Abstract
Heart failure is a major clinical problem in developed countries with about half of heart failure patients exhibiting decreased left ventricular systolic function. The correct identification and prompt treatment of some specific etiologies can reverse heart failure, and recognition of myocardial recovery may avoid long-term therapy. However, the echocardiographic patterns of patients with a variety of etiologies of heart failure are similar, so the selective use of other imaging techniques is necessary for identification of specific etiologies. The role of repeat imaging in monitoring the therapeutic response is controversial, as is the cessation of medical therapy in patients demonstrating recovery.
Keywords: Heart failure, Reversible cardiomyopathy, Echocardiography, Cardiac magnetic resonance imaging
Introduction
Heart failure is one of the most important health problems in both the developed and developing world.1) In developed countries, the incidence of heart failure is 1-2%,2) and the prevalence rises to > 10% among persons > 70 years of age.2) Hospital admissions for heart failure have markedly increased with rising life span and prolonged survival of patients with cardiac problems.3) Identification of the underlying etiology is important because the management of the underlying disease differs. However, heart failure is a clinical diagnosis; chest radiography and electrocardiography are insensitive in the detection of left ventricular (LV) systolic dysfunction, and only about 50% of heart failure patients show decreased LV ejection fraction.2) Imaging tools can give information about LV systolic function, additional cardiac structural abnormalities, hemodynamic status and sometimes the chance of reversibility. Moreover, repeated imaging studies can be used in the assessment of therapeutic responses.
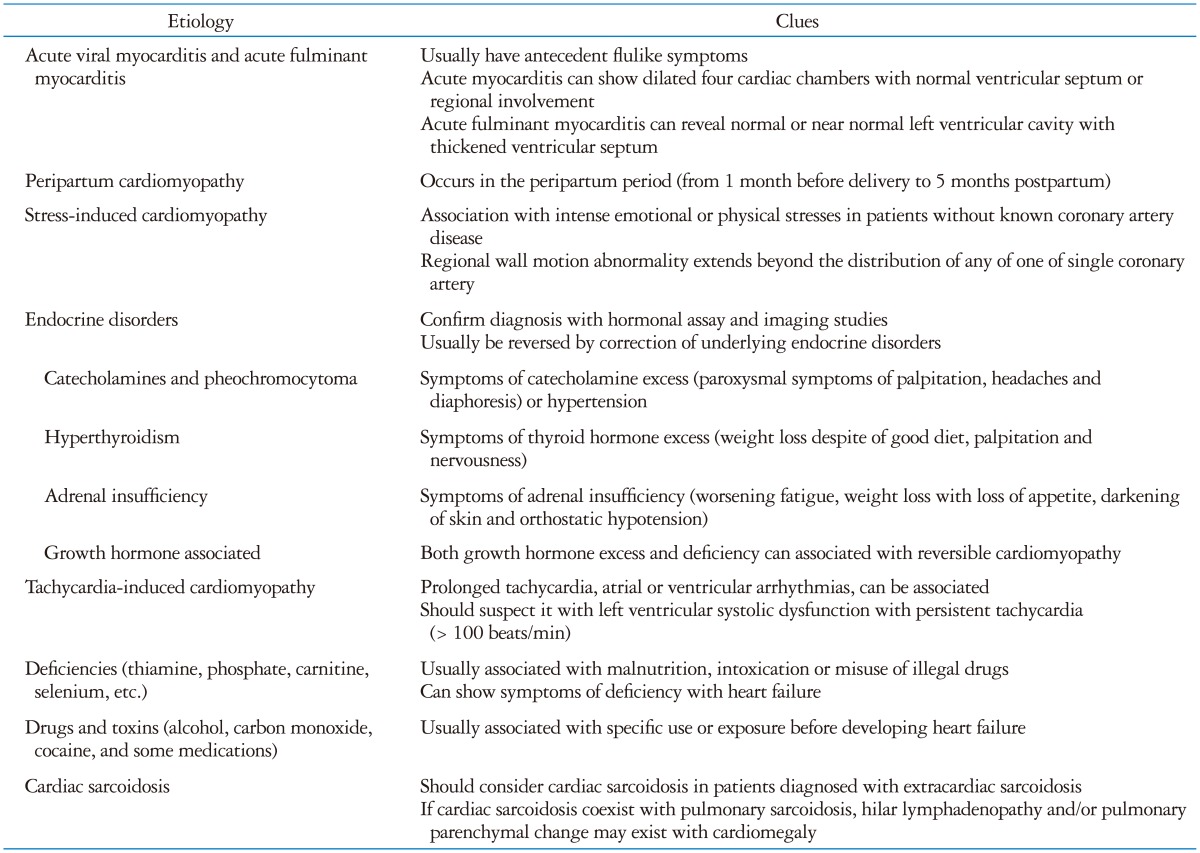
Although most patients with heart failure have a chronic, progressive and eventually fatal disease, a subgroup has a potentially reversible condition.4) Failure to recognize this may lead to patients not being given specific therapy, inappropriate insertion of implantable devices or continuing on heart failure therapy after resolution of the problem. As these patients with reversible cardiomyopathy have similar echocardiographic findings (dilatation of left, right or both ventricles with impaired systolic function), consideration of potential etiology should be the second diagnostic step in patients with impaired LV systolic function. This review focuses on the echocardiographic and cardiac magnetic resonance (CMR) imaging patterns and clinical outcomes of heart failure etiologies causing reversible LV systolic dysfunction (Table 1).
Table 1.
Possible causes of reversible cardiomyopathy
Diagnostic Tools for LV Evaluation in Heart Failure
Because some etiologies that lead to LV systolic dysfunction are potentially reversible, it is important to identify the underlying condition responsible for the cardiac abnormalities.5)
Echocardiography
This is usually the initial and preferred diagnostic test in the assessment of heart failure.5) Its use to distinguish systolic heart failure is critical in decision-making about drugs and devices such as implantable devices or ventricular assist device.6) Decreased LV ejection fraction has been associated with poor prognosis.6) In addition to assessment of ventricular size and systolic function, echocardiography can provide information about diastolic, valvular function and hemodynamic status.5) Sequential echocardiography is widely used for monitoring the evolution of the condition and response to therapy. While this latter application is considered appropriate especially in patients with a change in clinical status (appropriateness score 9),7) the large (> 10%) confidence intervals of ejection fraction measured with 2-dimensional echocardiography suggest this may not be a useful tool for identification of subtle changes.8)
Some echocardiographic features can provide clues to the causative etiology of heart failure, although tissue characterization with other imaging such as CMR is often required to clarify the differential diagnosis. For example, specific patterns of regional wall motion abnormalities suggest coronary artery disease. Distinction of this from the regional heterogeneity of dilated cardiomyopathy may be supported by ischemic or viable responses to either exercise or pharmacologic stress echocardiography,9) as well as coronary imaging with CT and scar imaging with CMR. Likewise, ambiguity about the cause of LV thickening in hypertensive heart failure may be elucidated by techniques characterizing myocardial infiltration. Finally, while apical ballooning or mid to basal ballooning can be a clue of stress-induced cardiomyopathy (SCMP),10),11) other testing may be needed to exclude acute myocardial infarction (AMI).
