Abstract
Protein misfolding disorders, such as Alzheimer's disease and Parkinson's disease, have in common that a protein accumulates in an insoluble form in the affected tissue. The process of aggregation follows a mechanism of seeded polymerization. Although the toxic species is still not well defined, the process, rather than the end product, of fibril formation is likely the main culprit in amyloid toxicity. These findings suggest that therapeutic strategies directed against the protein misfolding cascade should focus on depleting aggregation intermediates rather than on large fibrillar aggregates. Recent studies involving natural compounds have suggested new intervention strategies. The polyphenol epi-gallocatechine-3-gallate (EGCG), the main polyphenol in Camilla sinensis, binds directly to a large number of proteins that are involved in protein misfolding diseases and inhibits their fibrillization. Instead, it promotes the formation of stable, spherical aggregates. These spherical aggregates are not cytotoxic, have a lower β-sheet content than fibrils, and do not catalyze fibril formation. Correspondingly, epi-gallocatechine-3-gallate remodels amyloid fibrils into aggregates with the same properties. Derivatives of Orcein, which is a phenoxazine dye that can be isolated from the lichen Roccella tinctoria, form a second promising class of natural compounds. They accelerate fibril formation of the Alzheimer’s disease-related amyloid-beta peptide. At the same time these compounds deplete oligomeric and protofibrillar forms of the peptide. These compounds may serve as proof-of-principle for the strategies of promoting and redirecting fibril formation. Both may emerge as two promising new therapeutic approaches to intervening into protein misfolding processes.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0192-7) contains supplementary material, which is available to authorized users.
Keywords: Amyloid, Mechanism, Therapy, Polyphenol, EGCG, Orcein, Alzheimer, Parkinson
Many diseases that occur mostly with age are caused by protein misfolding. These include the most common neurodegenerative disorders, Alzheimer's disease (AD) and Parkinson's disease (PD), as well as systemic disorders in which the peripheral organs are serum amyloid A affected. Examples of these are amyloid light chain and amyloidoses and adult-onset (type II) diabetes. These disorders all have in common that a protein accumulates in an insoluble form in the affected tissue. The location of the deposits and the identity of the proteins are characteristic for each particular disorder and the deposited proteins share no obvious sequence homologies [1]. However, they do share two characteristics: the majority of the affected proteins or protein fragments are at least partially unfolded under physiological conditions [2], and all of them share a characteristic intramolecular cross-beta sheet conformation that results in the formation of insoluble fibrillar structures. These structures yield a characteristic pattern in powder X-ray diffraction measurements, reflecting the two characteristic atomic distances, 4.7 and ∼10 Å, of the cross-beta structure [3, 4]. Although the term “amyloid” has historically been defined in the context of histology, it will be used in this review as shorthand for all protein aggregates that share these structural properties.
AD, first described by Alois Alzheimer in 1907 [5] is the most frequent protein misfolding disease and, with 24 million cases per year worldwide, one of the most frequent aging-related diseases [6]. Extracelluar protein deposits in AD are formed by products of an alternative proteolytic processing of the amyloid precursor protein (APP). APP processing by beta- and gamma-secretase produces amyloid beta (Aβ) peptides of 36–43 amino acids [7], of which the 40-amino acid peptide Aβ 40 is the most abundant, with a total brain concentration of ∼1 nM, whereas the 42-amino acid peptide (Aβ 42) is formed at a 10-fold lower concentration, but has a higher aggregation propensity than Aβ 40 [8, 9]. Intraneuronal deposits in the form of neurofibrillary tangles are formed by the microtubule-associated protein tau [10, 11]. Tau found in neurofibrillary tangles is highly phosphorylated [12]. Mutations in the APP gene and in the APP processing proteins can increase Aβ 42 production leading to early-onset AD [13]. However, only a small percentage of AD cases can be traced to specific mutations. The second most frequent protein misfolding disease, PD, is characterized by the deposition of misfolded proteins in dopaminergic neurons of the substantia nigra [14]. These deposits primarily contain the alpha-synuclein protein (αS), but have tau and Aβ as secondary components. A number of autosomal dominant mutations in hereditary PD document the central role of alpha-synuclein protein in PD (for a review see [15]).
The process of amyloid formation in AD, PD, and other protein misfolding diseases follows a mechanism of seeded polymerization that has been compared to one-dimensional crystal growth [16–18]. The process is characterized by a lag phase, in which monomeric, di-, and oligomeric forms of the protein are in kinetic equilibrium, as oligomer formation is energetically unfavorable. Aggregates of a certain size or conformation, however, stabilize and catalyze the addition of monomers, which rapidly leads to the formation of large fibrillar aggregates [19, 20]. Aggregation processes in vivo are likely not as straightforward, but may involve numerous alternative pathways, in which oligomeric and protofibrillar aggregates are formed that may or may not be able to nucleate amyloid formation.
Secondary nucleation processes have a major effect on the kinetics of amyloid formation both in vitro and in vivo. In secondary nucleation, fibrillar aggregates that were initially formed in the aggregation pathway can nucleate further fibril growth if a mechanism exists to break long fibrils into shorter fragments [21]. These fragments can seed further fibril growth, thus turning a linear polymerization process into an autocatalytic replication of the amyloid structure [22]. This process can be replicated in vitro by subjecting prions to a cyclic process of fibril fragmentation and fibril growth, demonstrating that autocatalytic seeded aggregation is the molecular basis of prion replication [23, 24]. Recent data indicate that prion-like replication can occur in many, if not all, amyloid diseases [25, 26]. Inoculating young AD model mice with brain homogenate from late-stage disease mice accelerates their disease progression [27]. It is believed that internalization of amyloid aggregates and subsequent replication within the cell are crucial to the spread of amyloid pathology between cells and within tissues [25]. In cellular models, aggregates of the tau protein can spread between cells and accelerate tau aggregation in neighboring cells [28, 29].
Toxicity and Amyloid Formation
While macroscopic deposits of misfolded insoluble protein aggregates characterize protein misfolding diseases, their connection to cellular toxicity is not well understood. Alternative hypotheses of how protein misfolding induces toxicity include: 1) loss-of-function as the conversion to a misfolded conformation reduces the pool of functional protein; 2) disruption of cellular membranes; 3) disruption of mitochondrial function and generation of reactive oxygen species; and 4) overloading the regulatory networks of protein homeostasis [1, 30].
These mechanisms are not mutually exclusive, and all of them may contribute to amyloid toxicity. Although the toxic species is still poorly defined, there is growing consensus that the process, rather than the end product, of amyloid formation is the main culprit in amyloid toxicity[31–34]. Small oligomers of Aβ secreted from cell lines that overproduce Aβ 42 inhibit long-term potentiation in hippocampal synaptic transmission [32] and induces neuronal toxicity in mice [35]. Oligomeric forms of αS and Aβ inhibit mitochondrial activity [31, 36]. Toxicity of Aβ 42 aggregates ebbs as monomers are depleted, but can be restored by the addition of fresh monomeric protein [31].
These findings suggest that therapeutic strategies that target the amyloid formation process should focus on depleting aggregation intermediates such as oligomers and protofibrils, rather than on large fibrillar aggregates, and should also prevent the growth of these intermediate species. Alternative therapeutic strategies against amyloid toxicity could increase proteostatic capacity or target reactive oxygen species (ROS). It has been described that deposition of misfolded proteins increases the amount of ROS [37], by causing inflammation in neuroglia, which, in turn, catalyzes ROS formation [38, 39]. Aβ, αS, and PrP also complex metal ions, such as FeII and CuI, that strongly catalyze ROS formation [40, 41]. Concentrations of CuI and FeII are in amyloid plaques are about twice as high as in surrounding tissues [42].
Human amyloid deposits are rich in methionine and tryptophan [43]. Mouse Aβ, which lacks histidine and tyrosine, is less prone to aggregation than human Aβ [44]. Methionine and tyrosine are easily modified by ROS. Oxidation of methionine 35 increases Aβ aggregation propensity [45] and tri- and tetrameric Aβ oligomers that are cross-linked by radical-induced di-tyrosine bridges are toxic to cells [36]. It is not clear whether oxidative stress and the generation of ROS is the primary pathologic event or whether it is a consequence of protein misfolding [46]. However, both processes may well form a vicious cycle in which either process accelerates the other, and both may cause cellular toxicity in AD, PD, and other protein misfolding diseases via multiple pathways.
