Abstract
The Hunter Outcome Survey (HOS), an international, long-term observational registry of patients with Hunter syndrome, was used to develop a simple mnemonic screening tool (HUNTER) to aid in the diagnosis of Hunter syndrome. Data regarding the prediagnosis prevalence of ten specific signs and symptoms present in individual patients enrolled in the HOS were used to develop the HUNTER mnemonic screening tool. A total score of 6 or greater using a weighting scheme in which certain manifestations were assigned a weight of 2 (facial dysmorphism, nasal obstruction or rhinorrhea, enlarged tongue, enlarged liver, enlarged spleen, joint stiffness) and others assigned a weight of 1 (hernia, hearing impairment, enlarged tonsils, airway obstruction or sleep apnea) correctly identified 95 % of patients who had no family history of Hunter syndrome or who were not diagnosed prenatally. No association between age at diagnosis and HUNTER score was found. Conclusion: The HUNTER mnemonic appears to be a useful screening tool. Further validation in the clinical setting will be necessary to confirm its utility.
Keywords: Mucopolysaccharidosis type II, Lysosomal storage disease, Hunter syndrome, Diagnosis
Introduction
Hunter syndrome (mucopolysaccharidosis type II or MPS II) is an X-linked metabolic disorder caused by a deficiency in the lysosomal enzyme iduronate-2-sulfatase (I2S) [1, 12]. I2S catalyzes a step in the degradation of dermatan sulfate and heparan sulfate [12], and in affected patients, these enzyme substrates accumulate in cells and tissues and contribute to the multiorgan pathologies associated with Hunter syndrome. It has been estimated that the incidence is about 1 in 162,000 live male births [9]. Hunter syndrome patients usually appear normal at birth with clinical signs and symptoms emerging between the ages of 2 and 4 years [8, 12]. The presenting signs typically include coarse facial features; respiratory obstruction caused by enlarged tongue, tonsils, and adenoids; joint stiffness and skeletal abnormalities; and enlarged liver and spleen [15–17]. Two phenotypes are recognized—severe and attenuated. The severe phenotype is characterized by progressive central nervous system involvement that results in severe cognitive impairment and developmental regression, with death typically occurring in the second decade of life [7, 12, 17]. Patients with the attenuated phenotype do not experience cognitive impairment but may still demonstrate all of the somatic signs and symptoms of the disease [8, 12, 16]. These patients may survive into adulthood, although premature mortality occurs [7].
Diagnosis of Hunter syndrome can be challenging as signs and symptoms of Hunter syndrome are not specific to the disease, and not all clinical features will be present in the same order or equally rapidly in each patient. Diagnosis may therefore be delayed, especially in patients without a known family history of Hunter syndrome. Enzyme replacement therapy (ERT) with recombinant human I2S is available for the treatment of Hunter syndrome [10, 11]. In addition to ERT, there is a hope that Hunter syndrome may be responsive to bone marrow transplantation in the future [5]. Therefore, although a rare disease, pediatricians and other primary care physicians have an obligation to be aware of the signs and symptoms of Hunter syndrome and methods and tools to effectively establish a diagnosis. Mnemonics are commonly employed in clinical medicine as screening tools or to guide clinical management [2, 13], and here, we describe the development of a simple mnemonic screening tool that may aid pediatric specialists and primary care physicians in the recognition and diagnosis of Hunter syndrome.
Patients and methods
Hunter outcome survey
Hunter Outcome Survey (HOS) is an international, long-term observational registry designed to increase the knowledge of the natural history of Hunter syndrome and to evaluate the safety and effectiveness of long-term ERT with idursulfase (Elaprase®, Shire Human Genetic Therapies, Inc. (Shire HGT), Lexington, MA, USA) [15]. All participating centers receive approval from their local institutional review board or ethics committee before enrolling any patients into HOS. HOS is controlled by physicians and is overseen by national, regional, and international advisory boards comprising physicians experienced in the management and treatment of Hunter syndrome patients. Any patient with a biochemically or genetically confirmed diagnosis of Hunter syndrome is eligible to enroll in HOS independent of whether receiving treatment or not. All patients and/or their guardians provide informed written consent prior to participation in HOS.