Role of CMR
Although echocardiography is extremely versatile and readily accessible, image quality is often limited, and geometric assumptions are required to quantify LV systolic function. Furthermore, it lacks the ability to provide more detailed tissue characterization, which can be extremely important in defining the etiology of heart failure. CMR is currently considered the gold standard for the assessment of LV mass, systolic function, and assessment of myocardial fibrosis. CMR has the ability to image in any three dimensional plane, offering the ability to produce extremely accurate and reproducible assessment of LV and right ventricular (RV) volumes, ejection fraction, and mass, without relying on geometric assumptions that can result in significant miscalculations particularly in dilated ventricles.12),13)
In addition, CMR offers the ability to assess myocardial perfusion as well as implementing other imaging techniques [delayed hyperenhancement (DHE) imaging, T1-weighted, T2-weighted, and fat suppression imaging techniques] to assess for myocardial fibrosis as well as myocardial edema. DHE imaging allows for the identification of myocardial fibrosis with high resolution and offers the ability to differentiate between types of cardiomyopathies, based on patterns of fibrosis (Fig. 1).14),15) DHE-CMR can identify significant coronary artery disease and decrease the need of conventional coronary angiography in patients presenting with heart failure of uncertain etiology.16) T1- or T2-weighted image sequences provide the ability to differentiate between fat, muscle, and areas of inflammation, based on the different proton relaxation properties of these tissues. Tissue edema appears bright on T2-weighted images in both acute coronary syndromes as well as inflammatory processes such as cardiac sarcoidosis or myocarditis.17),18) Myocardial edema may occur in isolation, but is often accompanied by characteristic patterns of myocardial fibrosis, which has the ability to elucidate the etiology of decreased ventricular function. The standard T2-weighted image sequences use turbo spin-echo sequences, and have been limited by artifacts (e.g., posterior wall signal loss caused by through-plane motion or bright rim artifacts caused by stagnant blood along the endocardial surface).19) However, recent advances in T2-weighted methods have been developed which have demonstrated increased diagnostic accuracy and may improve the reliability of CMR edema imaging.18),19)
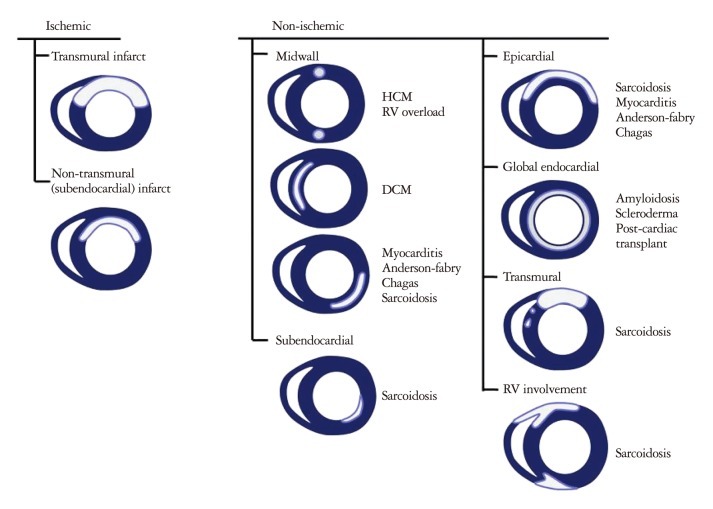
Fig. 1.
The patterns of delayed hyperenhancement of the heart. HCM: hypertrophic cardiomyopathy, RV: right ventricle, DCM: dilated cardiomyopathy.
Although DHE sequences on CMR is widely utilized for assessing regional myocardial fibrosis/scarring this relies on the relative difference in signal intensity between scarred and the presumed normal adjacent myocardium to generate image contrast. Scar formation in infarcted myocardium is due to replacement of the myocardium with collagen. Such areas of dense fibrosis have a much slower washout rate of gadolinium-based contrast than healthy myocardium, thus resulting in markedly increased signal intensity on T1-weighted imaging within the infarcted myocardium. A key shortcoming to delayed contrast-enhanced CMR is that it relies on the assumption that the surrounding and remote myocardium is truly normal and that there is a distinct difference in gadolinium washout kinetics. Because collagen deposition in nonischemic cardiomyopathy is commonly diffuse, the technique of delayed contrast enhancement often shows no regional scarring.
However, T1 mapping allows for the calculation of the relaxation time of each pixel within a parametric image, which can detect subtle differences in regional tissue characteristics. Therefore, contrast resolution not dependent on relative differences in signal intensity as it is with DHE scar imaging. Therefore, this newer CMR technique may prove to be useful in evaluating various reversible cardiomyopathies. Several techniques for measuring myocardial T1 to identify myocardial fibrosis with T1 mapping have been described in the literature.18),20),21)
Reversible Cardiomyopathies
Acute myocarditis
Myocarditis, immune and viral mediated cardiac damage, is about 15% of the patients with a new onset dilated cardiomyopathy or heart failure.22) Despite up to 50% of patients have no identifiable etiology with a full and complete evaluation, the determination of the etiology is important in the treatment and prediction of the prognosis.22)
Acute viral myocarditis
Acute viral myocarditis is a common cause of acute myocarditis and Coxsackie B virus is the most common cardiotropic virus. Although the clinical presentations are variable, the majority of patients have antecedent flulike symptoms.
Echocardiographic examination is helpful in the detection of heart failure. All cardiac chambers can be dilated in the severe and diffuse myocarditis. LV dysfunction with segmental involvement reflects the focal involvement of myocarditis. Echocardiography can detect other structural abnormalities including intracardiac thrombi, valvular regurgitation, and pericardial involvement. Endomyocardial biopsy showed myocardial inflammation and edema. CMR is a good diagnostic modality in the detection of acute myocarditis. T2-weighted imaging can detect tissue edema sensitively with the long T2 of water-bound protons as the contrast-generating mechanism. Edematous portion in the myocardium show in a high signal intensity.23)
Acute fulminant myocarditis
Acute fulminant myocarditis is defined on the basis of clinical pathological criteria. Patients with acute fulminant myocarditis have a history of an acute viral syndrome within 2 weeks of presentation.24) They usually present with profound heart failure with severe hemodynamic compromise, often requiring inotropic agents, intra-aortic balloon pump or ventricular assist device.24) Echocardiographic findings in these patients include decreased LV systolic function with near normal LV cavity dimensions and increased septal thickness, while those of acute myocarditis include increased LV cavity size and normal septal thickness.25)
Patients with fulminant myocarditis die or recover spontaneously within 2 weeks. They have better 5-year survival compared with those with acute myocarditis.24)
Peripartum cardiomyopathy
Peripartum cardiomyopathy is a rare cardiomyopathy of unknown etiology that occurs in the peripartum period (from 1 month before delivery to 5 months postpartum).26) The diagnosis requires both the absence of an identifiable cause for the heart failure and recognizable heart diseases prior to the last month of pregnancy. Because there are no available population-based studies and symptoms of early heart failure are similar to symptoms experienced by many women in the last month of a normal pregnancy, the incidence of peripartum cardiomyopathy is unknown. Risk factors include multiparity, advanced maternal age, multifetal pregnancy, preeclampsia, gestational hypertension, and African-American race.26) Because the multifetal pregnancy rate has risen rapidly over the last decades, the incidence of peripartum cardiomyopathy may have increased.27)
The possible mechanisms for this cardiomyopathy include myocarditis, abnormal immune responses to pregnancy, abnormal hemodynamic response to increased blood volume in pregnancy, and prolonged use of tocolytics.26)
Symptoms and signs that may suggest heart failure include paroxysmal nocturnal dyspnea, chest pain, cough, jugular venous distension, new onset cardiac murmurs and pulmonary crackles. As there are no specific criteria for differentiating the subtle symptoms of heart failure from symptoms of normal late pregnancy, the diagnosis of peripartum cardiomyopathy is usually based on the echocardiographic demonstration of new onset LV systolic dysfunction. Echo-cardiographic patterns include diffuse hypokinesia with increased LV diastolic dimensions.28) Patients with initial severe LV systolic dysfunction and larger LV dimensions seem unlikely to regain normal LV function on follow-up.28),29)
Myocardial inflammation and fibrosis can be detected by CMR in the acute stages. Several case studies and one case series demonstrated that patients with DHE had worse outcomes than those who did not.30),31) Furthermore, myocardial inflammation seen in the acute phases on T2-weighted images was transient in most cases, and DHE may regress in some patients.31)
In addition to the use of standard therapy for heart failure (diuretics, vasodilators and digoxin), prolactin inhibition32) may provide potential benefit to patients with peripartum cardiomyopathy. Careful attention must be paid to select drugs relatively safe for mother and fetus and to exclude those that can evoke fetal abnormalities. The duration of treatment is still unknown. Though the majority of peripartum cardiomyopathy patients recover partially or are completely improved with treatment, the reported mortality rates lie between 18% and 56%.26),33) Moreover, subsequent pregnancy in these patients can be associated with a recurrence of peripartum cardiomyopathy and can result in death.33)
Stress-induced cardiomyopathy
SCMP is a syndrome of reversible LV systolic dysfunction with characteristic apical ballooning in patients without significant epicardial coronary artery stenosis.10),34) This syndrome, also known as Takotsubo cardiomyopathy, is believed to be associated with various clinical scenarios, especially with intense mental or emotional stress.35) However, physical stress can cause this kind of cardiomyopathy. Park et al.35) reported their data of patients admitted to the medical intensive care unit. They reported 28% of patients showed apical ballooning and they were usually associated with hypotension, sepsis, cardiomegaly and use of inotropic agents.