Strategies for Intervention into the Amyloid Formation Cascade
Therapeutic approaches to AD or PD are, for the most part, still directed toward treating the symptoms of neurodegeneration. Approaches that target the underlying mechanisms of proteotoxicity are currently in the developmental stage, i.e., under clinical or preclinical evaluation [47, 48]. Recent studies involving natural compounds have suggested intervention strategies that target the protein misfolding cascade in new ways. This review will focus on catechins, especially the polyphenol epi-gallocatechine-3-gallate (EGCG), and phenoxazines, such as orcein and related compounds. The pharmacological intervention strategies discussed in this review are primarily directed against the process of protein misfolding and amyloid formation occurring in AD and PD. It should be noted, however, that many of the molecules discussed also have antioxidant characteristics. This is especially true for the polyphenol EGCG, which is a potent antioxidant that can also complex iron and copper ions and thus neutralize their catalytic effects in the production of ROS. The mechanisms involved in this activity have been described extensively elsewhere [41, 49, 50] .
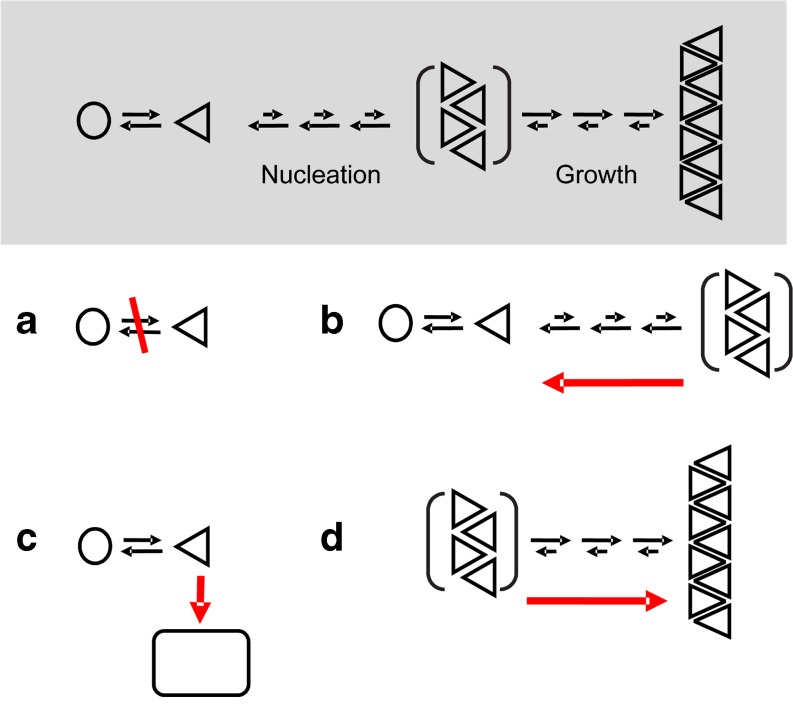
Multiple lines of evidence suggest that oligomeric intermediate stages of the amyloid cascade are the main culprits for the toxicity of misfolded proteins [31, 51]. Different strategies can be applied to reduce the amount of toxic oligomers (Fig. 1a). The first strategy is directed toward cutting off the supply of aggregation-competent monomeric protein; an example of this is the use of β-secretase inhibitors [52, 53] that block the formation of the Aβ peptide via cleavage of APP. A second approach is the inhibition of aggregation with substances that bind to and destabilize the amyloid structure (Fig. 1b). A detailed review of this approach is provided by Cohen and Kelly [54]. This review will examine two alternative strategies for reducing toxicity in protein misfolding: redirection of the amyloid cascade towards nontoxic aggregates (Fig. 1c) and the stabilization of large amyloid fibrils (Fig. 1d). Both approaches reduce the concentration of aggregation intermediates by shifting the equilibrium of the aggregation reaction to other, less toxic aggregate species.
Fig. 1.
Intervention strategies protein misfolding disorders. a Preventing formation of the aggregation competent monomers; b disaggregation of aggregation intermediates; c derailing amyloid formation; d accelerating formation of large fibrillar aggregates
Natural Compounds in Amyloid Intervention
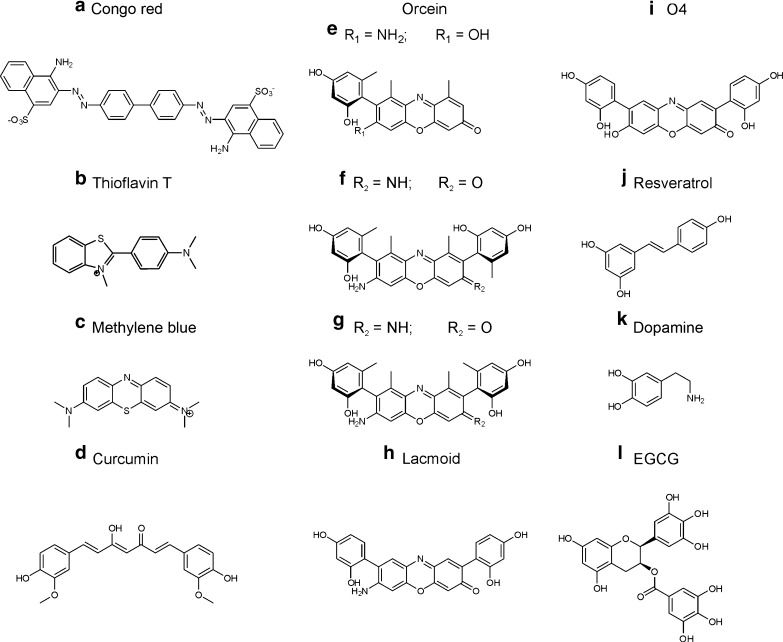
When analyzing the results of studies aimed at finding small molecules that can modulate amyloid formation, one cannot help but notice that natural compounds figure prominently. Many of these fall into either or both of the categories of polyaromatic dyes and antioxidants. The oldest dye that is used to stain amyloid deposits in histology is Congo red (Fig. 2a) [55]. At high concentrations, Congo red inhibits amyloid formation [56]. Its toxicity, however, limits therapeutic application. Dyes that inhibit amyloid formation include thioflavin T (Fig. 2b) [57], methylene blue (Fig. 2c) [58, 59], curcumin (Fig. 2d) [60]) and orcein (Fig. 2e–g) and its derivatives (Fig. 2h, i) [61], which will be discussed in more detail later. The class of antioxidants is mainly represented by polycyclic polyphenols. Curcumin (Fig. 2d), resveratrol (Fig. 2j) [62], dopamine (Fig. 2k) [63], and catechins of the tea plant Camilla sinensis fall into this category. In the following, EGCG (Fig. 2l), the most abundant of these catechins, will be discussed in detail.
Fig. 2.
Anti-amyloid natural compounds, many of which are polyaromatic polyphenols
New Strategies for Intervention in Amyloidogenesis: EGCG
Catechins from tea leaves and grape seeds, especially EGCG, have been a focus of intensive research for more than a decade. Tea leaves consist of 30 % catechins, one third of which is EGCG [64]. In addition to its antioxidant properties, EGCG shows promising effects on tumor growth in cell and mouse models. Thus, a possible application in cancer therapy has been extensively investigated. The various areas of potential EGCG applications have been summarized in [65].
A variety of epidemiologic data, as well as several experimental studies in cell and animal models of AD and PD, point to a beneficial effect of green tea, green tea extracts, or EGCG. EGCG reduced the toxicity of Aβ in pheochromocytoma and neuroblastoma cell models [66, 67]. Green tea extract decreased the formation of amyloid deposits [68, 69] and the loss of spatial learning ability in a mouse model of AD [70]. In a rat model, there was an increase in learning ability with long-term EGCG treatment [71]. EGCG or green tea extract inhibit the loss of dopaminergic neurons in the substantia nigra in animal models of PD [72]. Epidemiologic studies, in which patients were questioned about their lifestyles over many years, found a negative correlation between the consumption of green tea and loss of cognitive function [73]. The incidence of Parkinsonism is 5- to 10-fold lower in Asia than in Western societies [74]. Finally, the first clinical pilot studies demonstrated a reduction in light chain amyloid deposition and the thickness of the cardiac septum in myeloma patients after the intake of green tea extract [75, 76].
