Abstract
Increased α-synuclein levels and mutations in mitochondria-associated proteins both cause familial Parkinson’s disease (PD), and synuclein and mitochondria also play central, but poorly understood, roles in the pathogenesis of idiopathic PD. A fraction of synuclein interacts with mitochondria, and synuclein can produce mitochondrial fragmentation and impair mitochondrial complex I activity. However, the consequences of these mitochondrial changes for bioenergetic and other mitochondrial functions remain poorly defined, as does the role of synuclein–mitochondria interactions in the normal and pathologic effects of synuclein. Understanding the functional consequences of synuclein’s interactions with mitochondria is likely to provide important insights into disease pathophysiology, and may also reveal therapeutic strategies.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0182-9) contains supplementary material, which is available to authorized users.
Keywords: Mitochondria, Synuclein, Mitochondrial dynamics, Dynamin related protein 1 (Drp1), Parkinson’s disease, Neurodegeneration
Introduction
Parkinson’s disease (PD) is a common and debilitating neurodegenerative disorder involving the relatively selective degeneration of dopamine neurons in the substantia nigra [1]. Although dopamine replacement therapy ameliorates the motor symptoms of PD, it does not modify the underlying pathogenic mechanisms, and consequently loses efficacy as the disease progresses [2, 3]. The development of disease-modifying therapies will probably require a better understanding of disease pathophysiology. What is clear is that both α-synuclein and mitochondria are central to this disease process, as changes in either can cause familial forms of PD. Mutations or overexpression of wildtype α-synuclein produce rare autosomal dominant forms of the disease [4–7], while mutations in the mitochondrial kinase PINK1 or the mitochondrial-binding protein Parkin cause autosomal recessive PD [8–10]. There is also considerable evidence that both synuclein and mitochondria play central roles in idiopathic PD. First, α-synuclein accumulates in Lewy bodies and dystrophic neurites of essentially all patients with sporadic PD [11]. In PD, there are also selective impairments in mitochondrial complex I activity in the substantia nigra [12], and somatic mutations also accumulate with age and PD progression in the mitochondrial DNA of substantia nigra neurons [13]. Early in PD there is decreased expression of PGC1α and PGC1α-regulated genes that encode subunits of the electron transport chain and proteins involved in mitochondrial biogenesis and glucose metabolism [14]. These changes are suggestive of broad deficits in bioenergetic function in nigrostriatal dopamine (DA) neurons. These neurons are also particularly vulnerable to mitochondrial toxins, including 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rotenone, which are widely used to model PD.
Considering the central roles played by both synuclein and mitochondria, it seems likely that they impact disease pathogenesis through a convergent mechanism. In this review I discuss what we know about the interaction between synuclein and mitochondria, the impact of this interaction on mitochondria, and potential consequences of these changes on the functions of mitochondria, synuclein, and neuronal survival.
Co-localization of Synuclein and Mitochondria
Under basal conditions, both endogenous and heterologously expressed synuclein is primarily cytosolic, with no obvious enrichment on mitochondrial membranes [15–18]. Nonetheless, it is clear from ultrastructural studies that a fraction of synuclein normally associates with mitochondria, including in DA neurons in transgenic animals and humans [19–21]. Interestingly, the extent to which synuclein and mitochondria associate appears quite dynamic and can be increased markedly by a range of stressors, such as decreased cytosolic pH [18]. Notably, mitochondria isolated from PD patients also have a much higher content of synuclein that those from age-matched controls [20].
Curiously, the subcellular localization of synuclein in mitochondria varies across a range of in vitro and in vivo paradigms. Indeed, synuclein can be localized primarily to the outer mitochondrial membranes [18, 21–23], while, at other times, a substantial fraction is also present on the inner membranes [20, 23–25]. In some cases, localization to the inner membranes is associated with higher expression levels of synuclein [23], suggesting that the effects of synuclein on mitochondrial shape or function may contribute to the site of localization. Notably, in PD, a substantial fraction of synuclein localizes to inner membranes in mitochondria [20]. However, other undefined factors, including species and cell type, may also contribute to these discrepant findings.
Mechanism of Synuclein–Mitochondria Interactions
Although synuclein may interact with different membrane surfaces within cells, synuclein appears to have a preferential affinity for mitochondria over other organelles (Fig. 1). Indeed, we showed in fluorescence resonance energy transfer (FRET) reporter and biochemical assays that synuclein interacts preferentially with mitochondrial membranes over other native membranes, including endoplasmic reticulum, and synaptic plasma membrane and vesicle fractions [17]. The underlying mechanism remains unproven, but likely involves a direct interaction between synuclein and cardiolipin, which is enriched on both the outer and, especially, inner mitochondrial membranes [26–29]. Indeed, α-synuclein has a high affinity for cardiolipin, binding equally well to artificial membranes enriched in cardiolipin as to those enriched in phosphatidic acid [17]. In vitro, the interaction of synuclein with mitochondria can be blocked by the dye nonyl acridine orange, which binds cardiolipin with high affinity. Although this molecule has been criticized for nonspecific binding, nonyl acridine orange also blocks the binding of synuclein to liposomes enriched in cardiolipin without significantly affecting binding to those enriched in phosphatidic acid [18].