SCMP occurs usually in women over 50 years of age, and the typical features include profound mental stress immediately preceding and triggering the cardiac events, acute retrosternal chest pain with ST-segment change and/or T-wave inversion, absence of significant coronary artery stenosis by coronary angiography, and LV systolic dysfunction with abnormal wall motion of apex (apical ballooning).11) There is a variant of apical ballooning syndrome. Transient mid- and basal-ventricular ballooning is a new variant of the transient LV apical ballooning syndrome. The involvement of the LV's mid- and basal-ventricle with sparing of the apical segment is the unique finding of this variant.36) Because the guideline excludes the presence of pheochromocytoma in the diagnosis of SCMP, the presence of a specific pattern with pheochromocytoma should be classified as catecholamine-induced cardiomyopathy.37)
Echocardiography is a useful method to diagnose this type of cardiomyopathy. Transthoracic echocardiography usually demonstrates LV systolic dysfunction with typical apical ballooning and/or midventricular hypokinesia, and the wall-motion abnormality extends beyond the distribution of any of one single coronary artery.10),11) Transient LV hypokinesia can be restricted to the midventricular segment and/or basal segment without involvement of the apical segment in a minor population.36) The involvement of RV can be observed in 26% of patients, and seems to be associated with the presence of more severe LV systolic dysfunction and pleural effusion.38)
CMR can be useful in the assessment of SCMP. The distinction between an ischemic process vs. SCMP can be difficult at times. Patients who have the left anterior descending coronary artery thrombus which then recannulated can appear to have no significant disease on coronary angiogram, and may mistakenly be diagnosed with SCMP. Furthermore, patients with microvascular obstruction may have normal appearing epicardial vessels on coronary angiography. CMR can differentiate between ischemic etiologies and SCMP with perfusion imaging, T2-weighted imaging and DHE. Patients with SCMP typically have characteristic wall motion defects similar to that seen in anteroapical infarction. Patients with SCMP may have small areas of subtle DHE in a pattern that is distinctly different from DHE seen in patients with myocardial fibrosis.39),40) Myocardial edema may also be present in these segments on T2-weighted images.40),41) Furthermore, a recent multicenter study using CMR demonstrated that SCMP may also present in other patterns of myocardial dysfunction, such as biventricular dysfunction, mid-ventricular dysfunction, and basal dysfunction. Patients in this study who had no DHE demonstrated complete recovery of the LV function on follow-up CMR.
However, SCMP can be misdiagnosed as AMI, and AMI can be misdiagnosed as SCMP.42) The recent guidelines for the diagnosis of SCMP37) define this entity on the basis of acute LV apical ballooning, after exclusion of AMI. Although there is lack of agreement on the necessity of coronary angiography, either this or coronary CT is usually performed to exclude coronary occlusion. DHE-CMR may be used in the identification of the presence of a significant coronary artery disease and decrease the need of conventional coronary angiography.16)
The treatment of SCMP is similar to that of ischemic LV dysfunction. However, the prognosis of SCMP is good, with rapid recovery within a week.11),37) For this reason, not only is appropriate imaging an important step to confirm the diagnosis, but follow-up imagine is often performed to confirm resolution.
Endocrine etiologies
Catecholamine-induced cardiomyopathy and pheochromocytoma
Both endogenous and exogenous catecholamines have direct effects on the myocardium, including myocarditis and endocardial and myocardial hemorrhage.43) Subcutaneous injection of catecholamines and high dosage of some of sympathomimetic drugs, for example, methamphetamine, can cause catecholamine-induced cardiomyopathy.44),45) Pheochromocytoma is a well-known cause of catecholamine-induced cardiomyopathy.46)
The exact incidence of pheochromocytoma is unknown, but with the recent, widespread use of CT in routine screening, its incidence as an incidental finding is increasing, and the incidence of LV dysfunction associated with pheochromocytoma remains low.47)
Echocardiographic examinations of patients with pheochromocytoma have shown both dilated and hypertrophic cardiomyopathies, as well as obstruction of the LV outflow tract.46),48) However, variable types of SCMP can be associated with pheochromocytomas and iatrogenic catecholamine excess.44),47)
Elevated levels of circulating catecholamines may cause direct myocardial injury. CMR can be useful in identifying such injury resulting from adrenergic myocarditis. Myocardial edema on T2-weighted imaging and diffuse and patchy DHE can be seen. Increased myocardial wall thickness and areas of hypokinesis can also be seen in areas of edema and myocardial fibrosis.49)
The treatment of these patients includes normalization of circulating catecholamine levels, decreasing sympathetic response with alpha- and beta-blockers and conventional treatment of heart failure. LV function normalizes rapidly with decrease of circulating catecholamine levels.46) The prognosis of LV systolic dysfunction associated with pheochromocytoma is good.
