, the uncinate fasciculus was also segmented to have an internal control tract. In each hemisphere, the CST was reconstructed using as seed mask the cerebral peduncle on the first slice above the decussation of the superior cerebellar peduncle [3, 5, 14–16]. The target mask was placed on the motor cortex identified at the level of the omega. To increase accuracy of the tractography reconstruction and to standardize the size of the CST, we retained, whenever possible, only fiber tracts passing through a sphere with a diameter of 4 mm placed on the posterior limb of the ipsilateral internal capsule, where it has the largest diameter [3, 5, 14–16]. mTC fibers, which connect the right and the left primary motor cortices, were reconstructed using as seed and target masks the motor cortices. To standardize the size of this tract, an additional mask (a sphere with a diameter of 3 mm) was placed on its trajectory on the middle sagittal slice of the corpus callosum. The uncinate fasciculus was reconstructed in the nonaffected hemisphere using as seed mask the anterior temporal WM and as the target mask the orbitofrontal WM [3, 5, 14–16]. Additionally, T2-weighted images were coregistered to the distortion-free b = 0 image, and the calculated transformations were applied to the binary masks of detectable lesions. Then, average FA and MD were obtained from each tract and lesions using the Trackvis software.
Analysis of FC of Sensorimotor Resting State Network (RSN)
Before entering the analysis, RS fMRI scans of patients with lesions in the right hemisphere were flipped to have all the affected hemispheres on the left side. Using SPM8, RS fMRI scans were realigned to the first one of each session with a 6-degree rigid-body transformation to correct for minor head movements. None of the participants was excluded from the analysis because of motion, as, in all cases, the maximum cumulative translation was < 1.5 mm and the maximum rotation was < 0.3 degrees. The mean motion, computed as the root mean square of the 3 translational parameters [17] was 0.66 mm (S.D. = 0.29 mm) in healthy controls and 1.19 mm (S.D. = 0.50 mm) in patients (p = n.s.). Data were then normalized to the SPM8 default EPI template using a standard affine transformation through data subsampling with a resolution of 3 × 3 × 4 mm [18] and smoothed using a 3-dimensional 6-mm Gaussian kernel. The goodness of the normalization process was tested in 2 ways. First, we calculated the spatial correlation coefficient between the normalized images and the SPM8 EPI template. Second, we measured the volume of brain voxels of the normalized images lying outside the brain of the EPI template. Both these tests indicated that the normalization worked well on all study participants, with a slight trend for better results in controls than in patients: the average spatial correlation with the template was high in both groups (r = 0.98 in healthy volunteers and r = 0.97 in patients) and the volume of brain voxels lying outside the EPI template was very low in both groups (78.7 ml in healthy volunteers and 91.5 ml in patients). Linear de-trending and band-pass filtering between 0.01 and 0.08 Hz were performed using REST software (http://resting-fmri.sourceforge.net/). After these preprocessing steps, RS FC was assessed using an independent component analysis (ICA) with GIFT software [18] and following three main steps: 1) data reduction, 2) group ICA, and 3) back reconstruction. First, individual participants’ data were reduced to a lower dimensionality by using a 2-stage principal component analysis. Then, RS fMRI data from all participants were concatenated. The independent group components were estimated using the Infomax approach [19] and were used to compute spatial maps and temporal profiles of the individual participant’s components (back reconstruction). This latter stage was performed with the new optimized GICA3 algorithm [20]. The number of independent group components, i.e., the median number of independent components (ICs) in our study group determined by the minimum description length criterion, was 40 [18]. The statistical reliability of the IC decomposition was tested by using the ICASSO toolbox [21], and by running Infomax 10 times with different initial conditions and bootstrapped data sets. To obtain voxel values comparable across participant, individual functional maps were converted to Z-scores before entering group statistics. Visual inspection of the spatial patterns and a frequency analysis of the spectra of the estimated ICs allowed us to remove components clearly related to artifacts. Then, a frequency analysis of the IC time courses was performed to discard components without a high (≥50 %) spectral power at a low frequency (between 0.01 and 0.05 Hz). Finally, the sensorimotor network was selected on group ICA results using spatial correlation against an a priori defined network template [22], generated using the Wake Forest University (WFU) Pickatlas [23]. The components with the highest square correlation coefficients with this template were selected as networks of interest.