Fig. 1.
Hypothetical schema of synuclein interactions with mitochondria normally and in Parkinson’s disease (PD). Under normal conditions, most synuclein is cytosolic in transiently “closed” conformations. However, a fraction of synuclein binds to mitochondria and is stabilized in a relatively “open” conformation [17]. In PD, synuclein levels are increased and a higher proportion of synuclein may exist in an oligomeric conformation, which also interacts with mitochondria [25, 48]. Increased synuclein monomers and/or oligomers drive increased mitochondrial fission, leading to more fragmented mitochondrial morphology. ER = endoplasmic reticulum
The role of mitochondrial membrane potential in synuclein–mitochondria binding is also unclear. Using synuclein FRET reporters to monitor synuclein binding to isolated brain mitochondria, we found that mitochondrial membrane depolarization did not affect synuclein binding, suggesting that the functional state of mitochondria is less important than other factors such as lipid composition [17]. However, these assays did not assess subcellular localization and contrast with studies by Devi et al. [20], which found that normal mitochondrial membrane potential was required for synuclein import into isolated rat liver mitochondria. These seemingly discrepant findings require clarification, but may have resulted from differences in the source of mitochondria and/or dose of carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) used to depolarize mitochondria [17, 20]. Alternatively, it is possible that the membrane potential-independent affinity of synuclein for cardiolipin acts in conjunction with a membrane potential-dependent process involving the outer membrane translocase (Tom) complex to import synuclein into the mitochondria. In support of this assertion, the amino terminus of synuclein has been proposed to contain a cryptic mitochondrial-binding sequence, and synuclein transport into isolated mitochondria was shown to be blocked by antibodies against Tom40, an outer mitochondrial membrane import channel [20].
Effects of Synuclein on Mitochondrial Dynamics
What are the consequences of synuclein’s interactions with mitochondria? First, numerous studies have reported that supra-physiologic levels of synuclein disrupt mitochondrial morphology (Fig. 1). In cultured cells, increased synuclein levels produce fragmented mitochondria with a decreased length/width ratio [22, 23, 30–32]. Synuclein also alters mitochondrial morphology in vivo, including in midbrain DA neurons; however, the specific morphologic changes vary, sometimes including frank disruption versus remodeling of the mitochondrial membranes [19, 23, 33]. Determining the effects of synuclein on mitochondrial morphology in brain sections is also complicated by the ischemia that occurs during perfusion and fixation, which is certain to influence mitochondrial morphology. Nonetheless, it seems probable that the morphologic changes observed in vitro and in vivo represent the same process.
In addition, although moderate levels of synuclein are required to produce robust fragmentation [23], the effect does have some specificity for the α-synuclein homologue. Indeed, equivalent levels of β- and especially γ-synuclein produce little, if any, effect on fragmentation [23]. In addition, mitochondrial fragmentation develops in the absence of visible morphologic effects on other organelles, changes in respiration, or overt cell death—all of which might produce fragmentation through secondary mechanisms [22, 23].
An important open question is whether physiologic levels of synuclein also affect mitochondrial dynamics. This possibility is supported by a report from Kamp et al. [22] who used small interfering RNA to show that endogenous synuclein could produce fragmentation; whether this occurs in neurons in vivo remains unclear. Although we did not observe overt differences in mitochondrial morphology within the cell bodies of synuclein triple knock-out mice (lacking α- β- and γ- synuclein) [23], it is likely that in vivo studies of mitochondrial morphology lack the sensitivity to discern these changes for the reasons described above.
Mechanism of Morphologic Changes
The effects of synuclein on mitochondrial morphology appear to result from direct interactions between synuclein and mitochondrial membranes. Consistent with this, approaches that target synuclein binding away from mitochondria or mutations that disrupt the binding of synuclein to membranes both block the effects of synuclein on fragmentation [23]. In addition to direct interactions, it remains possible that synuclein also influences mitochondrial dynamics through indirect mechanisms that are independent of its interaction with mitochondrial membranes. For instance, although synuclein can produce mitochondrial fragmentation in the absence of Drp1, the dominant mitochondrial fission protein [23], synuclein can also increase Drp1 translocation to mitochondria [31], and this could also contribute to synuclein-dependent fission. Indeed, the overall level of mitochondrial fragmentation reflects the combined effects of synuclein and Drp1 [23, 31]. In addition, levels of mitofusins 1 and 2 are increased in the spinal cord of mice overexpressing A53T mutant synuclein [33], suggesting that synuclein might also affect morphology through effects on mitochondrial fusion proteins. However, synuclein does not affect mitofusin levels in vitro [22, 33], and increased mitofusin levels do not block the effects of synuclein on mitochondrial morphology [22], indicating that mitofusins are not required for fragmentation by synuclein. Lastly, synuclein may also affect more general aspects of mitochondrial function. For instance, under conditions of oxidative stress, an increased fraction of synuclein localizes to the nucleus and binds to the PGC1α promoter, and a consequent down-regulation in PGC1α-target genes could contribute indirectly to changes in mitochondrial morphology and/or function [34].