The HOS database consists of data collected from medical examinations conducted during the usual medical care of the patients. The signs, symptoms, and other clinical information (e.g., medical history, laboratory values, etc.) surveyed are described in detail by Wraith and colleagues [15] and cover all the major organ and system involvement that occurs in Hunter syndrome. Shire HGT maintains the database and provides statistical support to HOS physicians and advisory boards. For the purposes of this study, the investigators were given permission, by the global executive committee of the HOS participating investigators, to query the database regarding the prevalence of specific signs and symptoms present in individual patients. The preliminary assessment was based on the HOS database as of January 10, 2008, and the final assessment was based on the April 16, 2009 data extract.
Hunter mnemonic
The goal of the present study was to develop a sensitive screening tool to aid in the diagnosis of Hunter syndrome using medical history data collected in HOS. Table 1 presents the HUNTER mnemonic, which was based on the key clinical features observed in patients with Hunter syndrome. The January 2008 data extract of the HOS database was queried for the prevalence of the signs and symptoms before diagnosis within individual patients as shown in Table 2. A random sample of 25 patients in HOS with available information and who did not have a prenatal diagnosis was used to characterize the utility of a uniform weighting (Table 2, weighting 1) of these ten signs and symptoms to specifically identify patients with Hunter syndrome. A second weighting scheme was also evaluated (Table 2, weighting 2) using a second random sample of 25 patients in HOS with available information who were not included in the first sample and who did not have a prenatal diagnosis or family history of Hunter syndrome. With this scheme, signs and symptoms unlikely to be observed in a non-affected child were given a score of 2. These signs and symptoms included dysmorphic facial features, nasal obstruction, enlarged tongue, enlarged liver, enlarged spleen, and joint stiffness. The remaining signs and symptoms were given a score of 1. A total score between 0 and 16 was assigned to each patient. After this preliminary evaluation of the weighting schemes, weighting 2 was again evaluated using the April 2009 extract of the HOS database, which included 237 patients with available data who did not have a prenatal diagnosis or a family history of Hunter syndrome and who were not included in the two random samples of 25 patients used to assess the two weighting schemes.
Table 1.
The HUNTER mnemonic screening instrument for identifying patients with Hunter syndrome
| Mnemonic | Key clinical feature | |
|---|---|---|
| H |
Hernia Hearing |
Hernia Hearing loss or impairment, hearing aids, chronic or acute otitis |
| U | Unusual faces | Dysmorphic facial features |
| N | Nasal obstruction | Nasal obstruction, rhinorrhea |
| T | Tongue and tonsils | Enlarged tongue, enlarged tonsils |
| E | Enlarged liver and spleen | Hepatomegaly, splenomegaly |
| R |
Respiration Range of motion |
Airway obstruction, sleep apnea Joint stiffness |
Table 2.
Signs and symptoms used in the development of the HUNTER score screening tool
| Signs or symptoms | Weighting 1 | Weighting 2 |
|---|---|---|
| Hernia | 1 | 1 |
| Hearing loss, use of a hearing aid, acute or chronic otitis | 1 | 1 |
| Dysmorphic facial features | 1 | 2 |
| Nasal obstruction or rhinorrhea | 1 | 2 |
| Enlarged tongue | 1 | 2 |
| Enlarged tonsils | 1 | 1 |
| Hepatomegaly | 1 | 2 |
| Splenomegaly | 1 | 2 |
| Airway obstruction or sleep apnea | 1 | 1 |
| Joint stiffness | 1 | 2 |
| Maximum score for a patient | 10 | 16 |
Analysis
No formal statistical analysis of the weighting schemes was performed. The investigators relied on their clinical experience to determine if the results appeared to be specific enough to correctly identify patients with Hunter syndrome.
Results
As of April 16, 2009, a total of 541 prospective patients (i.e., alive at HOS entry) were enrolled in HOS. The mean patient age at their most recent examination as entered into HOS was 12.3 years (median 10.3 years, 10th–90th percentile 3.7–23.4 years). These patients had been enrolled in HOS for a mean of 10.4 months. The age at onset of signs and symptoms and the age at diagnosis are summarized in Table 3.