Statistical Analysis
Differences between patients and controls in demographic, clinical, and DTI variables at baseline were assessed using the Mann–Whitney U test. Using SPM8, 1-sample t tests were performed to derive maps of the sensorimotor network for control participants and patients, separately (p < 0.05, corrected for family-wise error). Between-group RS FC differences were tested by extracting with MarsBar [24] the average Z-scores of each significant SPM cluster at the 1-sample t test and by comparing these values between groups using the Mann–Whitney U test. To explore the influence of movement artifacts on RS FC findings, the between-group comparison was repeated by including the mean motion as a confounding covariate. Baseline correlations between clinical and MRI variables were assessed using the Spearman rank correlation coefficient. In patients, longitudinal generalized linear models for repeated measures were used to assess changes over time of the functional assessment scales. The ability of baseline MRI variables to predict the clinical outcome was assessed using multivariable linear models with step-wise variables selection using a significance level to entry into the model of 0.10 and a significance level to remain in the model of 0.05. Results were reported as partial R-squared. A p-value < 0.05 was considered as significant. All analyses were performed using SAS Release 9.1 (SAS Institute, Cary, NC, USA).
Results
Eighteen children (8 boys, 10 girls, mean age: 10.3 years) with hemiplegia were recruited. All ABI patients were right-handed [25] before injury. Four of them were unable to complete the MRI acquisition and, as a consequence, we report the results from the remaining 14 patients (6 boys, 8 girls, mean age:10.8 years; range: 7–17.6 years), who completed clinical, MRI, and rehabilitative assessment. Nine patients had a right-sided (dominant) and 5 a left-sided (nondominant) impairment. Five children had traumatic brain injury, 5 stroke, 2 vascular malformation, and 2 congenital CP. For patients with ABI, the mean age at injury onset was 8.5 years (range: 6.7–17.5 years) and mean disease duration at study enrolment 0.6 years (range: 0.1–2.2 years). The main clinical and demographic characteristics of the patients at study entry are summarized in Table 1.
Table 1.
Clinical and demographic data of the patients enrolled in the study
| Patient | Sex | Age (years) | Age of onset (years) | Onset | Cause | Lesion location | Clinical evaluation |
|---|---|---|---|---|---|---|---|
| 1 | M | 9.9 | 8.4 | Acute | TBI | Right hemispheric (parietal and temporal lobes) | Left hemiparesis |
| 2 | M | 12.9 | 12.1 | Acute | Stroke MCA | Left capsulo-lenticular | Right hemiparesis |
| 3 | F | 8.8 | 8.0 | Acute | Stroke in congenital cardiopathy | Right hemispheric (parietal and temporal lobes) | Left hemiparesis |
| 4 | F | 12.9 | 10.7 | Subacute | Bleeding cerebral cavernous angioma | Left capsulo-lenticular | Right hemiparesis |
| 5 | F | 10.2 | 9.9 | Acute | Stroke in antiphospholipid syndrome | Left capsulo-lenticular | Right hemiparesis |
| 6 | F | 10.3 | 10.1 | Acute | TBI | No visible focal lesion | Right hemiparesis |
| 7 | M | 8.2 | 7.3 | Acute | Stroke | Posterior periventricular white matter | Left hemiparesis |
| 8 | F | 8.6 | 8.4 | Acute | Stroke MCA | Left hemispheric (putamen, insula and parietal lobe) | Right hemiparesis |
| 9 | M | 12.6 | 12.5 | Acute | Rupture posterior communicating artery aneurysm | Right hippocampus, thalamus, and brainstem | Left hemiparesis |
| 10 | M | 17.6 | 17.5 | Acute | TBI | Left brainstem and hippocampus | Right hemiparesis |
| 11 | M | 11.6 | Congenital | Congenital | Congenital | Right parietal lobe and insula (associated atrophy) | Left hemiparesis |
| 12 | F | 12.4 | Congenital | Congenital | Congenital | Left hemispheric (parietal and temporal lobes) | Right hemiparesis |
| 13 | F | 7.6 | 7.5 | Acute | TBI | Left capsulo-lenticular | Right hemiparesis |
| 14 | F | 7.0 | 6.7 | Acute | TBI | Left capsulo-lenticular | Right hemiparesis |
M = male; F = female; TBI = traumatic brain injury; MCA = middle cerebral artery.