Synuclein might produce fragmentation by increasing the rate of mitochondrial fission or decreasing the rate of fusion. Surprisingly, these possibilities can be very difficult to distinguish experimentally. Although standard polyethylene glycol-based and photoactivation assays can distinguish decreased mitochondrial fusion from normal dynamics, it is unclear if they can accurately resolve decreased fusion from increased fission. In addition, although it is clear in vitro that recombinant synuclein can remodel liposomes enriched in acidic phospholipids, such as cardiolipin, synuclein’s specific effects on these membranes can resemble either tubulation or fission, presumably depending on the specific experimental conditions and preparations of synuclein used [22, 23, 35].
Interestingly, other PD proteins including PINK1, parkin, DJ1, and LRRK2 have also been found to influence mitochondrial dynamics [36–39]. Although the mechanisms have not been defined fully, they appear to involve effects on the levels and/or function of previously-defined components of the fusion and fission machinery [38, 40, 41], rather than direct effects on mitochondrial membranes, as appears to occur with synuclein. Interestingly, co-expression of wildtype, but not mutant, PINK1, Parkin or DJ-1 can all block mitochondrial fragmentation by synuclein, although the mechanisms remain unclear [22].
There are several biophysical mechanisms by which synuclein–mitochondria interactions could lead to fragmentation. For instance, insertion of synuclein’s N-terminal amphipathic helix into the lipid bilayer [42] and/or synuclein-induced clustering of acidic phospholipids could promote and stabilize mitochondrial membrane curvature [43]. Fission could also be promoted by energy-driven conformational changes in which synuclein transitions from an extended alpha-helical conformation—as observed upon binding to large liposomes and mitochondria [23, 44]—to a broken helix conformation, as found on small-diameter micelles [45]. Yet another interesting possibility is that synuclein may polymerize on mitochondrial membranes [46] and drive membrane remodeling [47]. Interestingly, the conformation and monomeric/oligomeric structure of synuclein that produces fragmentation is unclear (Fig. 1). Although both monomeric and oligomeric synuclein can associate with mitochondria [25, 48], only small synuclein oligomers—not monomers or aggregates—remodel liposomes enriched in acidic phospohlipids, such as cardiolipin [23, 49], suggesting that oligomers may be the active species. Notably, the prevalence of monomeric versus oligomeric synuclein in living cells under physiologic or pathologic conditions is also unclear [50–52], and remains an area of considerable controversy that cannot be resolved definitively with current approaches, which require either disruption of the cell membrane [50–52] or the use of fluorescent tags that disrupt synuclein’s properties [17].
Other Effects of Synuclein on Mitochondria
Complex I Activity
Several groups have found that increased synuclein protein levels can inhibit mitochondrial complex I activity. Indeed, wildtype or mutant A53T synuclein selectively inhibits complex I in purified mitochondria from cell lines and transgenic mouse brain [20, 24, 48, 53], although one group did not see this following acute incubation of isolated mitochondria with recombinant synuclein [54]. The level of synuclein in mitochondrial fractions from the substantia nigra of PD patients also correlates with decreased complex I activity in PD patients [20].
Interestingly, loss of synuclein expression can also adversely affect the function of respiratory enzymes by compromising electron flux between complex I and III, but not within complex I itself; these effects have been observed both in human dopaminergic neuronal cells by small interfering RNA [20] and synuclein knockout mice [55]. The mechanism of how synuclein interrupts complex I function is not clear, but may involve a direct interaction. Indeed, synuclein can reach the inner mitochondrial membrane and interact directly with complex I [20].
The temporal relationship between synuclein’s effects on complex I activity and mitochondrial dynamics are also not clear. Fragmentation precedes respiratory dysfunction in cell lines [23], but this relationship in neurons in vivo is unknown. Furthermore, mild complex I inhibition may also precede—and contribute to—synuclein-induced respiratory dysfunction. Interestingly, Loeb et al. [53] found that expression of A53T-synuclein compromised complex I activity in brain mitochondria isolated from 4–6-week-old mice prior to any evidence of cell toxicity, with the extent of the decrease being independent of mouse age. However, another study found no effect of A53T-synuclein on complex I activity or complex I-dependent respiration of dopaminergic synaptosomes isolated from the striata of high- versus low-expressing 4-month-old animals, but did find significant decreases in both parameters in high-expressing mice at 1 year of age [48]. Therefore, the relationship between synuclein expression level, age, complex I activity, and respiration remains to be clarified.