Mechanism of EGCG Intervention
Alternative hypotheses for the protective mechanism of EGCG on protein misfolding diseases have been discussed. Early hypotheses centered on the antioxidant activity of EGCG [49]. It has also been described that EGCG increases the degradation of APP into non-amyloidogenic peptides via α-secretase [77, 78] and inhibits the activity of β-secretase [79].
Recent evidence suggests that direct interaction of EGCG with misfolded proteins makes a major contribution to the beneficial effect of EGCG, if not being its primary mechanism of action. In 2006, Ehrnhoefer et al. [80] found that EGCG directly inhibits the aggregation of the huntingtin protein [80]. Since then, it has been shown that EGCG binds directly to a large number of proteins that are involved in protein misfolding diseases and that EGCG inhibits their fibrillization. Examples include αS [81, 82], Aβ [36, 82], transthyretin [83], lysozyme [84], the prion protein PrP [85], and the yeast prion Sup35 [86].
EGCG binds strongly to unfolded proteins [82, 87]. Examination by nuclear magnetic resonance (NMR) showed that resonances of 30 % of the amino acids were rapidly lost after incubation of monomeric αS with EGCG [82]. This finding would suggest a nonspecific interaction of EGCG with the peptide backbone or, possibly, with hydrophobic residues of the polypeptide chain. The binding could occur through hydrophobic interactions or through H-bonding. EGCG binds to the Aβ peptide via hydrophobic sequences at amino acids 14–24 and 27–37 [88, 89]. EGCG binding inhibits the formation of the β-sheet in the first β-sheet region (amino acids 14–24) [90]. Formation of this β-sheet is thought to initiate the formation of amyloid [91]. EGCG can also bind to the hydrophobic region of folded proteins, such as albumin [92] or transthyretin [93]. Several defined binding sites were localized on exposed hydrophobic residues of the proteins. The binding energy has an entropic, as well as an enthalpic, component, which indicates that stabilization of H-bonds occurs in addition to the hydrophobic interactions [92]. A thermodynamic analysis of the interaction of EGCG with Aβ40 confirmed that there are entropic and enthalpic contributions to the binding [87]. While most studies suggest that EGCG binding is mediated by hydrophobic and π stacking interactions reversible covalent conjugation, for example via the formation of Schiff bases, has also been discussed [94].
The analysis of aggregation reactions in the presence and absence of EGCG demonstrated that EGCG inhibits the formation of fibrillar aggregates that bind to amyloid dyes, such as Congo red or thioflavin T. Instead, in the case of αS, Aβ, and most other amyloidogenic proteins, stable, spherical aggregates are formed (Fig. 2) [82]. These spherical aggregates are not cytotoxic and have a lower β-sheet content than fibrils [90]. In particular, they lack the characteristic property of amyloid fibrils to catalyze their own formation. EGCG-induced aggregates do not seed fibril formation when they are added to a solution of monomeric Aβ or αS [82]. As EGCG aggregates cannot function as aggregation nuclei, they are, by definition, off-pathway in relation to the amyloid cascade (Fig. 2).
The general mechanism of EGCG has now been confirmed in various misfolded protein systems [81, 95, 96] and the yeast prion Sup35 [86]. However, mechanistic details seem to depend on the stability of the protein aggregates that are formed with the aid of EGCG. Carboxymethylated casein forms fibrils with β-sheet structure that bind to the amyloid dye thioflavin T; it has, therefore, been used as a generic model system for amyloid formation [97]. EGCG inhibits the fibril formation of casein through its binding to a β-sheet-turn-sheet motif of the protein [98]. The protein remains in solution as a monomer after EGCG binding. The casein model, however, is atypical for amyloidogenic proteins in that the β-sheet–turn–sheet motif structure, which is characteristic of many amyloid fibrils [99], is already present in the monomeric form of the protein [98]. In contrast, the Aβ peptide, as well as most other proteins, forms these structures during aggregation into oligomeric and protofibrillar intermediates of the amyloid cascade [100].
Disassembly and Remodeling of Amyloid Deposits
Hauber et al. [101] demonstrated that EGCG inhibits the formation of amyloid-like fibrils of a fragment of prostatic acidic phosphatase and that it dissolves fibrils that have already formed. The fibrils of this semen-derived enhancer of virus infection bind to human immunodeficiency virus and facilitate its entry into the body through mucous membranes [102]; here, a local application of EGCG helps to prevent human immunodeficiency virus infection [101]. Disassembly or transformation of fibrils by EGCG has also been observed for several other amyloid-forming proteins. After treatment with EGCG, the yeast prion Sup35 loses its ability to replicate the fibrillar structure [86]. EGCG degrades fibrils of transthyretin [96]; fibrils of merozoite surface protein 2 are also transformed into an amorphous structure by EGCG [103]. We were able to elucidate the mechanism by which EGCG acts on αS and Aβ fibrils [104].
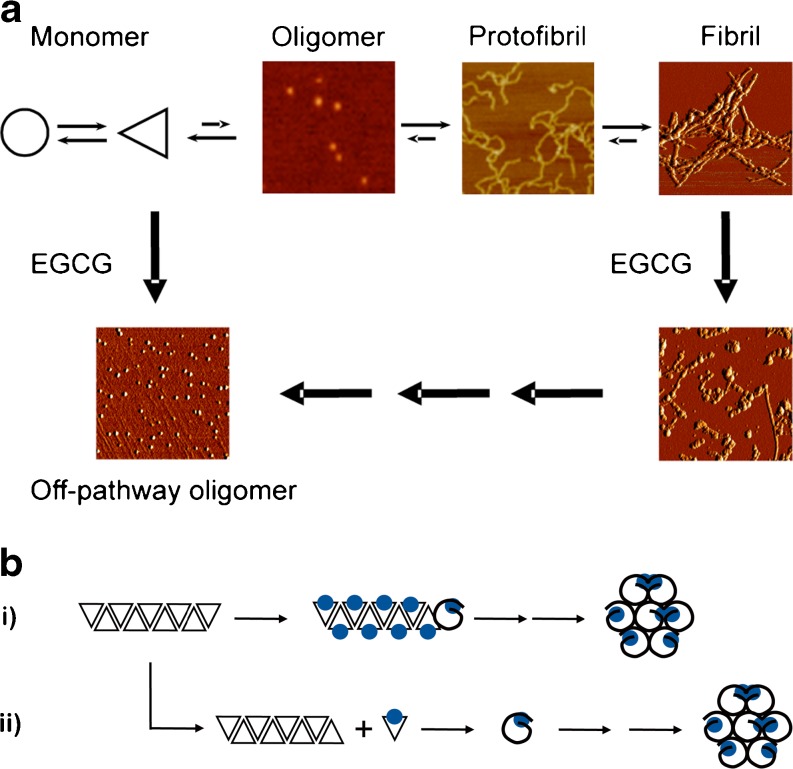
When EGCG is added to fibrils of Aβ or αS the fibrillar structure is gradually lost and sodium dodecyl sulfate (SDS)-resistant spherical or amorphous structures are formed (Fig. 3a) that do not bind to thioflavin T, do not catalyze seeding-induced aggregation, and that are not toxic in neuronal (SHSY5Y, PC12) cell models [104]. They possess the same properties as the off-pathway aggregates that are formed in the presence of monomeric αS and Aβ in the presence of EGCG. In the neuronal cell model Aβ aggregates are degraded after treatment with EGCG [104].
Fig. 3.
a Epi-gallocatechine-3-gallate (EGCG) redirects the amyloid cascade into the formation of globular off-pathway aggregates. b Alternative molecular mechanisms for the transformation of fibrillar Aβ into spherical aggregates. i = direct remodeling; ii = dissociation/re-aggregation
Mechanisms of Amyloid Fibril Remodeling
Two mechanisms may be postulated for the transformation of fibrillar into amorphous structures (Fig. 3a, b): either the fibrils are dissociated by EGCG or they are converted directly into amorphous structures, i.e., without passing through the monomer stage (Fig. 3b). Disassembly of the fibrils could be initiated by EGCG binding, or it could occur indirectly if EGCG binds to the monomeric form of the protein and shifts the equilibrium between monomeric and fibrillar phases by removing monomers irreversibly from the reaction (Fig. 3b).