Hyperthyroidism
Thyroid hormone excess has cardiovascular manifestations that include cardiomegaly, heart failure, or atrial fibrillation, both as de novo cardiac disease as well as aggravating pre-existing cardiac problems.50)
The mechanisms of LV systolic dysfunction include circulatory and cardiac factors. The circulatory changes include increased total blood volume, decreased systemic vascular resistance, and shortened circulation time. These changes decrease afterload and increase preload of the LV. Cardiac factors include increased cardiac output, increased heart rate and direct effects of thyroid hormones on cardiac muscles. Also, thyroid hormones can potentiate actions of catecholamines.51) Increased cardiac work, reduced cardiac contractile reserve, and sustained tachycardia can result in LV systolic dysfunction.50)
About 6% of patients with thyrotoxicosis show symptoms of heart failure, although the incidence of LV systolic dysfunction is < 1%.52) In these patients, echocardiography shows LV enlargement, diffuse LV hypokinesia or LV systolic dysfunction with apical ballooning.53) RV dysfunction and tricuspid regurgitation may occur. Patients with hyperthyroidism have not been shown to exhibit any myocardial edema, increased wall thickness, or DHE on CMR.54)
Management of thyrotoxicosis related LV systolic dysfunction includes identification of the underlying disease and the rapid reversal of adrenergic tone with use of beta-blocking agents. Other treatments are similar to the conventional treatment of heart failure.
Acute adrenal insufficiency
Acute adrenal insufficiency can be associated with reversible cardiomyopathy without regional wall motion abnormalities (diffuse hypokinesia).55) It can result from two hemodynamic profiles: shock with high cardiac output and low systemic vascular resistance. Massive intravenous fluid therapy may transform a patient in hypovolemic shock with myocardial incompetence into one with shock with high cardiac output.56) CMR can identify patients who have had coronary vasospasm or severe epicardial artery disease resulting in myocardial infarction with DHE imaging. T2-weighted imaging can also provide assessment of the area at risk. LV systolic dysfunction usually recovers rapidly with glucocorticoid therapy.
Acromegaly due to pituitary adenoma
There is a case report with severe dilated LV systolic dysfunction associated with acromegaly.57) In this case, LV systolic function improved with the removal of the pituitary adenoma. It has also shown to be associated with increased T2 values on CMR, which may represent myocardial edema. In addition, patients with acromegaly have significantly increased LV mass on CMR.58)
Acute growth hormone deficiency
Reversible LV systolic dysfunction has been reported in acute growth hormone deficiency due to Sheehan's syndrome.59)
Tachycardia-induced cardiomyopathy
Prolonged tachycardia can cause reversible cardiomyopathy.60) Sustained rapid atrial or ventricular pacing for about 24 hours can cause severe biventricular systolic and diastolic dysfunction in animal models.61) In a human series, various arrhythmias caused tachycardia-induced cardiomyopathy including atrial fibrillation, atrial tachycardia, accessory pathway associated tachycardias, atrioventricular node reentry tachycardia and ventricular tachycardia associated with LV systolic dysfunction. Frequent ventricular premature complexes can be associated with transient LV systolic dysfunction.62)
The precise mechanisms responsible for developing cardiomyopathy are unknown. The proposed mechanisms include myocardial energy depletion and impaired utilization of energy, myocardial ischemia, abnormal regulation of cardiac calcium metabolism, and remodeling of cardiomyocytes and extracellular matrix.60)
Tachycardia-induced cardiomyopathy can occur in any age group. Although the ventricular rate that causes tachycardia-induced cardiomyopathy has not been determined in humans, clinicians should suspect it when LV systolic dysfunction accompanies persistent tachycardia (> 100 beats/minute).63) The main differential diagnosis is increased sympathetic activity and tachycardia due to reduced stroke volume.
Echocardiography usually shows left and right ventricular dilatation and decreased systolic function, but this can occur in association with other forms of heart disease.64) CMR can provide precise assessment of LV and RV function and volumes. Tachycardia-induced cardiomyopathy should not result in DHE. The presence of DHE and the pattern of this finding should raise the suspicion of an alternative etiology for LV dysfunction, based on the pattern of fibrosis.
This type of LV systolic dysfunction can improve rapidly (often within 4 weeks) with intervention or correction of the underlying cause of their tachycardia,65) but complete reverse remodeling may be slow (6 months or more). Recognition of this entity allows institution of heart rate control, although this may be ineffective, especially in atrial fibrillation, perhaps reflecting a role of variation in RR interval as well as tachycardia.66) Therapies include medications lowering heart rate, cardioversion or radiofrequency ablation of arrhythmias, and antiarrhythmic surgery.63) Even though LV function normalizes rapidly with treatment, recurrent tachycardia can cause rapid decline in LV function, development of heart failure and sudden cardiac death.65)
Deficiencies
Deficiencies of nutrients, electrolytes or trace elements can cause LV systolic dysfunction. Sometimes these patients exhibit other symptoms of deficiencies, i.e., patients with hypophosphatemia show skeletal muscle weakness.67) These deficiencies are usually associated with malnutrition, intoxication or misuse of illegal drugs. Correction of these deficiencies can improve LV systolic function with time.
Thiamine deficiency
Thiamine is a coenzyme that assists in macronutrient oxidation and the production of cellular adenosine triphosphate.68) Because thiamine is a water soluble B-complex vitamin, thiamine deficiency is usually caused by malnutrition and/or excess diuretic use.68),69) One study reported thiamine deficiency in about one-third of patients with congestive heart failure.69) Thiamine deficiency is characterized by peripheral vasodilatation,69) and in addition to LV dilatation and dysfunction, these patients may have RV dysfunction with severe pulmonary hypertension.70) The presence of skeletal muscle edema in patients with thiamine deficiency induced myopathy can be identified with T2-weighted imaging on magnetic resonance imaging,71) suggesting the same process may be used to identify myocardial edema in patients with thiamine deficiency and LV dysfunction. With thiamine replacement, the improvement is dramatic and rapid.68),70)
Hypophosphatemia
Transient LV systolic dysfunction can occur as a consequence of chronic ingestion of large quantities of a phosphate, for example as antacid.67) Hypophosphatemia may cause diffuse LV dilatation and dysfunction as well as profound skeletal muscle weakness. After restoration of the serum phosphorus level, LV systolic dysfunction usually normalizes within 2 to 5 weeks.67)
Hypocalcemia and hypoparathyroidism
Reversible LV systolic dysfunction can be associated with hypocalcemia and hypoparathyroidism.72) The resultant hypocalcemia and hypomagnesemia are possible mechanisms of LV systolic dysfunction.72)
Carnitine deficiency
A small percentage of dilated cardiomyopathy and hypertrophic cardiomyopathy cases result from carnitine deficiency,73) and dietary supplement of L-carnitine improves mortality, especially in children.74)
Selenium deficiency
Selenium, an essential trace element, is a component of glutathione peroxidase and may prevent oxidative damage to cells. Selenium deficiency has been noted to cause dilated cardiomyopathy, especially in children with poor nutrition.75)
Cardiomyopathies due to drugs and toxins
Some patients with hypertensive and alcoholic cardiomyopathy recover with treatment. Also, some patients with myocardial infiltrative diseases like hemochromatosis76) and light chain deposition disease77) can be normalized with treatment. Though anthracycline-induced cardiomyopathy is largely irreversible and cumulative, some patients show a reversible course.78)
Alcoholic cardiomyopathy
Excessive ethanol use is associated with heart failure (alcoholic cardiomyopathy).79) Careful questioning for a history of alcohol use is an important part of the evaluation. Because of greater frequency of alcoholism in men, it is most common in young males. Direct toxic effect of ethanol to myocardium, coexisting nutritional deficiencies, heavy metal contamination and other comorbidities are proposed mechanisms of the alcoholic cardiomyopathy. Echocardiographic findings are similar to those of dilated cardiomyopathy including dilatation of four chambers, globally decreased ventricular function and mitral or tricuspid regurgitation. Abstinence of alcohol can lead to a dramatic improvement in ventricular function.80)
Cardiac sarcoidosis
Cardiac sarcoidosis is caused by the cardiac involvement of sarcoidosis, a multi-systemic granulomatous disease of unknown cause. With autopsy data, cardiac involvement can be found in about 20-50% of patients with sarcoidosis.81),82) However, the symptoms of cardiac sarcoidosis are in about 2-5% of patients with sarcoidosis.81),83) The clinical presentations are variable including conduction abnormalities, heart failure, and sudden cardiac death.83) Heart failure can be resulted from direct involvement of myocardium, valvular regurgitation, and/or RV dysfunction secondary to pulmonary disease and it is relatively common in patients with cardiac sarcoidosis (10-30%).81),84) Because of variable clinical presentations and potential benefit of treatment, antemortem diagnosis of cardiac sarcoidosis is challenging. All patients diagnosed with sarcoidosis should be screened for cardiac involvement. Echocardiography is an initial screening method and it can show regional wall motion abnormalities, ventricular aneurysm, LV systolic or diastolic dysfunction, valvular regurgitation, abnormal septal wall thickness and pericardial effusion.85),86) However, these finding are not specific to cardiac sarcoidosis. If with these findings, other imaging study like CMR or coronary angiography may be needed to exclude other etiologies.