Turnover
Overexpression of A53T mutant synuclein increases Parkin-dependent mitophagy in cortical neurons [30] and increases the number of mitochondria in autophagosomes in midbrain DA neurons [48]. The precipitating event may be a synuclein-dependent decrease in mitochondrial size, as studies have shown that mitophagy is blocked when mitochondrial shape is restored by overexpressing mitofusin 2 to increase fusion or by expressing the dominant-negative variant Drp1 to decrease fission [30]. The authors hypothesized that excessive mitophagy may remove functional mitochondria, thereby decreasing mitochondrial mass and producing bioenergetic failure. Consistent with this, blocking synuclein-mediated mitophagy in yeast protects against the toxicity of elevated synuclein [56]. However, the role of mitophagy in PD pathogenesis remains unclear: both increased and decreased mitophagy have been proposed to drive neuronal death under certain circumstances [30, 57]. Yet another possibility is that changes in mitophagy do not directly influence neural death; for instance, if mitophagy is up-regulated to remove mitochondria that are poorly functioning, but not otherwise contributing to neural death.
Endoplasmic Reticulum Interactions and Calcium Buffering
Synuclein has also been found to increase co-localization between the endoplasmic reticulum (ER) and mitochondria, and is proposed to increase calcium transfer from the ER to the mitochondria [58]. It will be important to better understand the consequences of this and other effects of synuclein on the ER for mitochondrial function and morphology, especially considering the emerging, but poorly understood, role of ER–mitochondria interactions in shaping bioenergetics [59] and mitochondrial morphology. Indeed, ER tubules have been found to encircle and constrict mitochondria—a step that may be required for Drp1-mediated mitochondrial fission [60].
Lipid Content
Synuclein also appears to exert broader effects on mitochondrial lipid metabolism and composition, although the mechanisms remain to be defined. Indeed, membranes from α-synuclein KO mice show, roughly, a 20 % decrease in cardiolipin and its precursor versus other phospholipids [55, 61], an intriguing finding considering the high affinity of synuclein for cardiolipin [17, 18], and the central roles of cardiolipin in electron transport [55] and cell death [62]. The effect appears to be specific to α-synuclein, as cardiolipin levels are not altered in γ-synuclein knockout mice [63].
Consequences of Synuclein–Mitochondria Interactions (in Normal and Disease Conditions)
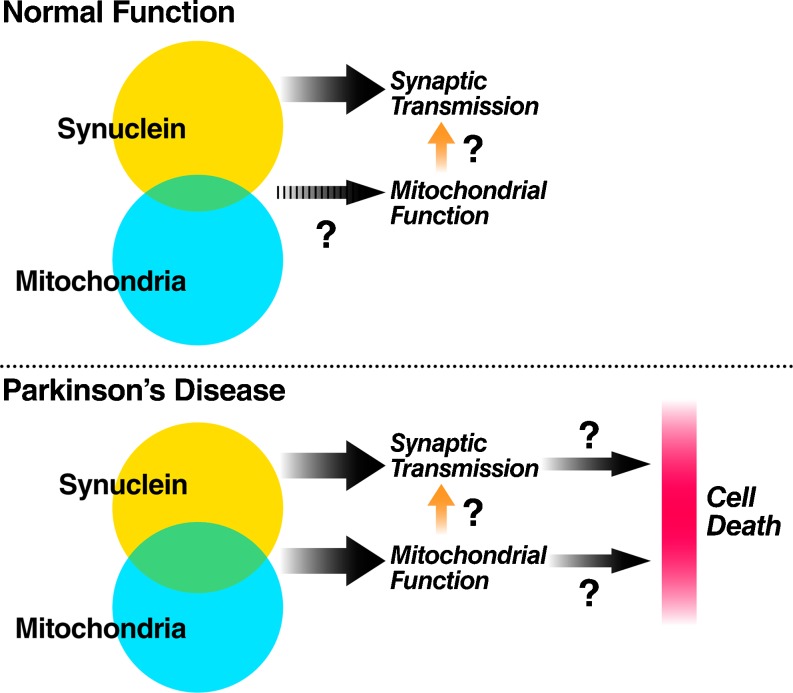
The effects of synuclein on mitochondria as described earlier may be initiating or early events in disease progression. These interactions may even be part of a normal physiologic process that becomes pathologic when in excess. However, very little is known about the biologic consequences of these interactions for mitochondrial function, synuclein function, or neuronal survival (Fig. 2).
Fig. 2.