In order to differentiate between these two possibilities, fluorescent markers were incorporated into Aβ fibrils [104]. If red-tagged fibrils are mixed with green-tagged fibrils, a mechanism in which dissociation of the fibrils is followed by re-aggregation should lead to amorphous aggregates containing similar amounts of both red- and green-tagged monomers. If the fibrils are transformed by EGCG in situ into amorphous structures without first being disassembled, then each structure would either be predominantly green or red. Surprisingly, this latter scenario was observed after treating Aβ with EGCG [104], indicating that EGCG does not disassemble the fibrils but rather remodels them directly into amorphous structures. The molecular details of this mechanism still need to be clarified. A plausible mechanism would begin with EGCG binding to the amino acids 14–24 β-sheet of Aβ in the fibril which could weaken the cross-β structure. Simultaneously, EGCG promotes formation of amorphous aggregates at the fibril ends through hydrophobic interactions, Π–Π stacking, or the formation of covalent networks (Fig. 3a).
Perspectives for Prevention and Therapy
The effects of EGCG in vitro were observed at low micromolar concentrations; reduction of Aβ deposits and rescue of metabolic inhibition occurred in the same concentration range [82, 89, 104]. Oral administration of EGCG (50 mg/kg body weight) to AD-model mice (tg2576) decreased plaque load and rescued cognitive performance [69]. Plasma concentrations close to 1 μM were achieved in mice after oral administration of EGCG (75 mg/kg) [105]. Oral administration of EGCG (450 mg) or green tea extract (1.2–4.5 g, corresponding to 100–300 mg EGCG) resulted in similar peak plasma concentrations in humans [106–108]. While EGCG has been recognized to be hepatotoxic in mice at concentrations of 500 mg/kg body weight and above and sporadic incidents of hepatotoxicity in humans have been reported [109, 110], daily doses of 800 mg EGCG have been used in clinical trials without adverse effects (clinical trial NCT00951834) [111].
However, a widely recognized problem in the therapeutic application of EGCG is the large variability in the bioavailability of orally administered EGCG (reviewed in [108] and [112]). This is owing to its high sensitivity to oxidation, the tendency to conjugate to proteins in the digestive tract (e.g., casein), and rapid metabolism in the body. With an especially conscientious oral regimen these problems can be overcome [76]. However, the variations in bioavailability confound the systematic evaluation of treatment outcome in clinical studies [108]. In addition, only 10–20% of the EGCG passes through the blood–brain barrier [113]. Several approaches aim to solve the problem of bioavailability; encapsulation of EGCG in nanoparticles increases its uptake and long-term bioavailability [114, 115]. The uptake of EGCG in the gastrointestinal tract can be increased via the simultaneous intake of vitamin C [116] and piperine [105]. Concomitant intake of fish oil has augmented the anti-amyloid effectiveness of EGCG in a mouse model of AD [117].
Encapsulation may also improve the cellular availability of EGCG. Enclosing EGCG in poly-(lactide-co-glycolide) nanoparticles increased its enhancing effect in chemotherapy with cisplatin compounds [118]. Enclosure in lipid nanoparticles increased the effect of EGCG on the cleavage of APP by α-secretase in neuroblastoma cells [119]. We have recently demonstrated that the transport of small molecules such as EGCG through the cell membrane can also be augmented by physical means through irradiation with a pulsed laser source in the red to the near infrared wavelength region [120]. Near infrared laser irradiation penetrates deeply into human tissue. It can stimulate mitochondrial metabolism [121] and enhance the effectiveness of EGCG in preventing the proliferation of tumor cells [120]. The combination of pulsed laser irradiation at 670 nm with the intake of EGCG decreased the amount of Aβ aggregates and improves the metabolic activity in a neuroblastoma cell model [122].
Reducing Proteotoxicity by Promoting Fibril Formation
The accumulation of extracellular amyloid plaques and intracellular neurofibrillary tangles characterizes AD pathology [123], and fibrillar protein deposits can be found in many, if not all, protein misfolding diseases [1]. However, as aggregation intermediates are the likely culprits in proteotoxicity [46], it should be possible to reduce toxicity by promoting the formation of larger fibrillar aggregates at the expense of oligomeric intermediates. We demonstrated that inhibition of the Daf-2 receptor by RNA interference, the equivalent of the insulin/insulin growth factor-receptor in Caenorhabditis elegans, reduced proteotoxicity and consequently paralysis in a C. elegans model that produced Aβ peptide in muscle cells [124]. At the same time, Aβ deposition increased when compared to the untreated control animals. Our interpretation of this finding supports the oligomer toxicity hypothesis, as we concluded that Daf-2 down-regulation likely reduced the amount of oligomeric aggregation intermediates by promoting the formation of large aggregates. Daf-2 negatively regulates Daf-16, the C. elegans homolog of the human transcription factor FOXO-3 [124], which controls the expression of a number of chaperone proteins.
While the details of the mechanistic connection between these pathways and opportunities for therapeutic intervention into the regulation of protein misfolding remain to be explored [30], the same concept can be leveraged for a direct therapeutic intervention into Aβ amyloid formation. A group of natural compounds, which can promote the formation of fibrillar Aβ, was found in a screening study in the laboratory of Erich Wanker [61]. At the same time, these compounds reduce oligomeric and protofibrillar forms of the peptide [61]. The compounds are derivatives of Orcein (Fig. 2), which, like litmus, is a phenoxazine dye that can be isolated from the lichen Roccella tinctoria [125]. Orcein is a mixture of closely related substances that share a phenoxazine backbone (Fig. 2e–g). It has been used as natural dye and food coloring, but also as a histological stain [126]. In testing a number of phenoxazines, O4 (Fig. 2i), a semi-synthetic derivative of Orcein had the strongest activity in promoting fibril formation. During extraction, the conjugated hydroxyl group of the phenoxazine backbone easily hydrolyzes into an amine (Fig. 1) [125]. Mixtures that contain either are also known as Lacmoid or Resorcin blue.
Quantitative analysis of electron micrographs finds an increase in fibrils and a decreased number of oligomeric and protofibrillar aggregates when Aβ 42 aggregates in the presence of O4 compared with untreated controls [61]. The amount of Aβ aggregates that remain insoluble when boiled in 2% SDS can be measured in a filter retardation assay as an indicator of mature amyloid fibrils [127]. SDS-resistant fibrils of Aβ 42 are formed faster in the presence of O4 than in its absence. While O4 increases the amount of mature fibrils, these fibrils show decreased inhibition metabolic activity in neuronal model cells and an increased survival of primary neurons when compared with treatment with the Aβ peptide in the absence of the compound [61]. At the same time O4 treatment rescued hippocampal long-term potentiation that is impaired by Aβ 42. The Aβ 42 fibrils that were formed in the presence of O4 were structurally similar to those formed by untreated Aβ peptide, which suggests that the compound binds to aggregated forms of the peptide and either promotes fibril growth or inhibits monomer dissociation.