Table 3.
Age at onset of signs and symptoms and diagnosis of Hunter syndrome
| Number | Mean (years) | Median (years) | 10th to 90th percentile | |
|---|---|---|---|---|
| Age at onset of symptoms | 405 | 2.0 | 1.5 | 0.2–4.0 |
| Age at diagnosis | 479 | 4.0 | 3.5 | 1.2–7.1 |
| Delay between onset of symptoms and diagnosis | 399 | 1.9 | 1.3 | 0–4.4 |
Based on prospective patients (i.e., alive at HOS entry) from the April 16, 2009 data extract
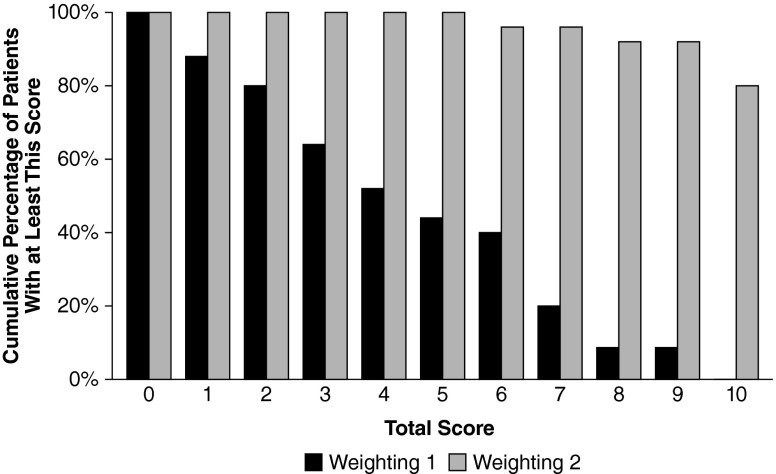
Figure 1 illustrates the results of the preliminary testing of the utility of uniform weighting (weighting 1) of the ten signs and symptoms presented in Table 2 as well as the second weighting scheme (weighting 2) using a second random sample. No threshold HUNTER score was found that could identify a Hunter patient without yielding too many false negative results. For example, a score of 1 failed to identify 12 % of the patients with Hunter syndrome and a score of 2 failed to identify 20 % of the patients. The failure of uniform weighting to identify Hunter syndrome patients was likely due in part to the fact that patients with a family history of Hunter syndrome were included in the test sample of 25 randomly selected patients. Because a family history of Hunter syndrome may result in diagnosis before the emergence of many signs and symptoms, this test sample included many patients with low scores. In addition, many of the signs and symptoms are common in children without Hunter syndrome, e.g., otitis and rhinorrhea.
Fig. 1.
Preliminary testing of weighting schemes for the HUNTER score. The black bars represent weighting 1, and the gray bars represent weighting 2. The maximum score for weighting 1 is 10, and the maximum score for weighting 2 is 16. N = 25 randomly selected patients from HOS. Based on two random samples of patients from the January 10, 2008 data extract. HOS Hunter Outcome Survey
Weighting scheme 2 (Table 2) was developed to give greater weight to those signs and symptoms that are more likely to be specifically associated with Hunter syndrome. Preliminary testing with 25 randomly selected patients from HOS who did not have a family history of Hunter syndrome suggested that a HUNTER score of 6 or 7 would correctly identify more than 95 % of patients with Hunter syndrome.
