Abstract
Many neurodegenerative diseases are characterized by the progressive accumulation of aggregated protein. Recent evidence suggests the prion-like propagation of protein misfolding underlies the spread of pathology observed in these diseases. This review traces our understanding of the mechanisms that underlie this phenomenon and discusses related therapeutic strategies that derive from it.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0196-3) contains supplementary material, which is available to authorized users.
Keywords: Trans-cellular propagation, Networks, Neurodegeneration, Prion, Templated conformational change
Introduction
The deposition of aggregated proteins defines virtually all neurodegenerative disorders, including Alzheimer disease (AD), Parkinson disease (PD), and amyotrophic lateral sclerosis (ALS). Protein accumulation and neurodegeneration typically proceeds in a relatively stereotypical fashion for these diseases, which suggests cell non-autonomous factors drive pathology. Recent studies have linked cell-to-cell propagation of pathology to molecular mechanisms reminiscent of prion pathogenesis. This review traces our understanding of neurodegenerative disease in light of trans-cellular propagation of aggregated proteins (Table 1). This occurs via templated conformational change, whereby an aggregated protein of a defined structure interacts with the native monomer and recruits it to a growing assembly. The recent recognition that protein aggregates transfer between cells to propagate pathology is helping to define new treatment strategies.
Table 1.
Evidence for prion-like mechanisms in neurodegenerative disease
| Protein | Seeded aggregation | Transcellular propagation | Induced spread of pathology in vivo | ||
|---|---|---|---|---|---|
| In cell culture | Transcellular movement | Propagation of aggregated state | Brain lysate | Synthetic/recombinant protein | |
| PrP | Yes | Yes | Yes | Yes | Yes |
| [113] | [114] | [114] | [115, 116] | [117, 118] | |
| Tau | Yes | Yes | Yes | Yes | Yes |
| [39–41] | [39] | [42] | [43] | [46] | |
| α-synuclein | Yes | Yes | Yes | Yes | Yes |
| [32, 41] | [32, 33, 65] | [32] | [32, 33]a | [34, 35] | |
| [25, 26]b | |||||
| β -amyloid | Yes | Yes | n.d. | Yes | Yes |
| [119] | [120] | [121]c | [17] | ||
| [15, 16]d | |||||
| Huntingtin | Yes | Yes | Yes | n.d. | n.d. |
| [80, 81] | [81] | [81] | |||
| SOD1 | Yes | Yes | n.d. | n.d. | n.d. |
| [77] | [77] | ||||
| TDP-43 | Yes | n.d. | n.d. | n.d. | n.d. |
| [122] | |||||
aMurine model
bHuman patients
cPeripheral application
dCortical application
Seeded aggregation is the process by which extracellular protein aggregates induce misfolding of native protein in an acceptor cell. Transcellular movement is the process by which protein aggregates escape one cell and enter a neighboring cell. Propagation of the aggregated state is the ability of those transferred aggregates to amplify the misfolded state. Induced spread of pathology is the ability of brain lysate or synthetic seeds to cause misfolding and progressive pathology in vivo. nd not determined, PrP prion protein, SOD superoxide dismutase, TDP-43 TAR DNA-binding protein-43
Prion Diseases
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are a family of progressive neurodegenerative diseases with a wide variety of clinical manifestations. The vast majority of TSEs are sporadic and not derived from “infection,” and more than 40 mutations in the prion protein (PrP) lead to autosomal dominant forms. Prusiner’s seminal studies on PrP demonstrated that PrP alone could act as a truly infectious agent, with the ability to transfer pathology from cell-to-cell and confer its pathological, “scrapie” conformation (PrPSC) onto naïve “cellular” PrPC.
Insight has come from structural analysis of PrP coupled with animal models of prion diseases. Native PrPC is composed of nonpathological alpha helices. Conversion to PrPSC, an amyloidogenic beta sheet conformation, causes neurodegeneration [1–3]. PrP knockout mice do not develop neuropathology upon inoculation with PrPSC, and those that are heterozygous for PrPC are more resistant to infection [4]. Thus, the propagation of pathological PrPSC to naïve cells, with templating of its pathological conformation onto endogenous PrPC, underlies the spread of prion pathology and neurodegeneration.
PrPSC must contain a high degree of sequence similarity to PrPC for efficient templating to occur. This “seeding barrier” is observed in murine PrPC, which is resistant to templated misfolding by the heterotypic hamster PrPSC. Seeding barriers also exist for human PrPC, which is resistant to sheep PrPSC, but not bovine PrPSC. Mice that express hamster PrPC in place of the murine protein are susceptible to conversion by hamster PrPSC [4, 5]. Such seeding barriers have also been confirmed in vitro, where a single amino acid substitution can affect the seeding specificity of PrP fibrils [6]. Thus, efficient templating of an amyloidogenic conformation requires primary amino acid sequences similar enough to allow templating of the native protein into a tertiary conformation compatible with the growing assembly.
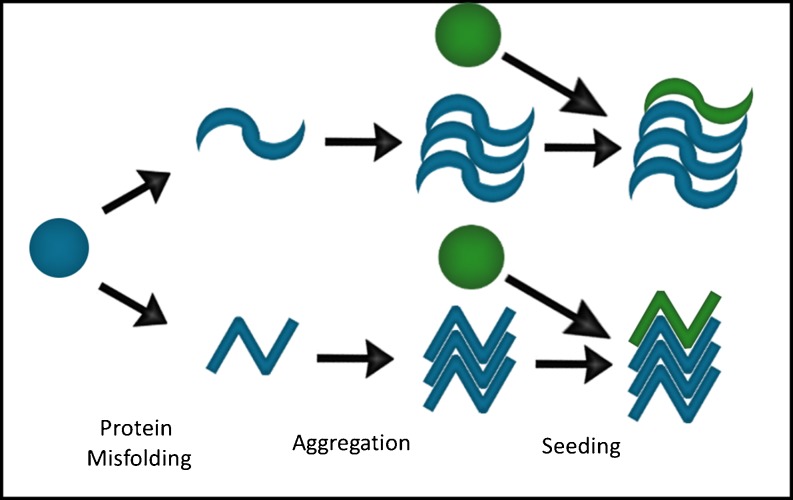
Importantly, multiple fibril conformations can be formed from a single PrP protein (Fig. 1). This is thought to explain some of the diversity of phenotypes observed in prion diseases [7, 8]. These different conformations, or strains, demonstrate great morphological and biochemical diversity, suggesting they are conformationally distinct from one another [9]. This structural diversity appears to underlie the different rates of spread and patterns of progression through the brain, and may prove important when designing effective therapies for these diseases [8, 9].
Fig. 1.
Amplification of a protein aggregate by templated conformational change. A native protein (blue circle) adopts a pathological conformation that facilitates aggregation into b-sheet rich structures. These structures can contact additional native proteins (green circles), adding them on to the aggregate by converting them to a specific aggregate structure
Expansion of the Prion Hypothesis to Aβ
The hypothesis that amyloid fibrils observed in AD act as prions dates back to original observations that PrP forms rod-like structures similar to Aβ [10]. At this time, it was recognized that both prion diseases and AD feature progressive cognitive decline and widespread brain amyloid deposition. Moreover, it was suggested that the aggregated proteins observed in AD may actually be accumulations of the toxic agent that underlies this disease. Aβ was later identified as the protein component of the plaques observed in AD, and familial forms of this disease were linked to mutations in the precursor of Aβ, amyloid precursor protein (APP) [11], and the presenilins [12, 13], which cleave APP to form Aβ. These findings suggested the deposition of Aβ is not an epiphenomenon but a cause of AD. Similar genetic/pathological correlations have now been described for most proteins associated with other major neurodegenerative disorders.