Intersecting effects of synuclein and mitochondria on synaptic transmission and neuronal death? In healthy neurons, synuclein is believed to regulate neurotransmitter release. A fraction of synuclein also interacts with mitochondria, but the consequences of this interaction for mitochondrial function or synaptic transmission are unknown. In Parkinson’s disease, a greater fraction of synuclein interacts with mitochondria, and subsequent impairments in mitochondrial function may adversely affect synaptic transmission and predispose to neuronal death
Mitochondrial Function
Bioenergetics (Global and Regional)
Synuclein might affect the bioenergetic function of mitochondria either globally or regionally. Global dysfunction might result from intrinsic impairments in respiratory capacity owing to decreased complex I activity and/or from mitochondrial fragmentation [64], or decreased respiratory capacity due to decreased mitochondrial mass from excessive mitophagy [30]. However, whether impairments in bioenergetic function actually cross the thresholds required to produce functionally significant decreases in adenosine triphosphate (ATP) within intact, living neurons is unknown. Indeed, although synuclein can cause mitochondrial depolarization in isolated mitochondria [54], even high levels of wildtype synuclein fail to affect mitochondrial membrane potential in intact cells [22, 23]. Initially, there are also no changes in respiration or ATP [22, 23], although, ultimately, mild impairments in respiration and decreases in ATP are observed [23, 30, 53].
Importantly, any effects of synuclein on bioenergetic function may be more prominent at the nerve terminal, as synuclein is present at much higher levels in this compartment, both normally and in disease [11, 65]. In addition, through its effects on mitochondrial morphology and/or degradation, synuclein might affect the mass and distribution of mitochondria in axons; in turn, this could result in regional deficits in ATP levels at the synapse, but not the cell body. Indeed, in alpha-synuclein-overexpressing mice, there is an accumulation of small axonal spheroids that are immunoreactive for alpha-synuclein and are enriched in autophagosome-like structures and deformed mitochondria [66]. Consistent with a potential adverse effect of synuclein on respiration at the nerve terminal, Chinta et al. [48] observed dose-dependent, selective decreases in complex I-dependent respiration of dopaminergic synaptosomes, isolated from the striata of synuclein-overexpressing mice.
Reactive Oxygen Species
Synuclein could affect reactive oxygen species (ROS) levels by virtue of its association with mitochondria, which are a major source of cellular ROS. For instance, this might occur through synuclein’s effects on the respiratory chain, and a number of studies have found that wildtype and/or mutant synuclein can increase ROS accumulation [25, 67–69] and oxidatively modify mitochondrial proteins [70]. Interestingly, in yeast, synuclein-induced increases in ROS and toxicity depend on the presence of mitochondrial DNA [71]. It remains unclear if the ROS are an epiphenomenon that result from synuclein’s effects on the respiratory chain or if they represent a key mechanism by which synuclein exerts its toxicity. Yet another possibility is that mitochondrial-driven ROS function upstream of synuclein to produce toxicity, for instance by regulating the rate of formation of toxic oligomeric and/or fibrillar synuclein species [72].
Synaptic Transmission
Although synuclein’s normal functions are not defined, it is known that elevated synuclein levels impair synaptic transmission, while decreased synuclein levels can enhance transmission. The mechanisms and relationship of these effects to neurodegeneration remain controversial [73–79]. At present, there is no information as to whether the effects of synuclein on mitochondria contribute to its effects on synaptic transmission (Fig. 2), for instance by compromising energy production or leading to other mitochondrial failure.
Neuronal Death
Understanding how or if the synuclein–mitochondria interaction contributes to neuronal death is a challenging, but critical, issue to resolve. Although both synuclein and mitochondria are clearly altered in idiopathic PD, it is possible that they promote neuronal death through independent mechanisms that converge only at the point of downstream cell death pathways. Alternatively, although changes in either can cause familial forms of PD, it is possible that only one causes idiopathic PD, and changes in the other are secondary epiphenomena with no functional consequences. In addition, as idiopathic PD is almost certainly heterogeneous, perhaps idiopathic PD is a mix of disorders, some caused by primary changes in synuclein and others by changes in mitochondria.
However, despite these other possibilities, it seems more likely that synuclein and mitochondria act together to produce disease (Fig. 2). Indeed, several studies found that even physiologic levels of synuclein sensitize DA neurons to a range of mitochondrial toxins [80–83], although others have found conflicting results [84, 85]. Indeed, synuclein has been found to both promote and protect against the activation of mitochondrial-dependent apoptotic pathways [69, 85]. Although the reasons for these discrepancies are not clear, mitochondria do appear to be critical to synuclein toxicity, as loss of mitochondrial DNA in yeast decreases ROS and blocks synuclein-induced death [71].
Do synuclein–mitochondria interactions contribute directly to death in neurons? We showed that synuclein-induced changes in mitochondrial morphology precede neuronal death from increased synuclein [23], but this does not establish causation. An important question is whether normalizing mitochondrial morphology by adjusting the levels of defined fusion/fission proteins (e.g., decreased Drp1 or increased fusion protein expression) can prevent neuronal death. This possibility is suggested by recent findings from Drosophila, in which increased levels of the mitochondrial chaperone TRAP1 blocked the effects of A53T-synuclein on mitochondrial fragmentation, complex I-dependent ATP production, and cell death [32].