CMR is useful not only in the assessment of LV function but also in the determining the presence of cardiac sarcoidosis. Because tissue edema appears bright on T2-weighted images, inflammatory lesions in cardiac sarcoidosis can be identified with bright lesion.17),18) Late gadolinium enhancement-CMR can detect regional wall motion abnormalities and areas of wall thickening precisely.87) In their study, basal and lateral LV segments are most commonly involved areas.
The treatment of cardiac sarcoidosis is similar to that of other heart failure including diuretics, angiotensin converting enzyme inhibitors or angiotensin-receptor blockers and beta-blockers. Using corticosteroid is mainstay of therapy to decrease the inflammatory process. Though there are no randomized trial, high dose corticosteroid therapy seemed to be effective in patients with LV ejection fraction was between 30 to 55%.88) Corticosteroid use was associated with improvement in about 87% of patients.89)
Carbon monoxide intoxication
Transient LV systolic dysfunction occurs in about 50% of patients with carbon monoxide poisoning,90) probably due to tissue hypoxia caused by left-ward shift of the oxygen-hemoglobin dissociation curve as well as an inflammatory reaction.90) In addition to diffuse hypokinesia and LV systolic dysfunction with regional wall motion abnormalities, an apical ballooning pattern of LV systolic dysfunction can occur. LV systolic dysfunction is and generally normalizes with conventional treatment within 3 days.91) Carbon monoxide poisoning can also result in myocardial fibrosis (detectable with DHE by CMR). This finding can occur even in the setting of normal LV function.92)
Cocaine intoxication
Cocaine intoxication can cause an acute, reversible cardiomyopathy,93) predominantly through production of a profoundly enhanced sympathomimetic state similar to catecholamine-induced cardiomyopathy. Mid-wall DHE has also been described, due to myocardial infarction secondary to cocaine related vasoconstriction of the coronary arterioles or cocaine-induced myocarditis.94)
Scorpion envenomation
Scorpion venom can cause diffuse LV hypokinesia and reduction of LV systolic function.95) The proposed mechanisms of LV systolic dysfunction are massive release of catecholamines and/or direct toxic effect to the cardiomyocytes. Usually, the laboratory and echocardiographic features were normalized within 1 week and the clinical course is satisfactory.
Interferon
Interferon can cause diffuse LV systolic dysfunction by an unknown mechanism. Possible mechanisms include stimulation of an autoimmune or inflammatory reaction, increased demand for oxygen as a result of tissue reaction, or interferon-induced coronary spasm.96)
Others
Reversible cardiomyopathy and ventricular tachycardia can be associated with chronic inhalation of marijuana,97) high dose interleukin-2 treatment,98) and some psychotropic drugs including tricyclic antidepressants, phenothiazine and lithium.
Conclusion
Clinicians should use imaging tools to decide if there is a chance of reversibility when they face with heart failure patients. Imaging tools can give specific diagnosis and guide the treatment. Echocardiography is the most effective noninvasive screening tool to identify patients with LV dysfunction. It can measure LV systolic function along with detection of subsequent valvular and pericardial pathologies. Moreover, it can give hemodynamic information with using Doppler technology. Echocardiography is an excellent imaging modality to measure the LV function repeatedly when heart failure patients with changes in their symptoms, occurring a new clinical events, or evaluation of treatment effects.5),99) Repeated imaging can avoid inappropriate use of aggressive medications or implantable devices. CMR is a good noninvasive modality that can give information about LV systolic function, valvular and pericardial pathologies and tissue characterizations. CMR can give important clues to identify the presence of reversible cardiomyopathy. Additional evidence is needed to understand how the detection of improvement at repeat imaging should be used to monitor the responsiveness to therapy and the implications this has for the duration of therapy.
Acknowledgements
Special thanks to Cathryn Brock for her assistance in writing the manuscript.
References
- 1.McMurray JJ. Clinical practice. Systolic heart failure. N Engl J Med. 2010;362:228–238. doi: 10.1056/NEJMcp0909392. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.Ghali JK, Cooper R, Ford E. Trends in hospitalization rates for heart failure in the United States, 1973-1986. Evidence for increasing population prevalence. Arch Intern Med. 1990;150:769–773. [PubMed] [Google Scholar]
- 4.McNamara DM, Starling RC, Cooper LT, Boehmer JP, Mather PJ, Janosko KM, Gorcsan J, 3rd, Kip KE, Dec GW IMAC Investigators. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J Am Coll Cardiol. 2011;58:1112–1118. doi: 10.1016/j.jacc.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 6.Senni M, Rodeheffer RJ, Tribouilloy CM, Evans JM, Jacobsen SJ, Bailey KR, Redfield MM. Use of echocardiography in the management of congestive heart failure in the community. J Am Coll Cardiol. 1999;33:164–170. doi: 10.1016/s0735-1097(98)00523-3. [DOI] [PubMed] [Google Scholar]
- 7.Douglas PS, Khandheria B, Stainback RF, Weissman NJ, Brindis RG, Patel MR, Khandheria B, Alpert JS, Fitzgerald D, Heidenreich P, Martin ET, Messer JV, Miller AB, Picard MH, Raggi P, Reed KD, Rumsfeld JS, Steimle AE, Tonkovic R, Vijayaraghavan K, Weissman NJ, Yeon SB, Brindis RG, Douglas PS, Hendel RC, Patel MR, Peterson E, Wolk MJ, Allen JM American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group; American Society of Echocardiography; American College of Emergency Physicians; American Society of Nuclear Cardiology; Society for Cardiovascular Angiography and Interventions; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; American College of Chest Physicians; Society of Critical Care Medicine. ACCF/ASE/ACEP/ASNC/SCAI/SCCT/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Coll Cardiol. 2007;50:187–204. doi: 10.1016/j.jacc.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Otterstad JE, Froeland G, St John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18:507–513. doi: 10.1093/oxfordjournals.eurheartj.a015273. [DOI] [PubMed] [Google Scholar]