Patients with AD develop progressive accumulation of Aβ plaques [14]. To test the possibility that Aβ seeds can promote subsequent pathology, several investigators injected AD patient brain homogenate into APP-expressing mice. This induced widespread senile plaques that were found as far as the contralateral hemisphere. In contrast, brain homogenate that lacked Aβ pathology did not induce plaque deposition [15, 16]. Brain lysate from a transgenic mouse model of AD also induced Aβ pathology in APP-expressing mice, demonstrating that the toxic agent was not specific to the human brain [15]. Immuno-depletion of Aβ prevented induction of plaque formation, indicating that the causative agent was Aβ itself [15]. Injection of a synthetic form of Aβ also accelerated plaque formation in APP-expressing mice as compared to controls, which confirmed that Aβ aggregates are sufficient to induce Aβ plaque formation [17]. These experiments suggested that Aβ could meet many of the experimental criteria typically applied to infectious PrP in terms of its ability to produce pathology in vivo. However, because pathogenic Aβ accumulates in the extracellular compartment, it was possible to explain these findings simply as a correlate of in vitro templated conformational change, as it does not require prion-like trans-cellular propagation.
Spread of α-Synuclein Pathology to Young Neurons in Human Patients
Studies of α-synuclein provided the first evidence for trans-cellular movement of a protein that forms intracellular aggregates. Patients with PD develop progressive Lewy body and neurite pathology composed of aggregated α-synuclein. Further, missense mutations in α-synuclein (A30P [18], E46K [19], and A53T [20]) as well as triplication of the wild-type (WT) α-synuclein gene [21] cause autosomal dominant forms of PD. PD pathology also exhibits a stereotypical deposition pattern that follows known anatomical connections. Early in the course of PD, α-synuclein accumulates in the brainstem and even the enteric nervous system before spreading to involve the midbrain and finally reaching the transentorhinal region and large areas of the neocortex [22]. This pattern of progressive accumulation of pathological inclusions is reminiscent of that seen with Aβ and PrP, and is consistent with a prion model of pathogenesis based on templated conformational change.
Intriguing findings in PD came from patients with advanced disease who received fetal mesencephalic grafts injected into the putamen [23, 24]. The patients who came to autopsy after only a few years had clusters of viable dopaminergic neurons that re-innervated areas of the striatum. But after longer incubation periods (12–16 years), α-synuclein and ubiqutin-positive Lewy body pathology was identified in the fetal grafts [25, 26]. The presence of metabolically and phenotypically normal dopaminergic grafts early in this process (18 months) suggested a long lag phase before induction of pathology [27–29]. Several hypotheses were presented to explain the spread of pathology into these young neurons, including the possibility that neuron-to-neuron spread of α-synuclein induced further aggregation in the fetal grafts.
This concept was further tested in numerous rodent models using viral expression of human α-synclein [30, 31] and transgenic mouse models of PD [32, 33]. In one case, neural stem cells were transplanted into transgenic mice expressing human α-synuclein. Human α-synuclein was detected in the grafted tissue as rapidly as 1 week after transplantation [33]. This was most consistent with α-synuclein transfer between neurons in vivo. Recombinant human α-synuclein fibrils also trigger the propagation of α-synuclein pathology in mice transgenic for human α-synuclein [34], and even in wild-type mice [35]. This confirms that α-synuclein fibrils alone are sufficient to induce PD pathology. The injected brain lysate or fibrils led to spread of pathology that followed known afferent/efferent neuronal pathways, whereas regions adjacent but not directly connect to the injected site showed fibril deposition [34]. These results support the hypothesis that the spread of misfolded α-synuclein may propagate pathology between cells.
Transcellular Spread of Tau Pathology In Vitro and In Vivo
Neurofibrillary tau pathology correlates with the neurodegeneration and cognitive decline observed in patients [36], suggesting it plays a role in the pathogenesis of AD. Tau deposition in AD has been known for years to progress through anatomically connected regions of the brain, beginning in the transentorhinal region, before involving the hippocampus and finally the neocortex [37, 38]. At the time of these original observations, no experimental evidence supported the concept of trans-cellular propagation of tau aggregates.
Evidence of such transmissible protein pathology has now been observed in cell culture and in animals. In cultured cells tau aggregates access the cytoplasm from the extracellular space and induce the fibrillization of native intracellular tau [39–41]. The intracellular tau fibrils can seed further fibrillization of recombinant protein ex vivo. Upon co-culture, intracellular tau fibrils, induced initially by exogenous tau fibrils, escape “donor” cells to be taken up by “recipient” cells [39]. More recent work has demonstrated true propagation of tau pathology in cell culture, whereby fibrils formed in one cell are released free into the media, gain entry to recipient cells, and directly contact the native protein to amplify aggregation [42]. A blocking antibody prevents this trans-cellular propagation, and can immunoprecipitate tau fibrils from conditioned media [42]. These data are most consistent with the idea that tau aggregates themselves serve as a “pathogenic agent” that can propagate pathology between cells.
Studies in vivo also support this idea. Injection of brain lysate containing aggregated forms of tau into mice that express WT human tau induced the formation of neurofibrillary tangles in the recipient mice. These spread beyond the injected hippocampus to more distant sites, including somatosensory cortex [43]. Immuno-depletion of tau from the injected material prevented induction of neurofibrillary tangles, confirming tau, and not a nonspecific “toxic factor” as the cause of pathology. Finally, injection of P301S-containing brain lysate into mice that only express murine tau produced very limited pathology that was confined to the injection site [43]. This was reminiscent of the seeding barriers observed for PrP.
To further explore the spread of tau pathology throughout the brain, two groups used a transgenic mouse model that restricts P301L tau expression to the entorhinal cortex (EC) [44, 45]. De Caglignon et al. [44] verified the limited expression of this transgene using in situ hybridization, which was further verified by qPCR on laser captured neurons. The group observed tau pathology in young mice that was limited to the medial entorhinal cortex (MEC). In aged mice, however, the granular layer of the dentate gyrus (DG), a region synaptically connected to the MEC, also exhibited tau pathology. Given tau expression relatively restricted to the MEC, this was consistent with tau transfer across synapses. On the other hand, Liu et al. [45] used qPCR analysis in these mice to determine that human tau was in fact expressed in a subset of neurons in the dentate gyrus. This group also crossed the neuropsin promoter driver line to a beta-galactosidase reporter mouse, and observed a few neurons stained positive in the DG. The authors concluded that the tau mRNA detected by qPCR could not explain the large amount of tau deposition observed in the DG in aged mice. Results of these studies are generally consistent with trans-neuronal spread of tau pathology, but it is still somewhat unclear how much of the pathology observed in this model is from trans-synaptic spread vs. transgene expression outside of the EC.
Recombinant tau protein also induces the spread of tau pathology in mice that express a human isoform of tau protein [46]. In mice injected with different amounts of recombinant fibrils, tau pathology occurred as far as the contralateral hippocampus. Thus purified tau protein is sufficient to trigger the propagation of tau pathology in vivo. Taken together with the cellular studies, these results strongly support the hypothesis that tau aggregates propagate pathology between cells.