With regard to bioenergetics, synuclein can clearly impair respiration under some circumstances, but it will be important to know if energy levels are actually compromised within individual living neurons, and if the magnitude of any such changes exceeds threshold levels needed to produce neuronal dysfunction or death. Equally important will be the extension of these findings into more physiologic systems, although at present, all animal models of synuclein overexpression and human neuronal models have important limitations, and it is unclear which, if any, are most predictive of disease pathogenesis or therapeutic efficiency in the human condition.
Summary
To summarize, the fraction of synuclein that interacts with mitochondria can disrupt normal mitochondrial morphology and function, and enhance susceptibility to mitochondrial toxins. The central role that both synuclein and mitochondria play in PD pathogenesis suggests that their interaction may represent an important point of convergence in disease pathogenesis. However, our current understanding of this process is limited, especially with regard to the functional consequences of synuclein–mitochondria interactions on mitochondrial bioenergetics and other functions, and how these changes ultimately affect synaptic transmission and neuronal survival. Addressing these questions will require new approaches to study mitochondrial functions in neurons in vitro and in vivo, especially at the nerve terminal where synuclein concentrates. A better understanding of the mechanisms by which synuclein and mitochondria contribute to PD should help guide the development of new therapeutic approaches.
Electronic supplementary material
(PDF 510 kb)
Acknowledgments
This work was supported by a Burroughs-Wellcome Medical Scientist Fund Career Award, the Michael J. Fox Foundation for Parkinson’s Research, and award numbers 1KO8NS062954-01A1 and P30NS069496 from the National Institute of Neurological Disorders And Stroke. I thank Anna Lisa Lucido for assistance editing the manuscript.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 2.de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, et al. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54(11 Suppl. 5):S21–S23. [PubMed] [Google Scholar]
- 3.Guttman M, Slaughter PM, Theriault ME, DeBoer DP, Naylor CD. Burden of parkinsonism: a population-based study. Mov Disord. 2003;18:313–319. doi: 10.1002/mds.10333. [DOI] [PubMed] [Google Scholar]
- 4.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 5.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 6.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 7.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 8.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 9.Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006;441:1162–1166. doi: 10.1038/nature04779. [DOI] [PubMed] [Google Scholar]
- 10.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- 11.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schapira AHV, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, et al. Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson's disease. J Neurochem. 1990;55:2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- 13.Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 14.Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, et al. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson's disease. Sci Transl Med. 2010;2:52–73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rideout HJ, Dietrich P, Savalle M, Dauer WT, Stefanis L. Regulation of alpha-synuclein by bFGF in cultured ventral midbrain dopaminergic neurons. J Neurochem. 2003;84:803–813. doi: 10.1046/j.1471-4159.2003.01574.x. [DOI] [PubMed] [Google Scholar]
- 16.Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, et al. Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol. 2003;62:644–654. doi: 10.1093/jnen/62.6.644. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura K, Nemani VM, Wallender EK, Kaehlcke K, Ott M, Edwards RH. Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria. J Neurosci. 2008;28:12305–12317. doi: 10.1523/JNEUROSCI.3088-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole NB, Dieuliis D, Leo P, Mitchell DC, Nussbaum RL. Mitochondrial translocation of alpha-synuclein is promoted by intracellular acidification. Exp Cell Res. 2008;314:2076–2089. doi: 10.1016/j.yexcr.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin LJ, Pan Y, Price AC, Sterling W, Copeland NG, Jenkins NA, et al. Parkinson's disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J Neurosci. 2006;26:41–50. doi: 10.1523/JNEUROSCI.4308-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li WW, Yang R, Guo JC, Ren HM, Zha XL, Cheng JS, et al. Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport. 2007;18:1543–1546. doi: 10.1097/WNR.0b013e3282f03db4. [DOI] [PubMed] [Google Scholar]
- 22.Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, et al. Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 2010;29:3571–3589. doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, et al. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem. 2011;286:20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, et al. alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci Lett. 2009;454:187–192. doi: 10.1016/j.neulet.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 25.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J Biochem Cell Biol. 2009;41:2015–2024. doi: 10.1016/j.biocel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985;822:1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- 27.Sperka-Gottlieb CD, Hermetter A, Paltauf F, Daum G. Lipid topology and physical properties of the outer mitochondrial membrane of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1988;946:227–234. doi: 10.1016/0005-2736(88)90397-5. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Dai Q, Chen J, Durrant D, Freeman A, Liu T, et al. Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Mol Cancer Res. 2003;1:892–902. [PubMed] [Google Scholar]