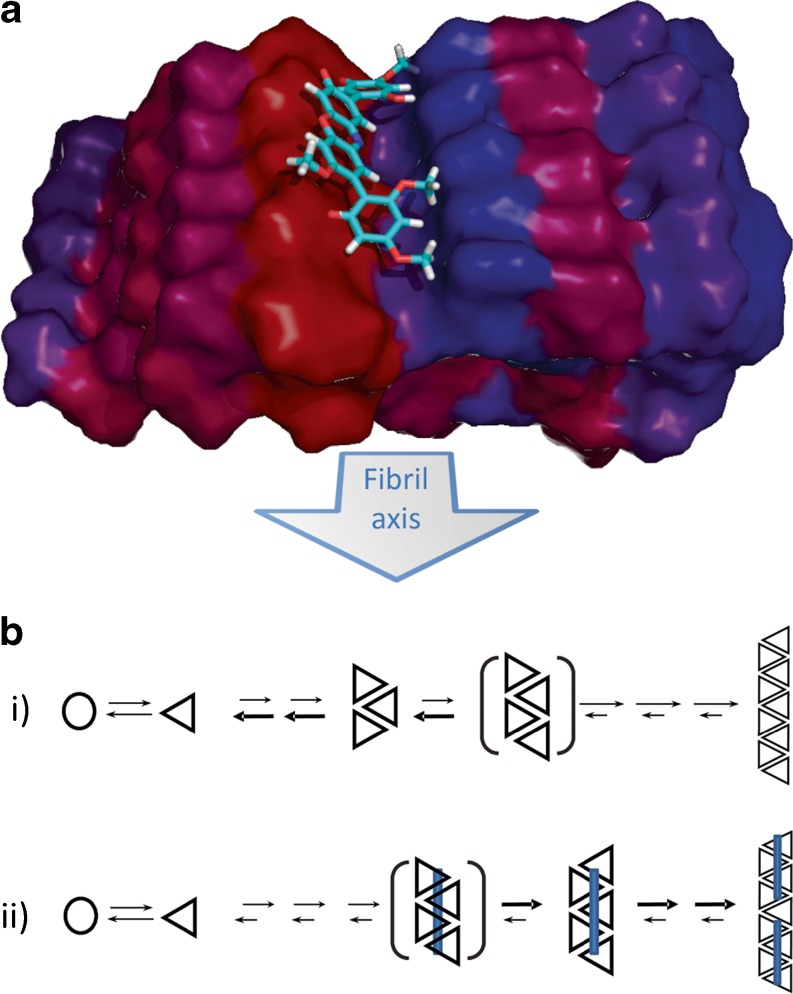
Binding of O4 to Aβ was analyzed by biochemical methods, NMR, and molecular modeling [61], which confirmed this hypothesis and resulted in the following model (Fig. 4): the small molecule binds with high affinity to oligomeric and fibrillar forms of the Aβ peptide, while affinity to the Aβ monomer is substantially lower. It preferentially interacts with two patches (amino acids 17–22 and 31–37). The combination of titration experiments, NMR, and docking models strongly suggests that O4 does not interact with a single Aβ monomer but rather binds along the fibril axis, bridging 4–5 Aβ molecules (Fig. 4a). This model strongly suggests that bridging the Aβ monomers is what inhibits monomer dissociation from the nascent fibril. Shifting the equilibrium towards monomer association promotes fibril growth (Fig. 4b). This, in turn, would shorten the lag phase and lowers the concentration of toxic oligomers.
Fig. 4.
a Model of O4 binding to fibrillar Aβ. The molecule bridges 4–5 peptides and thus stabilizes the fibril. Docking (B Grüning, S. Günther in [61]) uses the Aβ model from [99]. b Mechanistic model of intervention. Monomer dissociation exceeds growth in pre-nuclear assemblies (i), compound binding stabilizes early fibrillar aggregation intermediates and inhibits monomer dissociation (ii)
These data support the hypothesis that oligomeric forms, rather than mature fibrils, are responsible for amyloid toxicity [32]. It also fits the observation that functional amyloid, such as the Pmel17 protein that scaffolds melanin polymerization, forms fibrils much more rapidly that disease-associated proteins [128]. While the toxicity of oligomeric forms of Aβ has been established in multiple experiments in vitro [32, 51, 129], their role in pathogenesis in vivo is much less understood. In general, the deposition of macroscopic protein aggregates does track the progression of protein misfolding diseases. The amount of neurofibrillary tangles formed by the tau protein correlates with cognitive decline in AD [11]. The presence of characteristic protein deposits in brain tissue postmortem remains the definitive diagnostic proof of Creutzfeld–Jacob disease and AD [130, 131]. Drugs that have mechanisms of action similar to the Orcein derivatives could thus make a valuable contribution to validating the oligomer hypothesis in vivo. It would predict that these compounds should reduce neuronal loss and cognitive deficits, but, at the same time, increase the amount of protein deposited in large macroscopic aggregates.
There is plenty literature on a large number of small molecules, both natural and man-made, that intervene into the amyloid formation cascade (Fig. 2). Some of these have been reported to also accelerate fibril formation. Osmolytes like trimethylamine-N-oxide [132], but also short peptides [133], can accelerate the aggregation process. Likewise, scyllo-inositol stabilized fibrillar structures with high β-sheet content and low toxicity [134]. Their mechanism of action has not been fully explored yet, but it is likely that these substances also decrease the fraction of toxic aggregation intermediates. Interestingly, accelerated fibril formation was also observed in the case of methylene blue (Fig. 2c), which is structurally related to Orcein [59]. However, the authors concluded that methylene blue, unlike Orcein or O4 affects oligomer and fibril formation by two independent mechanisms.
These examples demonstrate that promoting fibril formation may emerge as a promising new therapeutic approach to intervening into protein misfolding processes. Many of the compounds studied so far are too large to effectively penetrate the blood–brain barrier, so their effectiveness in neurodegenerative diseases may be limited. Unlike green tea polyphenols, little data from animal models or from epidemiologic studies is available so far. Methylene blue showed promise in early studies on AD, which were attributed to its effect on tau aggregation [58]. Results from clinical studies, however, are inconclusive so far [135].
Conclusions
The misfolding of endogenous proteins and the formation of fibrillar structures, which are integral to the concept of amyloidogenesis, is a complex process involving the formation of many intermediate aggregate species. While the complexity of the aggregation process has confounded our understanding of the mechanisms of amyloid formation and toxicity, it also provides many potential therapeutic targets. Oligomeric intermediates play a central role in the pathology of disorders of protein misfolding. New intervention strategies involving natural compounds have emerged that target this point in the amyloid cascade. While some of these, for example, the polyphenol EGCG, show significant therapeutic potential, there are still numerous practical hurdles that have to be overcome before successful therapeutic application.
Understanding the basic mechanisms underlying pathology is the foundation on which every rational therapy is built. It offers a starting point for improving the pharmacokinetic properties of existing compounds or for the development of new substances that have the same mechanism of action as the tea polyphenols and other natural compounds that were discussed in this review. Mechanistic studies can open new treatment strategies for amyloid disorders and show as a proof-of-principle that detoxification of amyloid structures by redirecting the fibril formation process is possible. It is my express hope that their results will contribute to the development of an effective, cause-based therapy for AD and PD and other destructive forms of protein misfolding disorders.