Neuroimaging Evidence for Network Involvement
Advances in functional magnetic resonance imaging (fMRI), particularly resting-state functional connectivity MRI (rs-fcMRI), have provided insight into the neural networks of the brain. These new methods highlight the relationship of neurodegeneration patterns to existing brain networks. In a subject at rest, rs-fcMRI measures the temporal correlation in the blood-oxygen level dependent (BOLD) signal across different brain regions. Those regions with synchronous BOLD activity correspond to known anatomic networks, or areas commonly co-activated during a task [47–49].
The patterns of atrophy observed in patients with AD, behavioral variant frontotemporal dementia, semantic dementia, progressive non-fluent aphasia, and corticobasal syndrome matched five separate functional connectivity networks defined in a healthy control cohort [50]. Distinct disease “epicenters” were defined for each of the five syndromes, where the connectivity pattern in healthy controls precisely matched the atrophy pattern observed in patients. Further, the shortest functional path length to the identified “epicenter” of neurodegeneration, not spatial proximity, best predicted atrophy in a given region of the brain [51]. Association of atrophy with functional proximity, and the progression of these diseases along known networks is consistent with trans-neuronal spread of a toxic substrate between functionally connected brain regions, and with the atrophy patterns produced in mice following injection of pathogenic fibrils.
Escape of Aggregates from Neurons
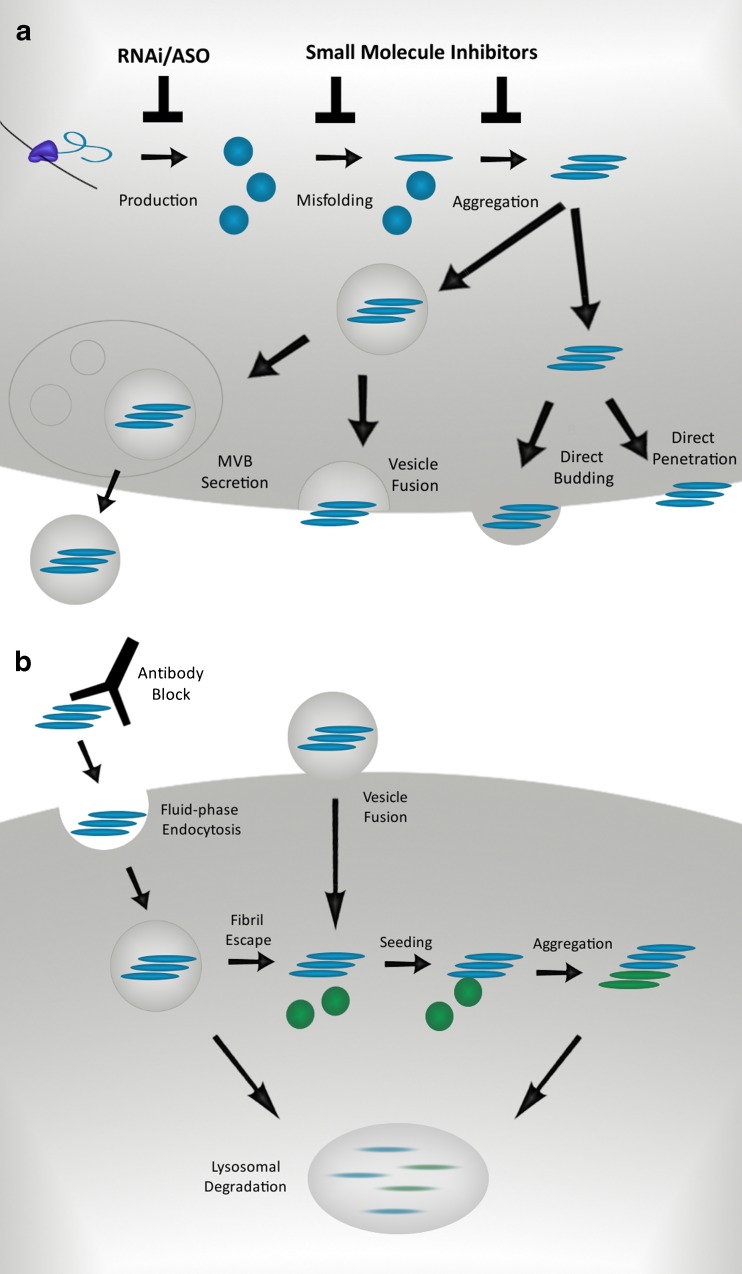
It is unknown how protein aggregates escape the cells in which they are formed, but studies have begun to uncover potential mechanisms (Fig. 2a). Monomeric tau is clearly released from cells, as tau has been found in the interstitial fluid (ISF) of wild-type mice in the absence of injury or neurodegeneration [52]. Mice expressing aggregation-prone mutant P301S tau have demonstrated a drop in soluble ISF tau and an increase in CSF levels, associated with intracellular aggregation (the microdialysis probe used in this study cannot measure tau aggregates). These results are consistent with a local shift of soluble tau to a more aggregated, insoluble form that would not be detected by microdialysis. In contrast, increased CSF tau seems to reflect the neurodegeneration observed in these mice, consistent with an increased cellular release of tau in disease [52]. However, tau levels do not correlate with lactate dehydrogenase or tubulin release into the media [53, 54], implying that release is not due to increased cell death. This observation is supported by evidence that monomeric tau secretion is an active process, as tau release is inhibited at low temperatures [53], and has been linked to synaptic activity [55]. It is still quite unclear whether the release of monomeric vs. aggregated tau is occurring via the same mechanism.
Fig. 2.
Mechanisms of aggregate release and uptake. a) Mechanisms of aggregate release. Proteins that misfold and aggregate might be secreted via several mechanisms. Direct penetration and vesicle fusion release aggregates directly into the media, whereas multivesicular body secretion and direct budding produce aggregate-laden vesicles. Therapeutic interventions can target the production of aggregate-prone proteins through RNA interference and antisense oligonucleotides. Small molecules might stabilize the native protein structure or inhibit templated misfolding of the cognate monomer. MVB, multivesicular body. b) Mechanisms of aggregate uptake. Fibrils are endocytosed by vesicle fusion or fluid-phase endocytosis. Aggregate escape into the cytosol allows further seeding and aggregation of endogenously expressed protein. Antibody blockade of cell attachment or endocytosis of aggregates will prevent the spread of misfolded protein, and therapies that induce relevant degradation pathways may increase the clearance of aggregates from the cell
Several explanations have been proposed for tau release. These include unconventional secretion directly into the media [53, 54], vesicle-associated release [56] and exosome-associated release [57, 58]. While certain studies show a subset of secreted tau is found in the exosomal fraction of conditioned media [58], others show little to no tau in this fraction [53, 54]. Importantly, free tau oligomers may copurify with exosomes, which would hinder accurate assessment of tau release by this mechanism. Protease digestion of this fraction could determine if tau is shielded within exosomes. Immuno-electron microscopy could also test whether tau aggregates colocalize with exosome markers.
In contrast to evidence for exosome-mediated release, immunoprecipitation of tau fibrils from culture media supports direct release of free fibrils into the extracellular space [42, 53]. Finally, an anti-tau antibody has been observed to block cell-cell propagation of aggregation “donor” to “recipient” cells by preventing cell uptake [42]. These findings suggest aggregated tau escapes directly into the extracellular space.