Patients showed a significant improvement at clinical scales following CIMT, with a marked improvement at the end of the treatment and a further amelioration 6 months after termination of therapy (Table 2). In particular, 12/14 patients improved at QUEST at week 10 and 10/14 had a further amelioration at month 6, whereas 11/14 patients improved at GMFM at week 10 and 9/14 had a further amelioration at month 6.
Table 2.
Clinical measures (means and S.D.) from patients with brain injury at the different time points of the study
| Clinical measures | Baseline | Week 10 | Week 10 vs baseline p-value (mean % of variation, range) | Month 6 | Month 6 vs week 10 p-value (mean % of variation, range) | p-value (global effect)* |
|---|---|---|---|---|---|---|
| QUEST | 73.3 (13.1) | 80.1 (13.8) | 0.006 (9.9, 0–49.9) | 82.3 (13.2) | 0.02 (3.2, –3 to 12.3) | 0.005 |
| GMFM | 237.6 (26.1) | 251.9 (14.7) | 0.001 (6.9, 0–35.2) | 256.0 (10.7) | 0.03 (1.7, 0–9.5) | 0.01 |
*Longitudinal generalized linear model for repeated measures.
QUEST = Quality of Upper Extremities Skills Test; GMFM = Gross Motor Function Measure.
Mean lesion T2 lesion volume was 47.3 ml (S.D. = 81.4 ml). The results of diffusion tensor tractography and RS FC analysis in patients and control volunteers are summarized in Table 3. Compared with healthy controls, patients had a decreased FA in the affected CST and mTC. No differences were found for the healthy CST and uncinate fasciculus. Compared with the affected CST, lesions had significantly decreased FA (mean = 0.15, S.D. = 0.06; p < 0.0001) and increased MD (mean = 1.47 mm2s-1 × 10-3, S.D. = 0.47 mm2s-1 × 10-3, p = 0.003).
Table 3.