- 29.Hovius R, Thijssen J, van der Linden P, Nicolay K, de Kruijff B. Phospholipid asymmetry of the outer membrane of rat liver mitochondria. Evidence for the presence of cardiolipin on the outside of the outer membrane. FEBS Lett. 1993;330:71–76. doi: 10.1016/0014-5793(93)80922-H. [DOI] [PubMed] [Google Scholar]
- 30.Choubey V, Safiulina D, Vaarmann A, Cagalinec M, Wareski P, Kuum M, et al. Mutant A53T alpha-synuclein induces neuronal death by increasing mitochondrial autophagy. J Biol Chem. 2011;286:10814–10824. doi: 10.1074/jbc.M110.132514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gui YX, Wang XY, Kang WY, Zhang YJ, Zhang Y, Zhou Y, et al. Extracellular signal-regulated kinase is involved in alpha-synuclein-induced mitochondrial dynamic disorders by regulating dynamin-like protein 1. Neurobiol Aging. 2012;33:2841–2854. doi: 10.1016/j.neurobiolaging.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Butler EK, Voigt A, Lutz AK, Toegel JP, Gerhardt E, Karsten P, et al. The mitochondrial chaperone protein TRAP1 mitigates alpha-Synuclein toxicity. PLoS Genet 8:e1002488. [DOI] [PMC free article] [PubMed]
- 33.Xie W, Chung KK. Alpha-synuclein impairs normal dynamics of mitochondria in cell and animal models of Parkinson's disease. J Neurochem 2012 Apr 28 [Epub ahead of print]. [DOI] [PubMed]
- 34.Siddiqui A, Chinta SJ, Mallajosyula JK, Rajagopolan S, Hanson I, Rane A, et al. Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson's disease. Free Radic Biol Med. 2012;53:993–1003. doi: 10.1016/j.freeradbiomed.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varkey J, Isas JM, Mizuno N, Jensen MB, Bhatia VK, Jao CC, et al. Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins. J Biol Chem. 2010;285:32486–32493. doi: 10.1074/jbc.M110.139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, Ouyang Y, Yang L, Beal MF, McQuibban A, Vogel H, et al. Pink1 regulates mitochondrial dynamics through interaction with the fission/fusion machinery. Proc Natl Acad Sci U S A. 2008;105:7070–7075. doi: 10.1073/pnas.0711845105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, et al. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet. 2012;21:1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Petrie TG, Liu Y, Liu J, Fujioka H, Zhu X. Parkinson's disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J Neurochem. 2012;121:830–839. doi: 10.1111/j.1471-4159.2012.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziviani E, Tao RN, Whitworth AJ. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc Natl Acad Sci U S A. 2010;107:5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, et al. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallop JL, Jao CC, Kent HM, Butler PJ, Evans PR, Langen R, et al. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006;25:2898–2910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandey AP, Haque F, Rochet JC, Hovis JS. Clustering of alpha-synuclein on supported lipid bilayers: role of anionic lipid, protein, and divalent ion concentration. Biophys J. 2009;96:540–551. doi: 10.1016/j.bpj.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jao CC, Der-Sarkissian A, Chen J, Langen R. Structure of membrane-bound alpha-synuclein studied by site-directed spin labeling. Proc Natl Acad Sci U S A. 2004;101:8331–8336. doi: 10.1073/pnas.0400553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. J Biol Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 46.Lee HJ, Choi C, Lee SJ. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J Biol Chem. 2002;277:671–678. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- 47.Bigay J, Gounon P, Robineau S, Antonny B. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature. 2003;426:563–566. doi: 10.1038/nature02108. [DOI] [PubMed] [Google Scholar]
- 48.Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett. 2010;486:235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Rooijen BD, Claessens MM, Subramaniam V. Lipid bilayer disruption by oligomeric alpha-synuclein depends on bilayer charge and accessibility of the hydrophobic core. Biochim Biophys Acta. 2009;1788:1271–1278. doi: 10.1016/j.bbamem.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Bartels T, Choi JG, Selkoe DJ. alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LT, Liao J, et al. A soluble alpha-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci U S A. 2011;108:17797–17802. doi: 10.1073/pnas.1113260108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fauvet B, Mbefo MK, Fares MB, Desobry C, Michael S, Ardah MT, et al. alpha-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem. 2012;287:15345–15364. doi: 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loeb V, Yakunin E, Saada A, Sharon R. The transgenic over expression of alpha-synuclein and not its related pathology, associates with complex I inhibition. J Biol Chem. 2010;285:7334–7343. doi: 10.1074/jbc.M109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banerjee K, Sinha M, Pham Cle L, Jana S, Chanda D, Cappai R, et al. Alpha-synuclein induced membrane depolarization and loss of phosphorylation capacity of isolated rat brain mitochondria: implications in Parkinson's disease. FEBS Lett. 2010;584:1571–1576. doi: 10.1016/j.febslet.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Ellis CE, Murphy EJ, Mitchell DC, Golovko MY, Scaglia F, Barcelo-Coblijn GC, et al. Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol Cell Biol. 2005;25:10190–10201. doi: 10.1128/MCB.25.22.10190-10201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sampaio-Marques B, Felgueiras C, Silva A, Rodrigues M, Tenreiro S, Franssens V, et al. SNCA (alpha-synuclein)-induced toxicity in yeast cells is dependent on sirtuin 2 (Sir2)-mediated mitophagy. Autophagy. 2012;8:1494–1509. doi: 10.4161/auto.21275. [DOI] [PubMed] [Google Scholar]