α-synuclein is also detectable in both human CSF and plasma in soluble, oligomeric forms that may have use as potential biomarkers for PD [59, 60]. α-synuclein secretion appears to be mediated by an unconventional mechanism, independent of the ER/Golgi [61, 62]. Evidence suggests that this secretion is an active process as it is temperature sensitive, independent of cell death, and the amount of α-synuclein released from cells does not correlate with the release of other cytosolic proteins into the media [62]. Release of α-synuclein may occur through multiple mechanisms, however. Free α-synuclein can be immunoprecipitated from media and human CSF [60], and at least a subset of the toxic species in conditioned media is removed via α-synuclein-specific immunoprecipitation [61]. Calcium-mediated exosome release may also contribute to secretion, as α-synuclein can be found in the exosomal fraction of conditioned media [61, 63]. Importantly, a subset of α-synuclein remains intact in the exosome fraction after trypsin digestion, but is absent after saponin-mediated membrane permeableization, confirming it is protected from protease digestion within exosomes [63].
Uptake of Aggregates into Neurons
Uptake of protein aggregates is almost certainly required to propagate aggregate pathology (Fig 2b). Conflicting data have been presented for tau, α-synuclein, and huntingtin peptides. α-synuclein uptake reportedly occurs via a temperature-sensitive mechanism that can be blocked by inhibiting endocytosis with a dominant negative dynamin mutant [32, 33, 64]. Proteinase K, which removes proteins from the extracellular surface, also abolishes the majority of α-synuclein uptake from the media [64]. This is consistent with a receptor-mediated mechanism. While the post-endocytic trafficking of α-synuclein is not yet clear, lysosomal inhibition with Bafilomycin A increases the levels of accumulated α-synuclein in the acceptor cell population [33, 64]. Thus, the internalized α-synuclein may be degraded via lysosomes.
Trans-synaptic movement of α-synuclein between neurons was recently demonstrated using a microfluidic chamber to separate first-order from second-order neurons in culture. First order neurons were incubated with α-synuclein fibrils, and these fibrils were observed to move along axons into the neighboring chamber. α-synuclein was observed in the soma of second order neurons after a 24-hour exposure of the first-order neurons. Spread to second-order neurons was not observed unless first-order neurons were present, thereby ruling out diffusion of aggregates through the microfluidic device. The kinetics of movement were consistent with slow component-b transport along the axon, rather than diffusion. These results support the idea that α-synuclein can be transported anterograde, released, and spread to second order neurons trans-synaptically [65]. However, the role of synaptic activity in this type of transfer is unknown.
Similar to α-synuclein, tau aggregates are taken into the cell through an active, temperature sensitive process [39, 40]. This appears to be mediated by fluid-phase endocytosis at the plasma membrane, rather than penetration through the lipid bilayer [39, 66]. While tau aggregates are readily taken into cells, monomeric tau does not gain access to the intracellular space [39, 66], implying higher-order oligomers may be required for efficient internalization. Microfluidic chambers have been used to show that tau aggregate uptake is possible at the somatodendritic compartment as well as axon terminals, and fibrils can be transported both anterograde and retrograde once inside neurons [66]. These results are consistent with the observation that tau pathology is associated with brain networks, but it has not yet been demonstrated that true propagation of aggregation across such networks occurs in vivo, as opposed to simple movement of protein aggregates.
Spreading Pathology in ALS
ALS has long been recognized as a disease of the motor network, since it involves progressive loss of both upper and lower motor neurons. Furthermore, the clinical symptoms and motor neuron pathology typically begin focally before spreading throughout the spinal cord. The spread appears to occur along neuroanatomically connected regions, and the rate and pattern are consistent with axonal spread of a toxic agent [67–69]. These observations are all consistent with cell-to-cell spread of misfolded proteins and subsequent templating of this conformation could underlie the spread of pathology, but does not rule out other possibilities. Familial forms of ALS (fALS) due to mutations in superoxide dismutase 1 (SOD1) [70, 71], fused in sarcoma (FUS) [72, 73], and TAR DNA-binding protein 43 (TDP-43) [74] are now widely described. These proteins accumulate in aggregates observed upon pathological examination of motor neurons, but have not been as extensively studied for potential prion-like mechanisms.
SOD1 is detectable in the CSF of control and fALS subjects [75], and is released from cells in culture [76]. Furthermore, it was recently observed that SOD1 fibrils can transfer between cells and be taken up via clathrin-independent macropinocytosis [77]. In neural cells, SOD1 localized to endosomes after internalization, but could escape to induce native cytosolic SOD1 to aggregate. This templated misfolding persisted for at least a month, well after the original internalized aggregates were no longer detectable [77]. SOD1 seeds can also be found in the CSF of mice that over-express an aggregate-prone mutant form of the protein, and these seeds can induce new fibril formation of recombinant SOD1 in vitro [78]. While this has not yet been tested in vivo, the cellular results suggest SOD1 fibrils might also propagate a misfolded, amyloid conformation between cells.
Prion-Like Properties of the Huntingtin Protein
Huntington disease (HD) is caused by an elongated polyglutamine (polyQ) tract in the huntingtin protein (Htt). The polyQ expansion causes Htt to form amyloids in neurons [79]. Expanded polyQ peptide amyloids gain access to the cytoplasm of a variety of mammalian cells upon introduction to the culture medium [80]. The fibrils that are taken into the cell can induce the aggregation of other proteins with polyQ tracts, and this misfolding is maintained for multiple passages, implying the aggregates formed from endogenously expressed protein can also seed further intracellular misfolding and aggregation, similar to yeast prions [81].
Htt aggregate release into the media has not been clearly documented, but cell lystates promote aggregation in an acceptor cell population [81]. Cells can therefore take up Htt, but cell-to-cell transfer of these aggregates appears relatively inefficient compared to the other aggregation-prone proteins discussed above. The exact amino acid sequence surrounding the polyglutamine tract can influence the propensity of uptake, as fibrils composed of KKQ44KK bind cells much more readily than fibrils formed from a fragment of the Htt protein [80]. Thus, polyQ fibrils can be taken into cells and seed the aggregation of other polyQ-containing proteins, but this phenomena depends on the sequences that flank this tract. It is unclear whether the relatively low propensity of polyQ aggregates to be released and enter neighboring cells represents an artifact of the experimental systems (which use shortened forms of Htt) or whether this process would be more efficient in vivo in the setting of full-length protein. This phenomenon may extend to other polyglutamine containing proteins, but the literature has focused on Htt-Exon1 and synthetic polyglutamine tracts.
Developing Effective Treatments for Neurodegenerative Disease
Classical treatment designs have focused on decreasing production, increasing intracellular clearance, and preventing aggregation of the proteins implicated in neurodegenerative diseases. Given the recent work regarding mechanisms of transcellular propagation, treatment strategies may soon be expanded to include extracellular clearance, inhibiting secretion and preventing uptake of pathological protein aggregate seeds. Endogenous expression of PrP is required for the spread of pathology, and decreasing either the endogenous or misfolded form of this protein is a viable treatment strategy [82, 83]. Given the current evidence for prion-like spread of the proteins implicated in other neurodegenerative diseases, decreased production may also have special benefit in this regard.