Electronic supplementary material
(PDF 510 kb)
Acknowledgments
The two main substances, EGCG and O4, that were discussed in this review were initially characterized by Dagmar Ehrnhöfer and Martin Herbst, respectively, in the laboratory of Professor Erich Wanker at the Max Delbrück Center for Molecular Medicine. I gratefully acknowledge his support, encouragement, and guidance in elucidating their underlying mechanisms of action. I would like to dedicate this work to the memory of Professor Werner Hunstein (1928–2012), who, in turning personal tragedy into research fervor, demonstrated that, through persistence and enthusiasm, the path from the bench to the bedside can be found.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Dobson CM. Protein folding and misfolding. Nature. 2003;426:884–90. doi: 10.1038/nature02261. [DOI] [PubMed] [Google Scholar]
- 2.Uversky VN. Intrinsic disorder in proteins associated with neurodegenerative diseases. Front Biosci. 2009;14:5188–238. doi: 10.2741/3594. [DOI] [PubMed] [Google Scholar]
- 3.Greenwald J, Riek R. Biology of amyloid: structure, function, and regulation. Structure. 2010;18:1244–60. doi: 10.1016/j.str.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Astbury WT, Dickinson S. The X-ray interpretation of denaturation and the structure of the seed globulins. Biochem J. 1935;29:2351–60 1. doi: 10.1042/bj0292351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alzheimer A. Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiat Psych-Gerichtl Med. 1907;64:146–8. [Google Scholar]
- 6.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D. Jones E. Alzheimer's disease. Lancet. 2011;377:1019–31. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 7.Selkoe DJ, Yamazaki T, Citron M, et al. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Ann N Y Acad Sci. 1996;777:57–64. doi: 10.1111/j.1749-6632.1996.tb34401.x. [DOI] [PubMed] [Google Scholar]
- 8.Gravina SA, Ho L, Eckman CB, et al. Amyloid beta protein (A beta) in Alzheimer's disease brain. Biochemical and immunocytochemical analysis with antibodies specific for forms ending at A beta 40 or A beta 42(43) J Biol Chem. 1995;270:7013–6. doi: 10.1074/jbc.270.13.7013. [DOI] [PubMed] [Google Scholar]
- 9.Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997;20:154–9. doi: 10.1016/S0166-2236(96)01030-2. [DOI] [PubMed] [Google Scholar]
- 10.Taylor JP, Hardy J, Fischbeck KH. Toxic proteins in neurodegenerative disease. Science. 2002;296:1991–5. doi: 10.1126/science.1067122. [DOI] [PubMed] [Google Scholar]
- 11.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–26. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 12.Vulliet R, Halloran SM, Braun RK, Smith AJ, Lee G. Proline-directed phosphorylation of human Tau protein. J Biol Chem. 1992;267:22570–4. [PubMed] [Google Scholar]
- 13.Campion D, Dumanchin C, Hannequin D, et al. Early-onset autosomal dominant Alzheimer disease: prevalence, genetic heterogeneity, and mutation spectrum. Am J Hum Genet. 1999;65:664–70. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–44. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- 15.Riess O, Kuhn W, Kruger R. Genetic influence on the development of Parkinson's disease. J Neurol. 2000;247(Suppl 2):II69–74. doi: 10.1007/PL00007764. [DOI] [PubMed] [Google Scholar]
- 16.Jarrett JT, Lansbury PT., Jr Seeding "one-dimensional crystallization" of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993;73:1055–8. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 17.Oosawa F, Asakura S. Thermodynamics of the Polymerization of Protein. London: Academic Press; 1975. [Google Scholar]
- 18.Ferrone F. Analysis of protein aggregation kinetics. Methods Enzymol. 1999;309:256–74. doi: 10.1016/S0076-6879(99)09019-9. [DOI] [PubMed] [Google Scholar]
- 19.Powers ET, Powers DL. The kinetics of nucleated polymerizations at high concentrations: amyloid fibril formation near and above the "supercritical concentration". Biophys J. 2006;91:122–32. doi: 10.1529/biophysj.105.073767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakur AK, Jayaraman M, Mishra R, et al. Polyglutamine disruption of the huntingtin exon 1 N terminus triggers a complex aggregation mechanism. Nat Struct Mol Biol. 2009;16:380–9. doi: 10.1038/nsmb.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knowles TP, Waudby CA, Devlin GL, et al. An analytical solution to the kinetics of breakable filament assembly. Science. 2009;326:1533–7. doi: 10.1126/science.1178250. [DOI] [PubMed] [Google Scholar]
- 22.Harper JD, Lansbury PT., Jr Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 23.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–3. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 24.Bieschke J, Weber P, Sarafoff N, Beekes M, Giese A, Kretzschmar H. Autocatalytic self-propagation of misfolded prion protein. Proc Natl Acad Sci U S A. 2004;101:12207–11. doi: 10.1073/pnas.0404650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–7. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frost B, Diamond MI. Prion-like mechanisms in neurodegenerative diseases. Nat Rev Neurosci. 2010;11:155–9. doi: 10.1038/nrn2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer-Luehmann M, Coomaraswamy J, Bolmont T, et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science. 2006;313:1781–4. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 28.Frost B, Diamond MI. The expanding realm of prion phenomena in neurodegenerative disease. Prion. 2009;3:74–7. doi: 10.4161/pri.3.2.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012;287:19440–51. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–9. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 31.Jan A, Adolfsson O, Allaman I, et al. Abeta42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete Abeta42 species. J Biol Chem. 2011;286:8585–96. doi: 10.1074/jbc.M110.172411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 33.Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–57. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein SL, Dupuis NF, Lazo ND, et al. Amyloid-beta protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer's disease. Nat Chem. 2009;1:326–31. doi: 10.1038/nchem.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brouillette J, Caillierez R, Zommer N, et al. Neurotoxicity and memory deficits induced by soluble low-molecular-weight amyloid-beta1-42 oligomers are revealed in vivo by using a novel animal model. J Neurosci. 2012;32:7852–61. doi: 10.1523/JNEUROSCI.5901-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ono K, Condron MM, Teplow DB. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc Natl Acad Sci U S A. 2009;106:14745–50. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glabe C. Intracellular mechanisms of amyloid accumulation and pathogenesis in Alzheimer's disease. J Mol Neurosci. 2001;17:137–45. doi: 10.1385/JMN:17:2:137. [DOI] [PubMed] [Google Scholar]
- 38.Meda L, Cassatella MA, Szendrei GI, et al. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–50. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 39.Wentworth P, Jr, Nieva J, Takeuchi C, et al. Evidence for ozone formation in human atherosclerotic arteries. Science. 2003;302:1053–6. doi: 10.1126/science.1089525. [DOI] [PubMed] [Google Scholar]
- 40.Hung YH, Bush AI, Cherny RA. Copper in the brain and Alzheimer's disease. J Biol Inorg Chem. 2009;15:61–76. doi: 10.1007/s00775-009-0600-y. [DOI] [PubMed] [Google Scholar]
- 41.Jomova K, Vondrakova D, Lawson M, Valko M. Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem. 2010;345:91–104. doi: 10.1007/s11010-010-0563-x. [DOI] [PubMed] [Google Scholar]
- 42.Rajendran R, Minqin R, Ynsa MD, et al. A novel approach to the identification and quantitative elemental analysis of amyloid deposits--insights into the pathology of Alzheimer's disease. Biochem Biophys Res Commun. 2009;382:91–5. doi: 10.1016/j.bbrc.2009.02.136. [DOI] [PubMed] [Google Scholar]
- 43.Glenner GG, Keiser HR, Bladen HA, et al. Amyloid. VI. A comparison of two morphologic components of human amyloid deposits. J Histochem Cytochem. 1968;16:633–44. doi: 10.1177/16.10.633. [DOI] [PubMed] [Google Scholar]
- 44.Dyrks T, Dyrks E, Masters CL, Beyreuther K. Amyloidogenicity of rodent and human beta A4 sequences. FEBS Lett. 1993;324:231–6. doi: 10.1016/0014-5793(93)81399-K. [DOI] [PubMed] [Google Scholar]
- 45.Bitan G, Tarus B, Vollers SS, et al. A molecular switch in amyloid assembly: Met35 and amyloid beta-protein oligomerization. J Am Chem Soc. 2003;125:15359–65. doi: 10.1021/ja0349296. [DOI] [PubMed] [Google Scholar]
- 46.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–12. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 47.Dasilva KA, McLaurin J. New therapeutic approaches for Alzheimer's disease. Discov Med. 2004;4:384–9. [PubMed] [Google Scholar]