Baseline structural and functional magnetic resonance imaging metrics from healthy volunteers and patients with brain injury. Note that average mean diffusivity (MD) is expressed in units of mm2s-1 × 10-3; fractional anisotropy (FA) is a dimensionless index
| Healthy controls (n = 10) | Brain injury patients (n = 14) | p* | p† | |
|---|---|---|---|---|
| Affected CST average FA (S.D.) | 0.54 (0.03) | 0.43 (0.04) | < 0.0001 | 0.01 |
| Affected CST average MD (S.D.) | 0.78 (0.02) | 0.86 (0.15) | n.s. | n.s. |
| Healthy CST average FA (S.D.) | 0.55 (0.02) | 0.52 (0.06) | n.s. | n.s. |
| Healthy CST average MD (S.D.) | 0.77 (0.02) | 0.77 (0.04) | n.s. | n.s. |
| mTC average FA (S.D.) | 0.52 (0.03) | 0.49 (0.05) | 0.01 | 0.06 |
| mTC average MD (S.D.) | 0.86 (0.06) | 0.95 (0.16) | n.s | n.s |
| Uncinate fasciculus average FA (S.D.) | 0.42 (0.02) | 0.43 (0.03) | n.s. | n.s. |
| Uncinate fasciculus average MD (S.D.) | 0.84 (0.02) | 0.83 (0.02) | n.s. | n.s. |
| Left cerebellum (lobule IV–V) mean Z-score (S.D.), sensorimotor network I | 1.07 (0.38) | 0.73 (0.49) | 0.05 | n.s. |
| Left cerebellum (lobule IV–V) mean Z-score (S.D.), sensorimotor network I | 1.22 (0.34) | 0.72 (0.39) | 0.006 | 0.05 |
| Left cerebellum (lobule VI) mean Z-score (S.D.), sensorimotor network II | 1.63 (0.49) | 1.18 (0.47) | 0.04 | n.s. |
| Right cerebellum (lobule VI) mean Z-score (S.D.), sensorimotor network II | 1.75 (0.55) | 0.69 (0.20) | < 0.0001 | 0.01 |
| Vermis mean Z-score (S.D.), sensorimotor network I | 1.16 (0.31) | 0.58 (0.27) | < 0.0001 | 0.01 |
| Right secondary sensorimotor cortex mean Z-score (S.D.), sensorimotor network I | 1.35 (0.47) | 1.02 (0.34) | 0.05 | n.s. |
| Supplementary motor area mean Z-score (S.D.), sensorimotor network I | 1.94 (0.12) | 1.99 (0.23) | n.s. | n.s. |
| Left precentral gyrus mean Z-score (S.D.), sensorimotor network II | 3.87 (0.39) | 2.22 (1.14) | 0.002 | 0.02 |
| Right precentral gyrus mean Z-score (S.D.), sensorimotor network II | 3.35 (0.55) | 2.84 (0.86) | n.s. | n.s. |
*Mann–Whitney U test.
†Adjusted for multiple comparisons following False Discovery Rate approach.
CST = corticospinal tract; mTC = motor transcallosal fibers of the corpus callosum; n.s. = not significant.
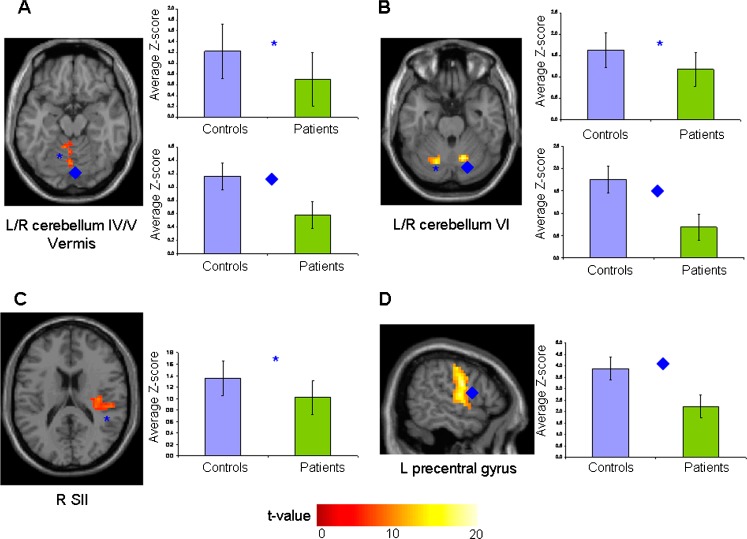
Two independent components (sensorimotor RSN I and sensorimotor RSN II) showed a high square spatial correlations (R2 = 0.33 and 0.21) with the sensorimotor network template. Compared with controls, patients had a significantly reduced FC of the bilateral cerebellar hemispheres (lobules IV, V, and VI), vermis, right secondary sensorimotor cortex (SII), and left precentral gyrus (Table 3, Fig. 1). These results did not change after correction for mean motion.
Fig. 1.