RNAi and Antisense Oligonucleotide Therapies
RNA interference (RNAi) and antisense oligonucleotides (ASOs) both have potential utility. These techniques target a highly specific sequence present in the mRNA of a target protein, leading to decreased translation (RNAi) or degradation of the mRNA transcript (RNAi/ASO) [84–86]. RNAi techniques can preferentially decrease the expression of a mutant allele over the wild-type transcript, as base pair mismatches between the siRNA and target RNA inhibit their binding and subsequent degradation [86]. Furthermore, these techniques allow for great versatility in the region targeted by these nucleotides, while retaining specificity due to unique mRNA sequences in the target transcripts. This form of treatment has been used in a mouse model of prion disease, and shRNA mediated knockdown of PrP in just a subset of neurons greatly extended the length of survival compared to control mice [82]
While RNAi based systems show great promise in treating several diseases [84, 86], limited uptake into cells and distribution to target tissues have hindered their use in vivo, and unforeseen off-target interactions may cause adverse effects in patients. Furthermore, high levels of shRNA appear to compete with endogenous miRNA processing, and have produced harmful effects in vivo [86]. Developing viable strategies to facilitate the delivery of shRNA and siRNA, as well as improving their safety in vivo would greatly improve the therapeutic options available for numerous neurodegenerative diseases.
Antisense oligonucleotides (ASOs) also pair with high specificity to target mRNA sequences and induce their degradation. These molecules have some advantages over RNAi-based approaches, as they are readily modified to improve their stability and decrease their susceptibility to exonuclease degradation, leading to increased longevity in vivo. Specific modifications can also improve their solubility and tissue distribution, which will allow for decreased concentrations to be used in vivo [83, 85].
The efficacy of ASOs has also been assessed with various animal models of neurodegenerative disease. ASOs were used to decrease PrP in a mouse model of prion disease, and led to a dramatic increase in the length of survival compared to control mice [83]. ASO-mediated decrease of huntingtin protein in a mouse model of Huntington’s disease delayed and even reversed pathology. This effect persisted for months after the mRNA and protein levels of huntingtin returned to baseline, indicating a robust and long-lasting effect [85]. ASOs also decreased mutant SOD1 in both the brain and the CSF in a mouse model of ALS [87]. Finally, a recent clinical trial was completed to verify the safety of intrathecal delivery of ASOs against SOD1 in patients with ALS (NCT01041222). Delivery of ASOs may have as yet undefined risks, and it is unknown how effectively their intrathecal delivery will knock down target genes throughout the brain. Despite these inherent limitations, antisense oligonucleotides carry great promise.
Preventing Aggregation of Amyloidogenic Proteins
Preventing the aggregate formation has long been sought as a therapeutic strategy for neurodegenerative diseases, with disappointing results. Most of the therapies have been designed to disrupt fibrillar species, without necessarily affecting stability of the native protein. An alternative strategy has been applied successfully to transthyretin. Transthyretin amyloidoses cause fatal, progressive peripheral neuropathy and cardiomyopathy, based on accumulation of oligomers that assemble into amyloid deposits [88, 89]. Transthyretin normally forms a tetramer whose dissociation into monomers is a critical first step in fibril formation. Thus, based on knowledge of tetramer structure, an alternative strategy was adopted to stabilize this form with a small molecule, Tafamidis. [90]. In a randomized, controlled trial it preserved nerve fiber function and slowed neurological deterioration in patients with amyloidosis [91]. The remarkable success of this approach will no doubt encourage exploration of similar treatments to prevent amyloidogenesis in other neurodegenerative diseases.
Extracellular Clearance, Degradation, and Prevention of Uptake of Aggregated Proteins
Clearance of extracellular aggregates and inhibition of cell uptake could work in tandem to block progression of diseases. Antibodies show promise in this regard. Both passive and active immunization against Aβ in mouse models of AD prevented memory loss and behavioral impairment, and reduced Aβ neuropathology [92–96]. These studies led to the development of several active and passive vaccination strategies in clinical trials. One of the earliest trials, based on active vaccination, was interrupted due to a subset of patients that developed meningoencephalitis [97], but the results of this trial proved informative. An over-exuberant T cell response appears to have been responsible for the meningoencephalitis that was observed in patients [98, 99]. New trials have therefore focused on active [100], or passive vaccination that does not stimulate a T cell response [101]. Two phase III trials using passive immunization against Aβ did not meet their primary endpoints, although Solanezumab did show a small but significant reduction of cognitive decline in those with mild dementia after pooling data from two clinical trials [102], and based on this it is being carried forward to Phase III studies. These results highlight the need for a deeper understanding of the complex mechanisms that underlie AD, and mark a push to treat patients before the pathological cascade of AD has begun to cause overt cognitive decline [103]. Multiple trials are attempting to apply these therapeutic interventions in those with dominantly inherited AD before the onset of clinical symptoms. This will provide a crucial test of the idea that removing Aβ in those who are predestined to develop pathology will prevent or slow disease.
Numerous mouse tauopathy models have also been used to assess the efficacy of active immunization [104–107] and passive immunization [54, 108] against tau protein. These studies are encouraging for the development of effective immune-based strategies. With new knowledge about the role of extracellular tau it may become easier to understand why these approaches might be working. Multiple mechanisms could be at play, including disaggregation of tau, promotion of extracellular degradation, and blocking cell entry. One antibody, for example blocks aggregate uptake in a cell culture model of aggregate propagation [42]. α-synuclein studies have provided further insight into mechanisms of aggregate clearance. In this work, antibodies against α-synuclein allowed more rapid clearance of these aggregates through Fc receptors on microglia. Antibody-bound aggregates traffic more readily to the lysosomes of microglia, allowing for more effective breakdown and decreased cell-cell transfer of α-synuclein in a transgenic mouse model [109]. Both active and passive immunization against α-synuclein in a transgenic mouse model of PD decreased α-synuclein accumulation and decreased neurodegeneration and functional decline [110, 111]. The first vaccination-based treatment for PD is currently in phase I clinical trials. This active vaccination was designed to be specific for α-synuclein while sparing β-synuclein, and the immunogen contained short stretches of amino acids to prevent a T-cell autoimmune response [112]. Immunotherapies for proteins associated with neurodegenerative disease thus have exciting potential, and are now being pursued by multiple pharmaceutical companies.
Conclusions
Despite vast differences in the clinical manifestations of neurodegenerative diseases linked to amyloid protein pathology, a clear pattern has emerged regarding their spread. Pathology emerges in a select area of the nervous system, and progresses along known neuroanatomical connections. Such diseases invariably include accumulation of aggregated protein(s), and genetic mutations that are linked to these amyloidogenic proteins are often identified as rare causes of familial forms of these disorders. Fibrillar protein aggregates or oligomers have been shown in several different disease models to be released and taken up by cells in vitro and in vivo. Once internalized, they can seed native protein, thereby propagating the pathological conformation to new cells. The mechanisms of pathological protein spread discussed here thus may have broad implications for the development of effective treatments to slow or stop the progression of these diseases.
Electronic supplementary material
(PDF 1225 kb)
Acknowledgements
This work was supported by the National Institutes of Health (NINDS); the Muscular Dystrophy Association; the American Health Assistance Foundation; the Ruth K. Broad Foundation; the Tau Consortium; a pilot grant from the Hope Center for Neurological Disorders at Washington University in St. Louis.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Conflicts of interest
MID has patents pending for novel diagnostic tests, and for therapeutic anti-tau antibodies that have been licensed by a pharmaceutical company.