- 57.Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci. 2012;125:795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cali T, Ottolini D, Negro A, Brini M. alpha-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J Biol Chem. 2012;287:17914–17929. doi: 10.1074/jbc.M111.302794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science. 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barcelo-Coblijn G, Golovko MY, Weinhofer I, Berger J, Murphy EJ. Brain neutral lipids mass is increased in alpha-synuclein gene-ablated mice. J Neurochem. 2007;101:132–141. doi: 10.1111/j.1471-4159.2006.04348.x. [DOI] [PubMed] [Google Scholar]
- 62.Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta. 2009;1788:2022–2031. doi: 10.1016/j.bbamem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Guschina I, Millership S, O'Donnell V, Ninkina N, Harwood J, Buchman V. Lipid classes and fatty acid patterns are altered in the brain of gamma-synuclein null mutant mice. Lipids. 2011;46:121–130. doi: 10.1007/s11745-010-3486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 65.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 66.Sekigawa A, Fujita M, Sekiyama K, Takamatsu Y, Rockenstein E, La Spada AR, et al. Distinct mechanisms of axonal globule formation in mice expressing human wild type alpha-synuclein or dementia with Lewy bodies-linked P123H Ss-synuclein. Mol Brain. 2012;5:34. doi: 10.1186/1756-6606-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Junn E, Mouradian MM. Human alpha-synuclein over-expression increases intracellular reactive oxygen species levels and susceptibility to dopamine. Neurosci Lett. 2002;320:146–150. doi: 10.1016/S0304-3940(02)00016-2. [DOI] [PubMed] [Google Scholar]
- 68.Jiang H, Wu YC, Nakamura M, Liang Y, Tanaka Y, Holmes S, et al. Parkinson's disease genetic mutations increase cell susceptibility to stress: mutant alpha-synuclein enhances H2O2- and Sin-1-induced cell death. Neurobiol Aging. 2007;28:1709–1717. doi: 10.1016/j.neurobiolaging.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 69.Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, et al. Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet. 2005;14:3801–3811. doi: 10.1093/hmg/ddi396. [DOI] [PubMed] [Google Scholar]
- 70.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci. 2008;65:1272–1284. doi: 10.1007/s00018-008-7589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buttner S, Bitto A, Ring J, Augsten M, Zabrocki P, Eisenberg T, et al. Functional mitochondria are required for alpha-synuclein toxicity in aging yeast. J Biol Chem. 2008;283:7554–7560. doi: 10.1074/jbc.M708477200. [DOI] [PubMed] [Google Scholar]
- 72.Nasstrom T, Fagerqvist T, Barbu M, Karlsson M, Nikolajeff F, Kasrayan A, et al. The lipid peroxidation products 4-oxo-2-nonenal and 4-hydroxy-2-nonenal promote the formation of alpha-synuclein oligomers with distinct biochemical, morphological, and functional properties. Free Radic Biol Med. 2011;50:428–437. doi: 10.1016/j.freeradbiomed.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 73.Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, et al. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scott DA, Tabarean I, Tang Y, Cartier A, Masliah E, Roy S. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010;30:8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greten-Harrison B, Polydoro M, Morimoto-Tomita M, Diao L, Williams AM, Nie EH, et al. alphabetagamma-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci U S A. 2010;107:19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anwar S, Peters O, Millership S, Ninkina N, Doig N, Connor-Robson N, et al. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J Neurosci. 2011;31:7264–7274. doi: 10.1523/JNEUROSCI.6194-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burre J, Sharma M, Sudhof TC. Systematic mutagenesis of alpha-synuclein reveals distinct sequence requirements for physiological and pathological activities. J Neurosci. 2012;32:15227–15242. doi: 10.1523/JNEUROSCI.3545-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, et al. Resistance of alpha -synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Orth M, Tabrizi SJ, Schapira AH, Cooper JM. Alpha-synuclein expression in HEK293 cells enhances the mitochondrial sensitivity to rotenone. Neurosci Lett. 2003;351:29–32. doi: 10.1016/S0304-3940(03)00941-8. [DOI] [PubMed] [Google Scholar]
- 82.Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, et al. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21:541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 83.Fountaine TM, Venda LL, Warrick N, Christian HC, Brundin P, Channon KM, et al. The effect of alpha-synuclein knockdown on MPP + toxicity in models of human neurons. EurJ Neurosci. 2008;28:2459–2473. doi: 10.1111/j.1460-9568.2008.06527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choong CJ, Say YH. Neuroprotection of alpha-synuclein under acute and chronic rotenone and maneb treatment is abolished by its familial Parkinson's disease mutations A30P, A53T and E46K. Neurotoxicology. 2011;32:857–863. doi: 10.1016/j.neuro.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 85.Musgrove RE, King AE, Dickson TC. alpha-synuclein protects neurons from apoptosis downstream of free-radical production through modulation of the MAPK signalling pathway. Neurotox Res 2012 Aug 31 [Epub ahead of print]. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)