- 9.Afridi I, Kleiman NS, Raizner AE, Zoghbi WA. Dobutamine echocardiography in myocardial hibernation. Optimal dose and accuracy in predicting recovery of ventricular function after coronary angioplasty. Circulation. 1995;91:663–670. doi: 10.1161/01.cir.91.3.663. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, Honda T, Hase M, Kai R, Morii I Angina Pectoris-Myocardial Infarction Investigations in Japan. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 11.Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, Maron BJ. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 12.Mahrholdt H, Wagner A, Holly TA, Elliott MD, Bonow RO, Kim RJ, Judd RM. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation. 2002;106:2322–2327. doi: 10.1161/01.cir.0000036368.63317.1c. [DOI] [PubMed] [Google Scholar]
- 13.Kilner PJ, Gatehouse PD, Firmin DN. Flow measurement by magnetic resonance: a unique asset worth optimising. J Cardiovasc Magn Reson. 2007;9:723–728. doi: 10.1080/10976640701465090. [DOI] [PubMed] [Google Scholar]
- 14.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J. 2005;26:1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 15.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 16.Assomull RG, Shakespeare C, Kalra PR, Lloyd G, Gulati A, Strange J, Bradlow WM, Lyne J, Keegan J, Poole-Wilson P, Cowie MR, Pennell DJ, Prasad SK. Role of cardiovascular magnetic resonance as a gatekeeper to invasive coronary angiography in patients presenting with heart failure of unknown etiology. Circulation. 2011;124:1351–1360. doi: 10.1161/CIRCULATIONAHA.110.011346. [DOI] [PubMed] [Google Scholar]
- 17.Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF, Jr, Arai AE. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 18.Abdel-Aty H, Boyé P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 19.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–897. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blume U, Lockie T, Stehning C, Sinclair S, Uribe S, Razavi R, Schaeffter T. Interleaved T(1) and T(2) relaxation time mapping for cardiac applications. J Magn Reson Imaging. 2009;29:480–487. doi: 10.1002/jmri.21652. [DOI] [PubMed] [Google Scholar]
- 21.Sparrow P, Messroghli DR, Reid S, Ridgway JP, Bainbridge G, Sivananthan MU. Myocardial T1 mapping for detection of left ventricular myocardial fibrosis in chronic aortic regurgitation: pilot study. AJR Am J Roentgenol. 2006;187:W630–W635. doi: 10.2214/AJR.05.1264. [DOI] [PubMed] [Google Scholar]
- 22.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCarthy RE, 3rd, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, Baughman KL. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 25.Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, Hare JM. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–232. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 26.Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, Ansari A, Baughman KL. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–1188. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 27.Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, Sutton SR. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 28.Witlin AG, Mabie WC, Sibai BM. Peripartum cardiomyopathy: a longitudinal echocardiographic study. Am J Obstet Gynecol. 1997;177:1129–1132. doi: 10.1016/s0002-9378(97)70028-0. [DOI] [PubMed] [Google Scholar]
- 29.Cooper LT, Mather PJ, Alexis JD, Pauly DF, Torre-Amione G, Wittstein IS, Dec GW, Zucker M, Narula J, Kip K, McNamara DM IMAC2 Investigators. Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail. 2012;18:28–33. doi: 10.1016/j.cardfail.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barone-Rochette G, Rodière M, Lantuejoul S. Value of cardiac MRI in peripartum cardiomyopathy. Arch Cardiovasc Dis. 2011;104:263–264. doi: 10.1016/j.acvd.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Kawano H, Tsuneto A, Koide Y, Tasaki H, Sueyoshi E, Sakamoto I, Hayashi T. Magnetic resonance imaging in a patient with peripartum cardiomyopathy. Intern Med. 2008;47:97–102. doi: 10.2169/internalmedicine.47.0316. [DOI] [PubMed] [Google Scholar]
- 32.Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, McMurray J, Yamac H, Labidi S, Struman I, Hilfiker-Kleiner D. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation. 2010;121:1465–1473. doi: 10.1161/CIRCULATIONAHA.109.901496. [DOI] [PubMed] [Google Scholar]
- 33.Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, Wani OR, Hameed A, Gviazda I, Shotan A. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344:1567–1571. doi: 10.1056/NEJM200105243442101. [DOI] [PubMed] [Google Scholar]
- 34.Pavin D, Le Breton H, Daubert C. Human stress cardiomyopathy mimicking acute myocardial syndrome. Heart. 1997;78:509–511. doi: 10.1136/hrt.78.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park JH, Kang SJ, Song JK, Kim HK, Lim CM, Kang DH, Koh Y. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest. 2005;128:296–302. doi: 10.1378/chest.128.1.296. [DOI] [PubMed] [Google Scholar]
- 36.Hurst RT, Askew JW, Reuss CS, Lee RW, Sweeney JP, Fortuin FD, Oh JK, Tajik AJ. Transient midventricular ballooning syndrome: a new variant. J Am Coll Cardiol. 2006;48:579–583. doi: 10.1016/j.jacc.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 37.Kawai S, Kitabatake A, Tomoike H Takotsubo Cardiomyopathy Group. Guidelines for diagnosis of takotsubo (ampulla) cardiomyopathy. Circ J. 2007;71:990–992. doi: 10.1253/circj.71.990. [DOI] [PubMed] [Google Scholar]
- 38.Haghi D, Athanasiadis A, Papavassiliu T, Suselbeck T, Fluechter S, Mahrholdt H, Borggrefe M, Sechtem U. Right ventricular involvement in Takotsubo cardiomyopathy. Eur Heart J. 2006;27:2433–2439. doi: 10.1093/eurheartj/ehl274. [DOI] [PubMed] [Google Scholar]
- 39.Haghi D, Fluechter S, Suselbeck T, Kaden JJ, Borggrefe M, Papavassiliu T. Cardiovascular magnetic resonance findings in typical versus atypical forms of the acute apical ballooning syndrome (Takotsubo cardiomyopathy) Int J Cardiol. 2007;120:205–211. doi: 10.1016/j.ijcard.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 40.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz-Menger J, Thiele H, Friedrich MG. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- 41.Abdel-Aty H, Cocker M, Friedrich MG. Myocardial edema is a feature of Tako-Tsubo cardiomyopathy and is related to the severity of systolic dysfunction: insights from T2-weighted cardiovascular magnetic resonance. Int J Cardiol. 2009;132:291–293. doi: 10.1016/j.ijcard.2007.08.102. [DOI] [PubMed] [Google Scholar]