References
- 1.Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl. Acad. Sci. U.S.A. 1993;90(23):10962–10966. [DOI] [PMC free article] [PubMed]
- 2.Caughey BW, Dong A, Bhat KS, Ernst D, Hayes SF, Caughey WS. Secondary structure analysis of the scrapie-associated protein PrP 27–30 in water by infrared spectroscopy. Biochemistry. 1991;30(31):7672–7680. [DOI] [PubMed]
- 3.Safar J, Roller PP, Gajdusek DC, Gibbs CJ. Thermal stability and conformational transitions of scrapie amyloid (prion) protein correlate with infectivity. Protein Sci. 1993;2(12):2206–2216. [DOI] [PMC free article] [PubMed]
- 4.Büeler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, et al. Mice devoid of PrP are resistant to scrapie. Cell. 1993;73(7):1339–1347. [DOI] [PubMed]
- 5.Scott M, Foster D, Mirenda C, Serban D, Coufal F, Wälchli M, et al. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989;59(5):847–857. [DOI] [PubMed]
- 6.Vanik DL, Surewicz KA, Surewicz WK. Molecular basis of barriers for interspecies transmissibility of mammalian prions. Mol. Cell. 2004;14(1):139–145. [DOI] [PubMed]
- 7.Telling GC, Parchi P, DeArmond SJ, Cortelli P, Montagna P, Gabizon R, et al. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274(5295):2079–2082. [DOI] [PubMed]
- 8.Peretz D, Williamson RA, Legname G, Matsunaga Y, Vergara J, Burton DR, et al. A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron. 2002;34(6):921–932. [DOI] [PubMed]
- 9.Safar J, Wille H, Itri V, Groth D, Serban H, Torchia M, et al. Eight prion strains have PrP(Sc) molecules with different conformations. Nat. Med. 1998;4(10):1157–1165. [DOI] [PubMed]
- 10.Prusiner SB. Some speculations about prions, amyloid, and Alzheimer's disease. N. Engl. J. Med. 1984;310(10):661–663. [DOI] [PubMed]
- 11.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349(6311):704–706. [DOI] [PubMed]
- 12.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375(6534):754–760. [DOI] [PubMed]
- 13.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269(5226):973–977. [DOI] [PubMed]
- 14.Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 1985;82(13):4531–4534. [DOI] [PMC free article] [PubMed]
- 15.Meyer-Luehmann M. Exogenous Induction of Cerebral-Amyloidogenesis Is Governed by Agent and Host. Science. 2006;313(5794):1781–1784. [DOI] [PubMed]
- 16.Kane MD, Lipinski WJ, Callahan MJ, Bian F, Durham RA, Schwarz RD, et al. Evidence for seeding of beta-amyloid by intracerebral infusion of Alzheimer brain extracts in beta-amyloid precursor protein-transgenic mice. Journal of Neuroscience. 2000;20(10):3606–3611. [DOI] [PMC free article] [PubMed]
- 17.Stöhr J, Watts JC, Mensinger ZL, Oehler A, Grillo SK, DeArmond SJ, et al. Purified and synthetic Alzheimer's amyloid beta (Aβ) prions. Proc. Natl. Acad. Sci. U.S.A. 2012;109(27):11025–11030. [DOI] [PMC free article] [PubMed]
- 18.Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, Kösel S, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat. Genet. 1998;18(2):106–108. [DOI] [PubMed]
- 19.Zarranz JJ, Alegre J, Gómez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55(2):164–173. [DOI] [PubMed]
- 20.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–2047. [DOI] [PubMed]
- 21.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841. [DOI] [PubMed]
- 22.Braak H, Del Tredici K, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 2003;24(2):197–211. [DOI] [PubMed]
- 23.Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann. Neurol. 2003;54(3):403–414. [DOI] [PubMed]
- 24.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N. Engl. J. Med. 2001;344(10):710–719. [DOI] [PubMed]
- 25.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat. Med. 2008;14(5):504–506. [DOI] [PubMed]
- 26.Li J-Y, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat. Med. 2008;14(5):501–503. [DOI] [PubMed]
- 27.Kordower JH, Rosenstein JM, Collier TJ, Burke MA, Chen EY, Li JM, et al. Functional fetal nigral grafts in a patient with Parkinson's disease: chemoanatomic, ultrastructural, and metabolic studies. J. Comp. Neurol. 1996;370(2):203–230. [DOI] [PubMed]
- 28.Kordower JH, Styren S, Clarke M, DeKosky ST, Olanow CW, Freeman TB. Fetal grafting for Parkinson's disease: expression of immune markers in two patients with functional fetal nigral implants. Cell Transplant. 1997;6(3):213–219. [DOI] [PubMed]
- 29.Kordower JH, Freeman TB, Snow BJ, Vingerhoets FJ, Mufson EJ, Sanberg PR, et al. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with Parkinson's disease. N. Engl. J. Med. 1995;332(17):1118–1124. [DOI] [PubMed]
- 30.Angot E, Steiner JA, Lema Tomé CM, Ekström P, Mattsson B, Björklund A, et al. Alpha-Synuclein Cell-to-Cell Transfer and Seeding in Grafted Dopaminergic Neurons In Vivo. PLoS ONE. 2012;7(6):e39465. [DOI] [PMC free article] [PubMed]
- 31.Kordower JH, Dodiya HB, Kordower AM, Terpstra B, Paumier K, Madhavan L, et al. Transfer of host-derived alpha synuclein to grafted dopaminergic neurons in rat. Neurobiology of Disease. 2011;43(3):552–557. [DOI] [PMC free article] [PubMed]
- 32.Hansen C, Angot E, Bergström A-L, Steiner JA, Pieri L, Paul G, et al. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 2011;121(2):715–725. [DOI] [PMC free article] [PubMed]
- 33.Desplats P, Lee H-J, Bae E-J, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. U.S.A. 2009;106(31):13010–13015. [DOI] [PMC free article] [PubMed]
- 34.Luk KC, Kehm VM, Zhang B, O'Brien P, Trojanowski JQ, Lee VMY. Intracerebral inoculation of pathological-synuclein initiates a rapidly progressive neurodegenerative-synucleinopathy in mice. Journal of Experimental Medicine. 2012;209(5):975–986. [DOI] [PMC free article] [PubMed]
- 35.Luk KC, Kehm V, Carroll J, Zhang B, O'Brien P, Trojanowski JQ, et al. Pathological-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science. 2012;338(6109):949–953. [DOI] [PMC free article] [PubMed]
- 36.Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001;24:1121–1159. [DOI] [PubMed]
- 37.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 38.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16(3):271–8; discussion 278–84. [DOI] [PubMed]
- 39.Frost B, Jacks RL, Diamond MI. Propagation of tau misfolding from the outside to the inside of a cell. J. Biol. Chem. 2009;284(19):12845–12852. [DOI] [PMC free article] [PubMed]
- 40.Guo JL, Lee VMY. Seeding of normal Tau by pathological Tau conformers drives pathogenesis of Alzheimer-like tangles. J. Biol. Chem. 2011;286(17):15317–15331. [DOI] [PMC free article] [PubMed]
- 41.Nonaka T, Watanabe ST, Iwatsubo T, Hasegawa M. Seeded aggregation and toxicity of -synuclein and tau: cellular models of neurodegenerative diseases. Journal of Biological Chemistry. 2010;285(45):34885–34898. [DOI] [PMC free article] [PubMed]
- 42.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of tau aggregation by fibrillar species. Journal of Biological Chemistry. 2012;287(23):19440–19451. [DOI] [PMC free article] [PubMed]
- 43.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11(7):909–913. [DOI] [PMC free article] [PubMed]