- 48.Jakob-Roetne R, Jacobsen H. Alzheimer's disease: from pathology to therapeutic approaches. Angew Chem Int Ed Engl. 2009;48:3030–59. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 49.Mandel SA, Amit T, Kalfon L, Reznichenko L, Weinreb O, Youdim MB. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: special reference to epigallocatechin gallate (EGCG) J Alzheimers Dis. 2008;15:211–22. doi: 10.3233/jad-2008-15207. [DOI] [PubMed] [Google Scholar]
- 50.Zhao B. Natural antioxidants protect neurons in Alzheimer's disease and Parkinson's disease. Neurochem Res. 2009;34:630–8. doi: 10.1007/s11064-008-9900-9. [DOI] [PubMed] [Google Scholar]
- 51.Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haass C, Koo EH, Mellon A, Hung AY, Selkoe DJ. Targeting of cell-surface beta-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature. 1992;357:500–3. doi: 10.1038/357500a0. [DOI] [PubMed] [Google Scholar]
- 53.Vassar R. The beta-secretase, BACE: a prime drug target for Alzheimer's disease. J Mol Neurosci. 2001;17:157–70. doi: 10.1385/JMN:17:2:157. [DOI] [PubMed] [Google Scholar]
- 54.Cohen FE, Kelly JW. Therapeutic approaches to protein-misfolding diseases. Nature. 2003;426:905–9. doi: 10.1038/nature02265. [DOI] [PubMed] [Google Scholar]
- 55.Sipe JD, Cohen AS. Review: history of the amyloid fibril. J Struct Biol. 2000;130:88–98. doi: 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 56.Feng BY, Toyama BH, Wille H, et al. Small-molecule aggregates inhibit amyloid polymerization. Nat Chem Biol. 2008;4:197–9. doi: 10.1038/nchembio.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011;472:226–9. doi: 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci U S A. 1996;93:11213–8. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–24. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- 60.Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res. 2005;2:131–6. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bieschke J, Herbst M, Wiglenda T, et al. Small-molecule conversion of toxic oligomers to nontoxic β-sheet-rich amyloid fibrils. Nat Chem Biol 2011;8:93-101. [DOI] [PubMed]
- 62.Ladiwala AR, Lin JC, Bale SS, et al. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Abeta into off-pathway conformers. J Biol Chem. 2010;285:24228–37. doi: 10.1074/jbc.M110.133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazzulli JR, Mishizen AJ, Giasson BI, et al. Cytosolic catechols inhibit alpha-synuclein aggregation and facilitate the formation of intracellular soluble oligomeric intermediates. J Neurosci. 2006;26:10068–78. doi: 10.1523/JNEUROSCI.0896-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–50. doi: 10.1016/0091-7435(92)90041-F. [DOI] [PubMed] [Google Scholar]
- 65.Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–80. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Choi YT, Jung CH, Lee SR, et al. The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70:603–14. doi: 10.1016/S0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]
- 67.Li MH, Jang JH, Sun B, Surh YJ. Protective effects of oligomers of grape seed polyphenols against beta-amyloid-induced oxidative cell death. Ann N Y Acad Sci. 2004;1030:317–29. doi: 10.1196/annals.1329.040. [DOI] [PubMed] [Google Scholar]
- 68.Rezai-Zadeh K, Shytle D, Sun N, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–14. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rezai-Zadeh K, Arendash GW, Hou H, et al. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–87. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 70.Wang J, Ho L, Zhao W, et al. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer's disease. J Neurosci. 2008;28:6388–92. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haque AM, Hashimoto M, Katakura M, Hara Y, Shido O. Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J Nutr Biochem. 2008;19:619–26. doi: 10.1016/j.jnutbio.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 72.Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem. 2001;78:1073–82. doi: 10.1046/j.1471-4159.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 73.Kuriyama S, Hozawa A, Ohmori K, et al. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr. 2006;83:355–61. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 74.Zhang ZX, Roman GC. Worldwide occurrence of Parkinson's disease: an updated review. Neuroepidemiology. 1993;12:195–208. doi: 10.1159/000110318. [DOI] [PubMed] [Google Scholar]
- 75.Mereles D, Buss SJ, Hardt SE, Hunstein W, Katus HA. Effects of the main green tea polyphenol epigallocatechin-3-gallate on cardiac involvement in patients with AL amyloidosis. Clin Res Cardiol. 2010;99:483–90. doi: 10.1007/s00392-010-0142-x. [DOI] [PubMed] [Google Scholar]
- 76.Hunstein W. Epigallocathechin-3-gallate in AL amyloidosis: a new therapeutic option? Blood. 2007;110:2216. doi: 10.1182/blood-2007-05-089243. [DOI] [PubMed] [Google Scholar]
- 77.Fernandez JW, Rezai-Zadeh K, Obregon D, Tan J. EGCG functions through estrogen receptor-mediated activation of ADAM10 in the promotion of non-amyloidogenic processing of APP. FEBS Lett. 2010;584:4259–67. doi: 10.1016/j.febslet.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JW, Lee YK, Ban JO, et al. Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J Nutr. 2009;139:1987–93. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- 79.Jeon SY, Bae K, Seong YH, Song KS. Green tea catechins as a BACE1 (beta-secretase) inhibitor. Bioorg Med Chem Lett. 2003;13:3905–8. doi: 10.1016/j.bmcl.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 80.Ehrnhoefer DE, Duennwald M, Markovic P, et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington's disease models. Hum Mol Genet. 2006;15:2743–51. doi: 10.1093/hmg/ddl210. [DOI] [PubMed] [Google Scholar]
- 81.Bae SY, Kim S, Hwang H, et al. Amyloid formation and disaggregation of alpha-synuclein and its tandem repeat (alpha-TR) Biochem Biophys Res Commun. 2010;400:531–6. doi: 10.1016/j.bbrc.2010.08.088. [DOI] [PubMed] [Google Scholar]
- 82.Ehrnhoefer DE, Bieschke J, Boeddrich A, et al. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat Struct Mol Biol. 2008;15:558–66. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 83.Ferreira N, Cardoso I, Domingues MR, et al. Binding of epigallocatechin-3-gallate to transthyretin modulates its amyloidogenicity. FEBS Lett. 2009;583:3569–76. doi: 10.1016/j.febslet.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 84.He M, Zhao L, Wei MJ, Yao WF, Zhao HS, Chen FJ. Neuroprotective effects of (-)-epigallocatechin-3-gallate on aging mice induced by D-galactose. Biol Pharm Bull. 2009;32:55–60. doi: 10.1248/bpb.32.55. [DOI] [PubMed] [Google Scholar]
- 85.Rambold AS, Miesbauer M, Olschewski D, et al. Green tea extracts interfere with the stress-protective activity of PrP and the formation of PrP. J Neurochem. 2008;107:218–29. doi: 10.1111/j.1471-4159.2008.05611.x. [DOI] [PubMed] [Google Scholar]
- 86.Roberts BE, Duennwald ML, Wang H, et al. A synergistic small-molecule combination directly eradicates diverse prion strain structures. Nat Chem Biol. 2009;5:936–46. doi: 10.1038/nchembio.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang SH, Liu FF, Dong XY, Sun Y. Thermodynamic analysis of the molecular interactions between amyloid beta-peptide 42 and (-)-epigallocatechin-3-gallate. J Phys Chem B. 2010;114:11576–83. doi: 10.1021/jp1001435. [DOI] [PubMed] [Google Scholar]
- 88.Lopez del Amo JM, Fink U, Dasari M, et al. Structural properties of EGCG-induced, nontoxic Alzheimer's disease Abeta oligomers. J Mol Biol. 2012;421:517–24. doi: 10.1016/j.jmb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 89.Grelle G, Albrecht O, Mario L, Frank RR, Wanker EE, Bieschke J. Black tea theaflavins inhibit formation of toxic amyloid-beta and alpha-synuclein fibrils. Biochemistry 2011:in press. [DOI] [PubMed]
- 90.Lopez Del Amo JM, Fink U, Dasari M, et al. Structural Properties of EGCG-Induced, Nontoxic Alzheimer's Disease Abeta Oligomers. J Mol Biol 2012. [DOI] [PubMed]
- 91.Kim W, Hecht MH. Generic hydrophobic residues are sufficient to promote aggregation of the Alzheimer's Abeta42 peptide. Proc Natl Acad Sci U S A. 2006;103:15824–9. doi: 10.1073/pnas.0605629103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maiti TK, Ghosh KS, Dasgupta S. Interaction of (-)-epigallocatechin-3-gallate with human serum albumin: fluorescence, fourier transform infrared, circular dichroism, and docking studies. Proteins. 2006;64:355–62. doi: 10.1002/prot.20995. [DOI] [PubMed] [Google Scholar]