- 42.Shin SK, Jin SA, Park YK, Park JH. A Case of Acute ST-Segment Elevation Myocardial Infarction Mimicking Stress Induced Cardiomyopathy; Demonstration of Typical Echocardiographic Finding Correlated with Unusual Distribution of Left Anterior Descending Coronary Artery. J Cardiovasc Ultrasound. 2010;18:101–103. doi: 10.4250/jcu.2010.18.3.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haft JI. Cardiovascular injury induced by sympathetic catecholamines. Prog Cardiovasc Dis. 1974;17:73–86. doi: 10.1016/0033-0620(74)90039-5. [DOI] [PubMed] [Google Scholar]
- 44.Kim EM, Park JH, Park YS, Lee JH, Choi SW, Jeong JO, Seong IW. Catecholamines may play an important role in the pathogenesis of transient mid- and basal ventricular ballooning syndrome. J Korean Med Sci. 2008;23:898–902. doi: 10.3346/jkms.2008.23.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobs LJ. Reversible dilated cardiomyopathy induced by methamphetamine. Clin Cardiol. 1989;12:725–727. doi: 10.1002/clc.4960121211. [DOI] [PubMed] [Google Scholar]
- 46.Imperato-McGinley J, Gautier T, Ehlers K, Zullo MA, Goldstein DS, Vaughan ED., Jr Reversibility of catecholamine-induced dilated cardiomyopathy in a child with a pheochromocytoma. N Engl J Med. 1987;316:793–797. doi: 10.1056/NEJM198703263161307. [DOI] [PubMed] [Google Scholar]
- 47.Park JH, Kim KS, Sul JY, Shin SK, Kim JH, Lee JH, Choi SW, Jeong JO, Seong IW. Prevalence and patterns of left ventricular dysfunction in patients with pheochromocytoma. J Cardiovasc Ultrasound. 2011;19:76–82. doi: 10.4250/jcu.2011.19.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam JB, Shub C, Sheps SG. Reversible dilatation of hypertrophied left ventricle in pheochromocytoma: serial two-dimensional echocardiographic observations. Am Heart J. 1985;109(3 Pt 1):613–615. doi: 10.1016/0002-8703(85)90580-0. [DOI] [PubMed] [Google Scholar]
- 49.Roghi A, Pedrotti P, Milazzo A, Bonacina E, Bucciarelli-Ducci C. Adrenergic myocarditis in pheochromocytoma. J Cardiovasc Magn Reson. 2011;13:4. doi: 10.1186/1532-429X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woeber KA. Thyrotoxicosis and the heart. N Engl J Med. 1992;327:94–98. doi: 10.1056/NEJM199207093270206. [DOI] [PubMed] [Google Scholar]
- 51.Levey GS, Klein I. Catecholamine-thyroid hormone interactions and the cardiovascular manifestations of hyperthyroidism. Am J Med. 1990;88:642–646. doi: 10.1016/0002-9343(90)90533-j. [DOI] [PubMed] [Google Scholar]
- 52.Dahl P, Danzi S, Klein I. Thyrotoxic cardiac disease. Curr Heart Fail Rep. 2008;5:170–176. doi: 10.1007/s11897-008-0026-9. [DOI] [PubMed] [Google Scholar]
- 53.Radhakrishnan A, Granato JE. An association between Takotsubo cardiomyopathy and thyroid storm. Postgrad Med. 2009;121:126–130. doi: 10.3810/pgm.2009.05.2012. [DOI] [PubMed] [Google Scholar]
- 54.Thebault C, Leurent G, Potier J, Bedossa M, Bonnet F. A case of thyroid storm following radioiodine therapy underlying usefulness of cardiac MRI. Eur J Intern Med. 2009;20:e136–e137. doi: 10.1016/j.ejim.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Derish M, Eckert K, Chin C. Reversible cardiomyopathy in a child with Addison's disease. Intensive Care Med. 1996;22:460–463. doi: 10.1007/BF01712167. [DOI] [PubMed] [Google Scholar]
- 56.Bouachour G, Tirot P, Varache N, Gouello JP, Harry P, Alquier P. Hemodynamic changes in acute adrenal insufficiency. Intensive Care Med. 1994;20:138–141. doi: 10.1007/BF01707669. [DOI] [PubMed] [Google Scholar]
- 57.Legrand V, Beckers A, Pham VT, Demoulin JC, Stevenaert A. Dramatic improvement of severe dilated cardiomyopathy in an acromegalic patient after treatment with octreotide and trans-sphenoidal surgery. Eur Heart J. 1994;15:1286–1289. doi: 10.1093/oxfordjournals.eurheartj.a060668. [DOI] [PubMed] [Google Scholar]
- 58.Gouya H, Vignaux O, Le Roux P, Chanson P, Bertherat J, Bertagna X, Legmann P. Rapidly reversible myocardial edema in patients with acromegaly: assessment with ultrafast T2 mapping in a single-breath-hold MRI sequence. AJR Am J Roentgenol. 2008;190:1576–1582. doi: 10.2214/AJR.07.2031. [DOI] [PubMed] [Google Scholar]
- 59.Frustaci A, Perrone GA, Gentiloni N, Russo MA. Reversible dilated cardiomyopathy due to growth hormone deficiency. Am J Clin Pathol. 1992;97:503–511. doi: 10.1093/ajcp/97.4.503. [DOI] [PubMed] [Google Scholar]
- 60.Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, Scheinman MM. Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol. 1997;29:709–715. doi: 10.1016/s0735-1097(96)00592-x. [DOI] [PubMed] [Google Scholar]
- 61.Moe GW, Stopps TP, Howard RJ, Armstrong PW. Early recovery from heart failure: insights into the pathogenesis of experimental chronic pacing-induced heart failure. J Lab Clin Med. 1988;112:426–432. [PubMed] [Google Scholar]
- 62.Chugh SS, Shen WK, Luria DM, Smith HC. First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol. 2000;11:328–329. doi: 10.1111/j.1540-8167.2000.tb01802.x. [DOI] [PubMed] [Google Scholar]
- 63.Umana E, Solares CA, Alpert MA. Tachycardia-induced cardiomyopathy. Am J Med. 2003;114:51–55. doi: 10.1016/s0002-9343(02)01472-9. [DOI] [PubMed] [Google Scholar]
- 64.Redfield MM, Kay GN, Jenkins LS, Mianulli M, Jensen DN, Ellenbogen KA. Tachycardia-related cardiomyopathy: a common cause of ventricular dysfunction in patients with atrial fibrillation referred for atrioventricular ablation. Mayo Clin Proc. 2000;75:790–795. doi: 10.4065/75.8.790. [DOI] [PubMed] [Google Scholar]
- 65.Nerheim P, Birger-Botkin S, Piracha L, Olshansky B. Heart failure and sudden death in patients with tachycardia-induced cardiomyopathy and recurrent tachycardia. Circulation. 2004;110:247–252. doi: 10.1161/01.CIR.0000135472.28234.CC. [DOI] [PubMed] [Google Scholar]
- 66.Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol. 1997;30:1039–1045. doi: 10.1016/s0735-1097(97)00254-4. [DOI] [PubMed] [Google Scholar]
- 67.Darsee JR, Nutter DO. Reversible severe congestive cardiomyopathy in three cases of hypophosphatemia. Ann Intern Med. 1978;89:867–870. doi: 10.7326/0003-4819-89-6-867. [DOI] [PubMed] [Google Scholar]
- 68.Shimon I, Almog S, Vered Z, Seligmann H, Shefi M, Peleg E, Rosenthal T, Motro M, Halkin H, Ezra D. Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am J Med. 1995;98:485–490. doi: 10.1016/s0002-9343(99)80349-0. [DOI] [PubMed] [Google Scholar]