- 44.de Calignon A, Polydoro M, Suárez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, et al. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73(4):685–697. [DOI] [PMC free article] [PubMed]
- 45.Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, et al. Trans-synaptic spread of tau pathology in vivo. PLoS ONE. 2012;7(2):e31302. [DOI] [PMC free article] [PubMed]
- 46.Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VMY. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer's-like tauopathy. Journal of Neuroscience. 2013;33(3):1024–1037. [DOI] [PMC free article] [PubMed]
- 47.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed]
- 48.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447(7140):83–86. [DOI] [PubMed]
- 49.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex. 2008;19(1):72–78. [DOI] [PMC free article] [PubMed]
- 50.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62(1):42–52. [DOI] [PMC free article] [PubMed]
- 51.Zhou J, Gennatas ED, Kramer JH, Miller BL, Seeley WW. Predicting regional neurodegeneration from the healthy brain functional connectome. Neuron. 2012;73(6):1216–1227. [DOI] [PMC free article] [PubMed]
- 52.Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. Journal of Neuroscience. 2011;31(37):13110–13117. [DOI] [PMC free article] [PubMed]
- 53.Plouffe V, Mohamed N-V, Rivest-McGraw J, Bertrand J, Lauzon M, Leclerc N. Hyperphosphorylation and cleavage at D421 enhance tau secretion. PLoS ONE. 2012;7(5):e36873. [DOI] [PMC free article] [PubMed]
- 54.Chai X, Dage JL, Citron M. Constitutive secretion of tau protein by an unconventional mechanism. Neurobiology of Disease. 2012;48(3):356–366. [DOI] [PubMed]
- 55.Pooler AM, Phillips EC, Lau DHW, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013. [DOI] [PMC free article] [PubMed]
- 56.Simón D, García-García E, Gómez-Ramos A, Falcón-Pérez JM, Díaz-Hernández M, Hernández F, et al. Tau overexpression results in its secretion via membrane vesicles. Neurodegenerative Dis. 2012;10(1–4):73–75. [DOI] [PubMed]
- 57.Lee S, Kim W, Li Z, Hall GF. Accumulation of vesicle-associated human tau in distal dendrites drives degeneration and tau secretion in an in situ cellular tauopathy model. International Journal of Alzheimer's Disease. 2012;2012(8504):1–16. [DOI] [PMC free article] [PubMed]
- 58.Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early alzheimer disease. journal of biological chemistry. 2012;287(6):3842–3849. [DOI] [PMC free article] [PubMed]
- 59.El-Agnaf OMA. Detection of oligomeric forms of -synuclein protein in human plasma as a potential biomarker for Parkinson's disease. FASEB J. 2006;20(3):419–425. [DOI] [PubMed]
- 60.El-Agnaf OMA. -Synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003. [DOI] [PubMed]
- 61.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, et al. Cell-produced -synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. Journal of Neuroscience. 2010;30(20):6838–6851. [DOI] [PMC free article] [PubMed]
- 62.Lee HJ. Intravesicular localization and exocytosis of -synuclein and its aggregates. Journal of Neuroscience. 2005;25(25):6016–6024. [DOI] [PMC free article] [PubMed]
- 63.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegeneration. 2012;7(1):1–1. [DOI] [PMC free article] [PubMed]
- 64.Lee H-J, Suk J-E, Bae E-J, Lee J-H, Paik SR, Lee S-J. Assembly-dependent endocytosis and clearance of extracellular α-synuclein. The International Journal of Biochemistry & Cell Biology. 2008;40(9):1835–1849. [DOI] [PubMed]
- 65.Freundt EC, Maynard N, Clancy EK, Roy S, Bousset L, Sourigues Y, et al. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Ann. Neurol. 2012;72(4):517–524. [DOI] [PMC free article] [PubMed]
- 66.Wu JW, Herman M, Liu L, Simoes S, Acker CM, Figueroa H, et al. Small misfolded tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J. Biol. Chem. 2013;288(3):1856–1870. [DOI] [PMC free article] [PubMed]
- 67.Brooks BR. The role of axonal transport in neurodegenerative disease spread: a meta-analysis of experimental and clinical poliomyelitis compares with amyotrophic lateral sclerosis. Can J Neurol Sci. 1991;18(3 Suppl):435–438. [DOI] [PubMed]
- 68.Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007;68(19):1571–1575. [DOI] [PubMed]
- 69.Ravits J, Laurie P, Fan Y, Moore DH. Implications of ALS focality: rostral-caudal distribution of lower motor neuron loss postmortem. Neurology. 2007;68(19):1576–1582. [DOI] [PubMed]
- 70.Shibata N, Hirano A, Kobayashi M, Siddique T, Deng HX, Hung WY, et al. Intense superoxide dismutase-1 immunoreactivity in intracytoplasmic hyaline inclusions of familial amyotrophic lateral sclerosis with posterior column involvement. J. Neuropathol. Exp. Neurol. 1996;55(4):481–490. [DOI] [PubMed]
- 71.Kato S, Takikawa M, Nakashima K, Hirano A, Cleveland DW, Kusaka H, et al. New consensus research on neuropathological aspects of familial amyotrophic lateral sclerosis with superoxide dismutase 1 (SOD1) gene mutations: inclusions containing SOD1 in neurons and astrocytes. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1(3):163–184. [DOI] [PubMed]
- 72.Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208–1211. [DOI] [PMC free article] [PubMed]
- 73.Kwiatkowski TJ, Bosco DA, LeClerc AL, Tamrazian E, Vanderburg CR, Russ C, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. [DOI] [PubMed]
- 74.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–1672. [DOI] [PMC free article] [PubMed]
- 75.Jacobsson J, Jonsson PA, Andersen PM, Forsgren L, Marklund SL. Superoxide dismutase in CSF from amyotrophic lateral sclerosis patients with and without CuZn-superoxide dismutase mutations. Brain. 2001;124(Pt 7):1461–1466. [DOI] [PubMed]
- 76.Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien J-P. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2005;9(1):108–118. [DOI] [PubMed]
- 77.Münch C, O'Brien J, Bertolotti A. Prion-like propagation of mutant superoxide dismutase-1 misfolding in neuronal cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108(9):3548–3553. [DOI] [PMC free article] [PubMed]
- 78.Chia R, Tattum MH, Jones S, Collinge J, Fisher EMC, Jackson GS. Superoxide dismutase 1 and tgSOD1G93A mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PLoS ONE. 2010;5(5):e10627. [DOI] [PMC free article] [PubMed]
- 79.Duyao M, Ambrose C, Myers R, Novelletto A, Persichetti F, Frontali M, et al. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat. Genet. 1993;4(4):387–392. [DOI] [PubMed]
- 80.Trevino RS, Lauckner JE, Sourigues Y, Pearce MM, Bousset L, Melki R, et al. Fibrillar structure and charge determine the interaction of polyglutamine protein aggregates with the cell surface. Journal of Biological Chemistry. 2012;287(35):29722–29728. [DOI] [PMC free article] [PubMed]
- 81.Ren P-H, Lauckner JE, Kachirskaia I, Heuser JE, Melki R, Kopito RR. Cytoplasmic penetration and persistent infection of mammalian cells by polyglutamine aggregates. Nat Cell Biol. 2009;11(2):219–225. [DOI] [PMC free article] [PubMed]