- 93.Miyata M, Sato T, Kugimiya M, et al. The crystal structure of the green tea polyphenol (-)-epigallocatechin gallate-transthyretin complex reveals a novel binding site distinct from the thyroxine binding site. Biochemistry. 2010;49:6104–14. doi: 10.1021/bi1004409. [DOI] [PubMed] [Google Scholar]
- 94.Ishii T, Ichikawa T, Minoda K, et al. Human serum albumin as an antioxidant in the oxidation of (-)-epigallocatechin gallate: participation of reversible covalent binding for interaction and stabilization. Biosci Biotechnol Biochem. 2011;75:100–6. doi: 10.1271/bbb.100600. [DOI] [PubMed] [Google Scholar]
- 95.He J, Xing YF, Huang B, Zhang YZ, Zeng CM. Tea catechins induce the conversion of preformed lysozyme amyloid fibrils to amorphous aggregates. J Agric Food Chem. 2009;57:11391–6. doi: 10.1021/jf902664f. [DOI] [PubMed] [Google Scholar]
- 96.Ferreira N, Saraiva MJ, Almeida MR. Natural polyphenols inhibit different steps of the process of transthyretin (TTR) amyloid fibril formation. FEBS Lett. 2011;585:2424–30. doi: 10.1016/j.febslet.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 97.Ecroyd H, Koudelka T, Thorn DC, et al. Dissociation from the oligomeric state is the rate-limiting step in fibril formation by kappa-casein. J Biol Chem. 2008;283:9012–22. doi: 10.1074/jbc.M709928200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hudson SA, Ecroyd H, Dehle FC, Musgrave IF, Carver JA. (-)-epigallocatechin-3-gallate (EGCG) maintains kappa-casein in its pre-fibrillar state without redirecting its aggregation pathway. J Mol Biol. 2009;392:689–700. doi: 10.1016/j.jmb.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 99.Luhrs T, Ritter C, Adrian M, et al. 3D structure of Alzheimer's amyloid-beta(1-42) fibrils. Proc Natl Acad Sci U S A. 2005;102:17342–7. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mastrangelo IA, Ahmed M, Sato T, et al. High-resolution atomic force microscopy of soluble Abeta42 oligomers. J Mol Biol. 2006;358:106–19. doi: 10.1016/j.jmb.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 101.Hauber I, Hohenberg H, Holstermann B, Hunstein W, Hauber J. The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection. Proc Natl Acad Sci U S A. 2009;106:9033–8. doi: 10.1073/pnas.0811827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Munch J, Rucker E, Standker L, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–71. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 103.Chandrashekaran IR, Adda CG, Macraild CA, Anders RF, Norton RS. EGCG disaggregates amyloid-like fibrils formed by Plasmodium falciparum merozoite surface protein 2. Arch Biochem Biophys. 2011;513:153–7. doi: 10.1016/j.abb.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bieschke J, Russ J, Friedrich RP, et al. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc Natl Acad Sci U S A. 2010;107:7710–5. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS. Piperine enhances the bioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J Nutr. 2004;134:1948–52. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- 106.Lee MJ, Wang ZY, Li H, et al. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol Biomarkers Prev. 1995;4:393–9. [PubMed] [Google Scholar]
- 107.Yang CS, Chen L, Lee MJ, Balentine D, Kuo MC, Schantz SP. Blood and urine levels of tea catechins after ingestion of different amounts of green tea by human volunteers. Cancer Epidemiol Biomarkers Prev. 1998;7:351–4. [PubMed] [Google Scholar]
- 108.Mereles D, Hunstein W. Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? Int J Mol Sci. 2011;12:5592–603. doi: 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (-)-epigallocatechin-3-gallate in mice. Food Chem Toxicol. 2010;48:409–16. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mazzanti G, Menniti-Ippolito F, Moro PA, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–41. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 111.Clinical trial Sunphenon EGCg (Epigallocatechin-Gallate) in the Early Stage of Alzheimer´s Disease (SUN-AK). 2013. (Accessed at http://clinicaltrials.gov/show/NCT00951834.)
- 112.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem Pharmacol 2011. [DOI] [PMC free article] [PubMed]
- 113.Singh M, Arseneault M, Sanderson T, Murthy V, Ramassamy C. Challenges for research on polyphenols from foods in Alzheimer's disease: bioavailability, metabolism, and cellular and molecular mechanisms. J Agric Food Chem. 2008;56:4855–73. doi: 10.1021/jf0735073. [DOI] [PubMed] [Google Scholar]
- 114.Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur J Pharm Sci. 2010;41:219–25. doi: 10.1016/j.ejps.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 115.Siddiqui IA, Adhami VM, Ahmad N, Mukhtar H. Nanochemoprevention: sustained release of bioactive food components for cancer prevention. Nutr Cancer. 2010;62:883–90. doi: 10.1080/01635581.2010.509537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peters CM, Green RJ, Janle EM, Ferruzzi MG. Formulation with ascorbic acid and sucrose modulates catechin bioavailability from green tea. Food Res Int. 2010;43:95–102. doi: 10.1016/j.foodres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Giunta B, Hou H, Zhu Y, et al. Fish oil enhances anti-amyloidogenic properties of green tea EGCG in Tg2576 mice. Neurosci Lett. 2010;471:134–8. doi: 10.1016/j.neulet.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Singh M, Bhatnagar P, Srivastava AK, Kumar P, Shukla Y, Gupta KC. Enhancement of cancer chemosensitization potential of cisplatin by tea polyphenols poly(lactide-co-glycolide) nanoparticles. J Biomed Nanotechnol. 2011;7:202. doi: 10.1166/jbn.2011.1268. [DOI] [PubMed] [Google Scholar]
- 119.Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer's disease. Int J Pharm. 2010;389:207–12. doi: 10.1016/j.ijpharm.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sommer AP, Zhu D, Scharnweber T. Laser modulated transmembrane convection: Implementation in cancer chemotherapy. J Control Release. 2010;148:131–4. doi: 10.1016/j.jconrel.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 121.Eells JT, Henry MM, Summerfelt P, et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003;100:3439–44. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sommer A, Bieschke J, Friedrich R, Wanker EE, Zhu D, Hunstein W. 670 nm Laser Light and EGCG Complementarily Reduce Amyloid-beta Aggregates in Human Neuroblastoma Cells. Photomedicine and Laser Surgery 2011:in press. [DOI] [PubMed]
- 123.Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–81. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 124.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–10. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 125.Beecken H, Gottschalk E-M v, Gizycki U, Kramer H, Maassen D, Matthies HG, Musso H, Rathjen C, Zahorsky UI. Orcein und Lackmus. Angewandte Chemie. 1967;73:665–88. doi: 10.1002/ange.19610732002. [DOI] [Google Scholar]
- 126.Henwood A. Current applications of orcein in histochemistry. A brief review with some new observations concerning influence of dye batch variation and aging of dye solutions on staining. Biotech Histochem. 2003;78:303–8. doi: 10.1080/10520290410001671335. [DOI] [PubMed] [Google Scholar]
- 127.Wanker EE, Scherzinger E, Heiser V, Sittler A, Eickhoff H, Lehrach H. Membrane filter assay for detection of amyloid-like polyglutamine-containing protein aggregates. Methods Enzymol. 1999;309:375–86. doi: 10.1016/S0076-6879(99)09026-6. [DOI] [PubMed] [Google Scholar]
- 128.Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE, Kelly JW. Functional amyloid formation within mammalian tissue. PLoS Biol. 2006;4:e6. doi: 10.1371/journal.pbio.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bieschke J, Herbst M, Wiglenda T, et al. Small-molecule conversion of toxic oligomers to nontoxic beta-sheet-rich amyloid fibrils. Nat Chem Biol. 2012;8:93–101. doi: 10.1038/nchembio.719. [DOI] [PubMed] [Google Scholar]
- 130.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132:2659–68. doi: 10.1093/brain/awp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang DS, Yip CM, Huang TH, Chakrabartty A, Fraser PE. Manipulating the amyloid-beta aggregation pathway with chemical chaperones. J Biol Chem. 1999;274:32970–4. doi: 10.1074/jbc.274.46.32970. [DOI] [PubMed] [Google Scholar]
- 133.Ghanta J, Shen CL, Kiessling LL, Murphy RM. A strategy for designing inhibitors of beta-amyloid toxicity. J Biol Chem. 1996;271:29525–8. doi: 10.1074/jbc.271.47.29525. [DOI] [PubMed] [Google Scholar]
- 134.McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid beta peptide and inhibit abeta -induced toxicity. J Biol Chem. 2000;275:18495–502. doi: 10.1074/jbc.M906994199. [DOI] [PubMed] [Google Scholar]
- 135.Schirmer RH, Adler H, Pickhardt M, Mandelkow E. "Lest we forget you--methylene blue…". Neurobiol Aging. 2011;32:2325 e7–16. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)