- 69.Hanninen SA, Darling PB, Sole MJ, Barr A, Keith ME. The prevalence of thiamin deficiency in hospitalized patients with congestive heart failure. J Am Coll Cardiol. 2006;47:354–361. doi: 10.1016/j.jacc.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 70.Park JH, Lee JH, Jeong JO, Seong IW, Choi SW. Thiamine deficiency as a rare cause of reversible severe pulmonary hypertension. Int J Cardiol. 2007;121:e1–e3. doi: 10.1016/j.ijcard.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 71.Koike H, Watanabe H, Inukai A, Iijima M, Mori K, Hattori N, Sobue G. Myopathy in thiamine deficiency: analysis of a case. J Neurol Sci. 2006;249:175–179. doi: 10.1016/j.jns.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 72.Bashour T, Basha HS, Cheng TO. Hypocalcemic cardiomyopathy. Chest. 1980;78:663–665. doi: 10.1378/chest.78.4.663. [DOI] [PubMed] [Google Scholar]
- 73.Ino T, Sherwood WG, Benson LN, Wilson GJ, Freedom RM, Rowe RD. Cardiac manifestations in disorders of fat and carnitine metabolism in infancy. J Am Coll Cardiol. 1988;11:1301–1308. doi: 10.1016/0735-1097(88)90296-3. [DOI] [PubMed] [Google Scholar]
- 74.Helton E, Darragh R, Francis P, Fricker FJ, Jue K, Koch G, Mair D, Pierpont ME, Prochazka JV, Linn LS, Winter SC. Metabolic aspects of myocardial disease and a role for L-carnitine in the treatment of childhood cardiomyopathy. Pediatrics. 2000;105:1260–1270. [PubMed] [Google Scholar]
- 75.Reeves WC, Marcuard SP, Willis SE, Movahed A. Reversible cardiomyopathy due to selenium deficiency. JPEN J Parenter Enteral Nutr. 1989;13:663–665. doi: 10.1177/0148607189013006663. [DOI] [PubMed] [Google Scholar]
- 76.Rahko PS, Salerni R, Uretsky BF. Successful reversal by chelation therapy of congestive cardiomyopathy due to iron overload. J Am Coll Cardiol. 1986;8:436–440. doi: 10.1016/s0735-1097(86)80063-8. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura M, Satoh M, Kowada S, Satoh H, Tashiro A, Sato F, Masuda T, Hiramori K. Reversible restrictive cardiomyopathy due to light-chain deposition disease. Mayo Clin Proc. 2002;77:193–196. doi: 10.4065/77.2.193. [DOI] [PubMed] [Google Scholar]
- 78.Keefe DL. Anthracycline-induced cardiomyopathy. Semin Oncol. 2001;28(4 Suppl 12):2–7. [PubMed] [Google Scholar]
- 79.Regan TJ. Alcoholic cardiomyopathy. Prog Cardiovasc Dis. 1984;27:141–152. doi: 10.1016/0033-0620(84)90001-x. [DOI] [PubMed] [Google Scholar]
- 80.Demakis JG, Proskey A, Rahimtoola SH, Jamil M, Sutton GC, Rosen KM, Gunnar RM, Tobin JR. The natural course of alcoholic cardiomyopathy. Ann Intern Med. 1974;80:293–297. doi: 10.7326/0003-4819-80-3-293. [DOI] [PubMed] [Google Scholar]
- 81.Matsui Y, Iwai K, Tachibana T, Fruie T, Shigematsu N, Izumi T, Homma AH, Mikami R, Hongo O, Hiraga Y, Yamamoto M. Clinicopathological study of fatal myocardial sarcoidosis. Ann N Y Acad Sci. 1976;278:455–469. doi: 10.1111/j.1749-6632.1976.tb47058.x. [DOI] [PubMed] [Google Scholar]
- 82.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation. 1978;58:1204–1211. doi: 10.1161/01.cir.58.6.1204. [DOI] [PubMed] [Google Scholar]
- 83.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Jr, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, McLennan G, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki B, Weinberger SE, Terrin ML, Knatterud GL, Cherniak R Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 84.Roberts WC, McAllister HA, Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11) Am J Med. 1977;63:86–108. doi: 10.1016/0002-9343(77)90121-8. [DOI] [PubMed] [Google Scholar]
- 85.Burstow DJ, Tajik AJ, Bailey KR, DeRemee RA, Taliercio CP. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol. 1989;63:478–482. doi: 10.1016/0002-9149(89)90323-8. [DOI] [PubMed] [Google Scholar]
- 86.Gibbons WJ, Levy RD, Nava S, Malcolm I, Marin JM, Tardif C, Magder S, Lisbona R, Cosio MG. Subclinical cardiac dysfunction in sarcoidosis. Chest. 1991;100:44–50. doi: 10.1378/chest.100.1.44. [DOI] [PubMed] [Google Scholar]
- 87.Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol. 2005;45:1683–1690. doi: 10.1016/j.jacc.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 88.Chiu CZ, Nakatani S, Zhang G, Tachibana T, Ohmori F, Yamagishi M, Kitakaze M, Tomoike H, Miyatake K. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95:143–146. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 89.Chapelon-Abric C, de Zuttere D, Duhaut P, Veyssier P, Wechsler B, Huong DL, de Gennes C, Papo T, Blétry O, Godeau P, Piette JC. Cardiac sarcoidosis: a retrospective study of 41 cases. Medicine (Baltimore) 2004;83:315–334. doi: 10.1097/01.md.0000145367.17934.75. [DOI] [PubMed] [Google Scholar]
- 90.Satran D, Henry CR, Adkinson C, Nicholson CI, Bracha Y, Henry TD. Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J Am Coll Cardiol. 2005;45:1513–1516. doi: 10.1016/j.jacc.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 91.Ahn KT, Park JH, Kim MS, Park YS, Kim YJ, Lee IS, Kim JH, Lee JH, Choi SW, Jeong JO, Seong IW. Prevalence and clinical outcomes of left ventricular systolic dysfunction after carbon monoxide exposure. Int J Cardiol. 2011;153:108–110. doi: 10.1016/j.ijcard.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 92.Henry TD, Lesser JR, Satran D. Myocardial fibrosis from severe carbon monoxide poisoning detected by cardiac magnetic resonance imaging. Circulation. 2008;118:792. doi: 10.1161/CIRCULATIONAHA.107.750778. [DOI] [PubMed] [Google Scholar]
- 93.Chokshi SK, Moore R, Pandian NG, Isner JM. Reversible cardiomyopathy associated with cocaine intoxication. Ann Intern Med. 1989;111:1039–1040. doi: 10.7326/0003-4819-111-12-1039. [DOI] [PubMed] [Google Scholar]
- 94.Robaei D, Grieve SM, Nelson GC, Bhindi R, Figtree GA. Cocaine-induced epicardial coronary artery thrombosis resulting in extensive myocardial injury assessed by cardiac magnetic resonance imaging. Eur Heart J. 2010;31:2446. doi: 10.1093/eurheartj/ehq229. [DOI] [PubMed] [Google Scholar]
- 95.Hering SE, Jurca M, Vichi FL, Azevedo-Marques MM, Cupo P. Reversible cardiomyopathy in patients with severe scorpion envenoming by Tityus serrulatus: evolution of enzymatic, electrocardiographic and echocardiographic alterations. Ann Trop Paediatr. 1993;13:173–182. doi: 10.1080/02724936.1993.11747642. [DOI] [PubMed] [Google Scholar]
- 96.Sonnenblick M, Rosenmann D, Rosin A. Reversible cardiomyopathy induced by interferon. BMJ. 1990;300:1174–1175. doi: 10.1136/bmj.300.6733.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ting JY. Reversible cardiomyopathy associated with acute inhaled marijuana use in a young adult. Clin Toxicol (Phila) 2007;45:432–434. doi: 10.1080/15563650601073587. [DOI] [PubMed] [Google Scholar]
- 98.Goel M, Flaherty L, Lavine S, Redman BG. Reversible cardiomyopathy after high-dose interleukin-2 therapy. J Immunother (1991) 1992;11:225–229. doi: 10.1097/00002371-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 99.Hunt SA American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]