- 82.Pfeifer A, Eigenbrod S, Al-Khadra S, Hofmann A, Mitteregger G, Moser M, et al. Lentivector-mediated RNAi efficiently suppresses prion protein and prolongs survival of scrapie-infected mice. J. Clin. Invest. 2006;116(12):3204–3210. [DOI] [PMC free article] [PubMed]
- 83.Nazor Friberg K, Hung G, Wancewicz E, Giles K, Black C, Freier S, et al. Intracerebral infusion of antisense oligonucleotides into prion-infected mice. Mol Ther Nucleic Acids. 2012;1(2):e9. [DOI] [PMC free article] [PubMed]
- 84.Castanotto D, Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457(7228):426–433. [DOI] [PMC free article] [PubMed]
- 85.Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, et al. Sustained therapeutic reversal of Huntington's disease by transient repression of huntingtin synthesis. Neuron. 2012;74(6):1031–1044. [DOI] [PMC free article] [PubMed]
- 86.Seyhan AA. RNAi: a potential new class of therapeutic for human genetic disease. Hum Genet. 2011;130(5):583–605. [DOI] [PubMed]
- 87.Winer L. SOD1 in cerebral spinal fluid as a pharmacodynamic marker for antisense oligonucleotide therapy SOD1 in CSF as a pharmacodynamic marker. JAMA Neurol. 2013;70(2):201. [DOI] [PMC free article] [PubMed]
- 88.Reixach N, Deechongkit S, Jiang X, Kelly JW, Buxbaum JN. Tissue damage in the amyloidoses: transthyretin monomers and nonnative oligomers are the major cytotoxic species in tissue culture. Proc. Natl. Acad. Sci. U.S.A. 2004;101(9):2817–2822. [DOI] [PMC free article] [PubMed]
- 89.Andersson K, Olofsson A, Nielsen EH, Svehag S-E, Lundgren E. Only amyloidogenic intermediates of transthyretin induce apoptosis. Biochemical and Biophysical Research Communications. 2002;294(2):309–314. [DOI] [PubMed]
- 90.Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, Fleming JA, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc. Natl. Acad. Sci. U.S.A. 2012;109(24):9629–9634. [DOI] [PMC free article] [PubMed]
- 91.Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Plante-Bordeneuve V, Lozeron P, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: A randomized, controlled trial. Neurology. 2012;79(8):785–792. [DOI] [PMC free article] [PubMed]
- 92.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. [DOI] [PubMed]
- 93.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408(6815):982–985. [DOI] [PubMed]
- 94.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408(6815):979–982. [DOI] [PubMed]
- 95.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6(8):916–919. [DOI] [PubMed]
- 96.Wilcock DM, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, et al. Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. Journal of Neuroscience. 2004;24(27):6144–6151. [DOI] [PMC free article] [PubMed]
- 97.Orgogozo J-M, Gilman S, Dartigues J-F, Laurent B, Puel M, Kirby LC, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. [DOI] [PubMed]
- 98.Buckwalter MS, Coleman BS, Buttini M, Barbour R, Schenk D, Games D, et al. Increased T cell recruitment to the CNS after amyloid beta 1–42 immunization in Alzheimer's mice overproducing transforming growth factor-beta 1. Journal of Neuroscience. 2006;26(44):11437–11441. [DOI] [PMC free article] [PubMed]
- 99.Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of A immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64(9):1553–1562. [DOI] [PubMed]
- 100.Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, et al. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer's disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11(7):597–604. [DOI] [PubMed]
- 101.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–2070. [DOI] [PMC free article] [PubMed]
- 102.Gerald Z, Ockert W. Alzheimer's disease market: hope deferred. Nature. 2013;12(1):19–20. [DOI] [PubMed]
- 103.Mullard A. Sting of Alzheimer's failures offset by upcoming prevention trials. Nat Rev Drug Discov. 2012;11(9):657–660. [DOI] [PubMed]
- 104.Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp. Neurol. 2010;224(2):472–485. [DOI] [PubMed]
- 105.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. Journal of Neuroscience. 2007;27(34):9115–9129. [DOI] [PMC free article] [PubMed]
- 106.Troquier L, Caillierez R, Burnouf S, Fernandez-Gomez FJ, Grosjean M-E, Zommer N, et al. Targeting phospho-Ser422 by active Tau Immunotherapy in the THYTau22 mouse model: a suitable therapeutic approach. Curr Alzheimer Res. 2012;9(4):397–405. [DOI] [PMC free article] [PubMed]
- 107.Bi M, Ittner A, Ke YD, Götz J, Ittner LM. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS ONE. 2011;6(12):e26860. doi: 10.1371/journal.pone.0026860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J. Neurochem. 2011;118(4):658–667. [DOI] [PMC free article] [PubMed]
- 109.Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, et al. Antibody-aided clearance of extracellular -synuclein prevents cell-to-cell aggregate transmission. Journal of Neuroscience. 2012;32(39):13454–13469. [DOI] [PMC free article] [PubMed]
- 110.Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS ONE. 2011;6(4):e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46(6):857–868. [DOI] [PubMed]
- 112.Schneeberger A, Mandler M, Mattner F, Schmidt W. Vaccination for Parkinson's disease. Parkinsonism Relat. Disord. 2012;18 Suppl 1:S11–3. [DOI] [PubMed]
- 113.Magalhães AC, Baron GS, Lee KS, Steele-Mortimer O, Dorward D, Prado MAM, et al. Uptake and neuritic transport of scrapie prion protein coincident with infection of neuronal cells. Journal of Neuroscience. 2005;25(21):5207–5216. [DOI] [PMC free article] [PubMed]
- 114.Kanu N, Imokawa Y, Drechsel DN, Williamson RA, Birkett CR, Bostock CJ, et al. Transfer of scrapie prion infectivity by cell contact in culture. Curr. Biol. 2002;12(7):523–530. [DOI] [PubMed]
- 115.Chandler RL. Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet. 1961;1(7191):1378–1379. [DOI] [PubMed]
- 116.Prusiner SB. Prions. Proc. Natl. Acad. Sci. U.S.A. 1998;95(23):13363–13383. [DOI] [PMC free article] [PubMed]
- 117.Legname G. Synthetic mammalian prions. Science. 2004;305(5684):673–676. [DOI] [PubMed]
- 118.Makarava N, Kovacs GG, Bocharova O, Savtchenko R, Alexeeva I, Budka H, et al. Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol. 2010;119(2):177–187. [DOI] [PMC free article] [PubMed]
- 119.Bahr BA, Hoffman KB, Yang AJ, Hess US, Glabe CG, Lynch G. Amyloid beta protein is internalized selectively by hippocampal field CA1 and causes neurons to accumulate amyloidogenic carboxyterminal fragments of the amyloid precursor protein. J. Comp. Neurol. 1998;397(1):139–147. [PubMed]
- 120.Nath S, Agholme L, Kurudenkandy FR, Granseth B, Marcusson J, Hallbeck M. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of -amyloid. Journal of Neuroscience. 2012;32(26):8767–8777. [DOI] [PMC free article] [PubMed]
- 121.Eisele YS, Obermüller U, Heilbronner G, Baumann F, Kaeser SA, Wolburg H, et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science. 2010;330(6006):980–982. [DOI] [PMC free article] [PubMed]
- 122.Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. J. Biol. Chem. 2011;286(21):18664–18672. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)