Illustrative spatial patterns, color-coded for t values, and average Z-scores of clusters of resting state (RS) functional connectivity (FC) significantly different between healthy controls (light blue boxes) and patients with brain injury (green boxes). (A) left (L) and right (R) cerebellum, lobules IV–V, and R vermis (*, ♦) (sensorimotor network I); (B) L and R cerebellum, lobule VI (*, ♦) (sensorimotor network II); (C) R secondary sensorimotor cortex (SII) (*) (sensorimotor network I); (D) L precentral gyrus (♦) (sensorimotor network II)
At baseline, mTC average FA (r = 0.64, p = 0.02) and right SII FC (r = 0.63, p = 0.02) were correlated with GMFM score. An inverse correlation was found between lesion volume and FC of the left precentral gyrus (r = −0.63, p = 0.02) and right cerebellum (r = −0.64, p = 0.02).
The results of the multivariable analyses are summarized in Table 4. A higher baseline average lesion FA was the only predictor of clinical improvement at week 10 measured with QUEST and GMFM. Lower FC of the right SII and right cerebellum at baseline predicted GMFM improvement at month 6.
Table 4.
Results of the multivariate analyses
| Outcomes | Time | Predictors | Beta | p | r2 |
|---|---|---|---|---|---|
| QUEST improvement* | Week 10 | Baseline average lesion FA | 133.6 | 0.02 | 0.39 |
| GMFM improvement* | Week 10 | Baseline average lesion FA | 114.6 | 0.01 | 0.49 |
| Month 6 | Baseline right secondary sensorimotor Z-score | −4.97 | 0.01 | 0.28 | |
| Baseline right cerebellum (lobule VI) Z-score | −9.75 | 0.007 | 0.37 |
*Improvement defined as relative variation vs baseline value (see Table 2).
QUEST = Quality of Upper Extremities Skills Test; GMFM = Gross Motor Function Measure; FA = fractional anisotropy.
The results of all the previous analyses did not change significantly when excluding the 2 patients with congenital CP (data not shown).
Discussion
In this study, we quantified structural and functional abnormalities of the sensorimotor network to identify potential predictors of response to CIMT in pediatric patients with brain injury. This is of paramount importance for therapeutic decisions and patient management, as well as for the economic burden related to such treatment. Indeed, CIMT has a cost in terms of time and effort not only for the medical therapists involved during the rehabilitation sessions but also for the family, who has to continue the treatment at home. Nevertheless, despite the effort required to complete the CIMT treatment, the absence of relevant adverse effects, and its effectiveness fully legitimize the approach [26, 27].
Consistent with a few previous studies in pediatric patients with brain injury of different etiologies [8, 28, 29], CIMT resulted in an improved performance at the functional scales administered. Although such an improvement was more evident at the end of the rehabilitation period, a further amelioration of the affected upper limb function was also detected 6 months after cessation of therapy. This “delayed” effect might be secondary to a relearned use of the affected limb in patients during daily-life activities and is known to occur after CIMT.
The multivariable analyses revealed that baseline intrinsic tissue damage of symptomatic lesions is associated with clinical improvement, as measured by QUEST and GMFM, at the end of the treatment, and that reduced RS FC of the right SII and cerebellum predict additional functional gaining 6 months later.
Owing to its ability to measure microstructural damage beyond the resolution of conventional imaging, several studies have applied DTI to quantify the severity and distribution of central nervous system damage in pediatric patients with congenital or ABI [3–5]. Similarly to ours, these studies have demonstrated that, when compared with matched healthy controls, these patients have abnormal DTI indexes (decreased FA and increased MD) not only in the symptomatic lesions but also in the corresponding CST [3–5]. Conversely, the analysis of the contralateral CST gave conflicting results: a few authors found this to be injured [15], but others did not [3, 5]. The notion that a relative preservation of the structural integrity of the affected hemisphere might be associated with a better clinical outcome is supported by previous correlative studies, which found higher FA values in the affected CST in patients with a better clinical performance [3–5]. This study demonstrates that measures of lesional damage predict clinical improvement in patients treated with CIMT. Clearly, this has important clinical implications, as it can contribute to select patients who might benefit most from specific rehabilitation programmes. In adult patients with hemiparesis, the volume of the symptomatic lesion was not related to the degree of motor deficits, whereas the extent of lesion intersection with the CST was related to the presence of a residual motor ability [30, 31]. Intrinsic lesion damage was not quantified by previous investigations. Of note, lesional volume and the severity of CST damage were not associated with functional motor improvement following CIMT in 10 adult patients with stroke [31]. This is in agreement with our results, which showed that only average lesion FA was able to predict functional improvement at the end of treatment, while lesion volume and DTI indexes of the affected CST were not. The notion that measuring lesion FA may be a rewarding exercise for predicting clinical outcome and assessing the efficacy of interventions is supported by 2 studies of infants with motor dysfunction in whom baseline FA values of affected areas predicted functional outcome after 2 [32] and 3 [16] years of follow up.
An enhancement of the cortical representation of upper limb movement is believed to be among the factors contributing to the efficacy of CIMT [33]. In line with this, previous studies in pediatric patients with hemiparesis due to different causes have shown that CIMT increases task-related brain activations of the motor areas of the lesioned hemisphere [2, 6, 34].
We assessed baseline functional integrity of the sensorimotor network by applying an RS FC analysis. This strategy enabled us to obtain fMRI results unbiased by task performance and showed that, when compared with matched healthy control participants, children with brain injury experienced a distributed reduction of RS FC of several areas of the sensorimotor network, including the cerebellum, bilaterally; right SII; and left precentral gyrus. The reduced RS FC of the right SII was related to a worse performance at clinical scales. In patients with spastic diplegia, the severity of motor impairment has been associated with a reduction of cortical connectivity of the motor control areas and the degree of CST damage quantified using DTI. This suggests that our findings might be interpreted as a reflection of relatively unspecific phenomena occurring after tissue damage to the motor system [35]. The novel finding of our study is that baseline FC of the right SII and cerebellum predicted clinical improvement at month 6. The notion that an increased recruitment of SII and cerebellum might contribute to recovery after CIMT is in line with the results of previous fMRI studies [36] in adult patients with stroke. Indeed, both these regions are involved in motor learning [37], which is known to occur during CIMT. A previous active fMRI study in adult patients with stroke [38] showed that an early cerebral reorganization involving SII was likely to be the neuronal substrate of motor recovery. Other studies have also suggested a role of SII in improving motor function in the chronic phase following stroke [39]. Although our results are not comparable with these previous ones owing to the different characteristics of the cohorts studied and methods applied, they confirm the pivotal role of SII in the recovery of motor function after brain damage has occurred.
Our study is not without limitations. First, the study group was relatively small and this did not allow us to perform powered subanalyses according to the location and etiology of lesions, or to define responders/nonresponders at the individual level. Second, the presence of movement artifacts and of extensive brain lesions might affect the results of coregistration and normalization to the standard template. However, an accurate check of the results of coregistration and normalization showed that both these preprocessing steps worked well in our study participants. Moreover, the use of ICA for the analysis of RS data ensures an automatic exclusion of all noise sources, including those related to participants’ movements, from the components of interest [40]. Third, owing to ethical considerations, and similarly to the majority of previous studies [8, 28, 29], we did not monitor a group of untreated patients with similar clinical and imaging characteristics. As a consequence, we cannot differentiate the effect of CIMT from spontaneous recovery following brain injury. Nevertheless, as all our patients were studied in a chronic phase, spontaneous recovery is likely to have had a modest effect, if any, on our findings.
Electronic supplementary material
(PDF 511 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Maria A. Rocca and Anna C. Turconi contributed equally to the study and should be considered joint first authors.
References
- 1.Taub E. Movement in nonhuman primates deprived of somatosensory feedback. Exerc Sport Sci Rev. 1976;4:335–374. doi: 10.1249/00003677-197600040-00012. [DOI] [PubMed] [Google Scholar]
- 2.Walther M, Juenger H, Kuhnke N, et al. Motor cortex plasticity in ischemic perinatal stroke: a transcranial magnetic stimulation and functional MRI study. Pediatr Neurol. 2009;41:171–178. doi: 10.1016/j.pediatrneurol.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Glenn OA, Ludeman NA, Berman JI, et al. Diffusion tensor MR imaging tractography of the pyramidal tracts correlates with clinical motor function in children with congenital hemiparesis. AJNR Am J Neuroradiol. 2007;28:1796–1802. doi: 10.3174/ajnr.A0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida S, Hayakawa K, Yamamoto A, et al. Quantitative diffusion tensor tractography of the motor and sensory tract in children with cerebral palsy. Dev Med Child Neurol. 2010;52:935–940. doi: 10.1111/j.1469-8749.2010.03669.x. [DOI] [PubMed] [Google Scholar]
- 5.Holmstrom L, Lennartsson F, Eliasson AC, et al. Diffusion MRI in corticofugal fibers correlates with hand function in unilateral cerebral palsy. Neurology. 2011;77:775–783. doi: 10.1212/WNL.0b013e31822b0040. [DOI] [PubMed] [Google Scholar]
- 6.Juenger H, Linder-Lucht M, Walther M, Berweck S, Mall V, Staudt M. Cortical neuromodulation by constraint-induced movement therapy in congenital hemiparesis: an FMRI study. Neuropediatrics. 2007;38:130–136. doi: 10.1055/s-2007-985904. [DOI] [PubMed] [Google Scholar]
- 7.Facchin P, Rosa-Rizzotto M, Turconi AC, et al. Multisite trial on efficacy of constraint-induced movement therapy in children with hemiplegia: study design and methodology. Am J Phys Med Rehabil. 2009;88:216–230. doi: 10.1097/PHM.0b013e3181951382. [DOI] [PubMed] [Google Scholar]
- 8.Cimolin V, Beretta E, Piccinini L, et al. Constraint-induced movement therapy for children with hemiplegia after traumatic brain injury: a quantitative study. J Head Trauma Rehabil. 2012;27:177–187. doi: 10.1097/HTR.0b013e3182172276. [DOI] [PubMed] [Google Scholar]
- 9.DeMatteo C, Law M, Russell D, Pollock N, Rosenbaum P, Walter S. QUEST: Quality of Upper Extremity Skills Test Manual. Neurodevelopmental Research Unit, Chedoke Campus, Chedoke-McMasters Hospital: Hamilton, Ontario; 1992. [Google Scholar]
- 10.Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol. 1989;31:341–352. doi: 10.1111/j.1469-8749.1989.tb04003.x. [DOI] [PubMed] [Google Scholar]
- 11.Law M, Cadman D, Rosenbaum P, Walter S, Russell D, DeMatteo C. Neurodevelopmental therapy and upper-extremity inhibitive casting for children with cerebral palsy. Dev Med Child Neurol. 1991;33:379–387. doi: 10.1111/j.1469-8749.1991.tb14897.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang R, Benner T, Sorensen A, Wedeen V. Diffusion Toolkit: A Software Package for Diffusion Imaging Data Processing and Tractography. ISMRM abstract. Proc Intl Soc Mag Reson Med 2007;15.
- 13.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36:893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 14.Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 15.Thomas B, Eyssen M, Peeters R, et al. Quantitative diffusion tensor imaging in cerebral palsy due to periventricular white matter injury. Brain. 2005;128:2562–2577. doi: 10.1093/brain/awh600. [DOI] [PubMed] [Google Scholar]
- 16.Ludeman NA, Berman JI, Wu YW, et al. Diffusion tensor imaging of the pyramidal tracts in infants with motor dysfunction. Neurology. 2008;71:1676–1682. doi: 10.1212/01.wnl.0000304084.59964.e2. [DOI] [PubMed] [Google Scholar]
- 17.Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 20.Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–2095. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 24.Brett M AJ, Valabregue R, Poline JP. Region of interest analysis using an SPM toolbox. Neuroimage 2002;16:372.
- 25.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 26.Wolf SL. Revisiting constraint-induced movement therapy: are we too smitten with the mitten? Is all nonuse "learned"? and other quandaries. Phys Ther. 2007;87:1212–1223. doi: 10.2522/ptj.20060355. [DOI] [PubMed] [Google Scholar]
- 27.French B, Leathley M, Sutton C, et al. A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness. Health Technol Assess 2008;12:iii, ix-x, 1–117. [DOI] [PubMed]
- 28.Hoare BJ, Wasiak J, Imms C, Carey L. Constraint-induced movement therapy in the treatment of the upper limb in children with hemiplegic cerebral palsy. Cochrane Database Syst Rev 2007:CD004149. [DOI] [PubMed]
- 29.Wallen M, Ziviani J, Herbert R, Evans R, Novak I. Modified constraint-induced therapy for children with hemiplegic cerebral palsy: a feasibility study. Dev Neurorehabil. 2008;11:124–133. doi: 10.1080/17518420701640897. [DOI] [PubMed] [Google Scholar]
- 30.Pineiro R, Pendlebury ST, Smith S, et al. Relating MRI changes to motor deficit after ischemic stroke by segmentation of functional motor pathways. Stroke. 2000;31:672–679. doi: 10.1161/01.STR.31.3.672. [DOI] [PubMed] [Google Scholar]
- 31.Sterr A, Shen S, Szameitat AJ, Herron KA. The role of corticospinal tract damage in chronic motor recovery and neurorehabilitation: a pilot study. Neurorehabil Neural Repair. 2010;24:413–419. doi: 10.1177/1545968309348310. [DOI] [PubMed] [Google Scholar]
- 32.Drobyshevsky A, Bregman J, Storey P, et al. Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci. 2007;29:289–301. doi: 10.1159/000105470. [DOI] [PubMed] [Google Scholar]
- 33.Mark VW, Taub E, Morris DM. Neuroplasticity and constraint-induced movement therapy. Eura Medicophys. 2006;42:269–284. [PubMed] [Google Scholar]
- 34.Sutcliffe TL, Logan WJ, Fehlings DL. Pediatric constraint-induced movement therapy is associated with increased contralateral cortical activity on functional magnetic resonance imaging. J Child Neurol. 2009;24:1230–1235. doi: 10.1177/0883073809341268. [DOI] [PubMed] [Google Scholar]
- 35.Lee JD, Park HJ, Park ES, et al. Motor pathway injury in patients with periventricular leucomalacia and spastic diplegia. Brain. 2011;134:1199–1210. doi: 10.1093/brain/awr021. [DOI] [PubMed] [Google Scholar]
- 36.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 37.Ramnani N, Toni I, Josephs O, Ashburner J, Passingham RE. Learning- and expectation-related changes in the human brain during motor learning. J Neurophysiol. 2000;84:3026–3035. doi: 10.1152/jn.2000.84.6.3026. [DOI] [PubMed] [Google Scholar]
- 38.Loubinoux I, Carel C, Pariente J, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20:2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Askim T, Indredavik B, Vangberg T, Haberg A. Motor network changes associated with successful motor skill relearning after acute ischemic stroke: a longitudinal functional magnetic resonance imaging study. Neurorehabil Neural Repair. 2009;23:295–304. doi: 10.1177/1545968308322840. [DOI] [PubMed] [Google Scholar]
- 40.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 511 kb)