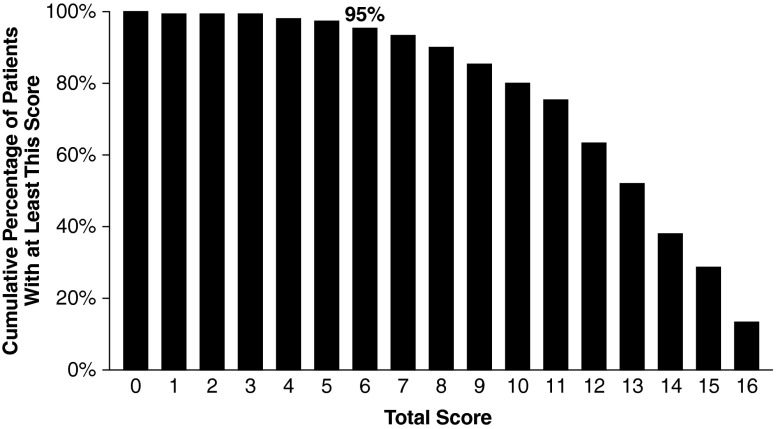
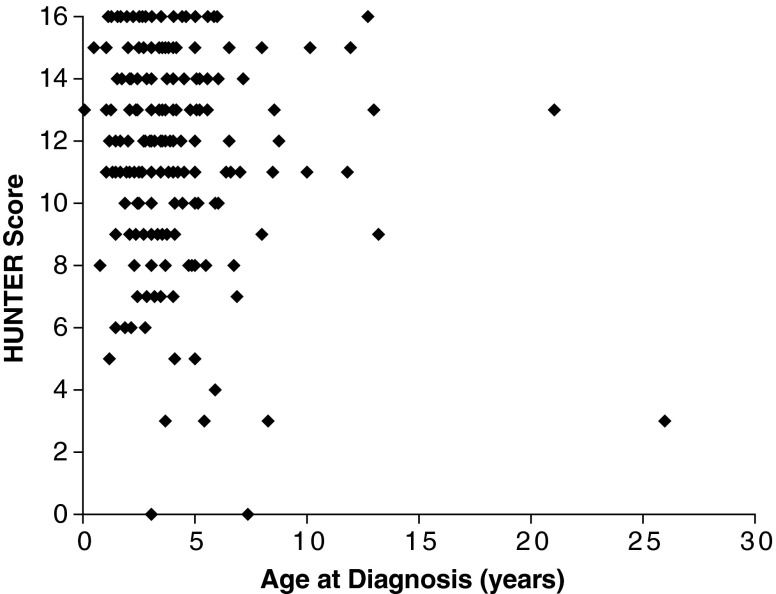
Weighting 2 was further evaluated by using the 237 patients in HOS who had complete information regarding the prevalence of signs and symptoms and who had no prenatal diagnosis and no family history of Hunter syndrome. A score of 6 or greater correctly identified 95 % of this population as having Hunter syndrome (Fig. 2). No association between age of diagnosis and HUNTER score at diagnosis was observed (Fig. 3). The median (10th–90th percentile) HUNTER score for patients <2, 2 to 4, and >4 years old at diagnosis was 12 (7–16), 13 (8–16), and 13 (7–16), respectively.
Fig. 2.
HUNTER evaluation with patients in HOS with complete information. N = 237 patients enrolled in HOS with complete information. Based on the April 16, 2009 data extract and excluding patients with missing information, prenatal diagnosis, or family history of Hunter. HOS Hunter Outcome Survey
Fig. 3.
The association between HUNTER score and age at diagnosis. N = 237 patients in HOS with complete information. Based on the April 16, 2009 data extract and excluding patients with missing information or with prenatal diagnosis or family history of Hunter. HOS Hunter Outcome Survey
Discussion
This simple mnemonic screening tool correctly identified patients as having Hunter syndrome with 95 % accuracy at a HUNTER score of 6 or above. This score appears to be unlikely to be attained by a person without Hunter syndrome or other MPS disorders.
Mnemonics are frequently used in clinical practice as screening tools or to guide clinical management, for example, mnemonic or scoring systems have been employed to screen newborns for Down syndrome [6], to identify infants in need of resuscitation [4], and to describe heart sounds [14].
The early diagnosis of Hunter syndrome remains a challenge [3]. A newborn screening test is not yet available, and the low awareness of rare, genetic diseases hinders the recognition and diagnosis of this syndrome. Most pediatricians will never encounter a child with this syndrome during their entire careers. The recognition and diagnosis of Hunter syndrome is often delayed because its complex and diverse signs and symptoms are not specific to Hunter syndrome [8], making a simple tool to help narrow the differential diagnosis is an asset in pediatric and primary care settings.
The goal of the present study was to develop a simple, sensitive, and unified screening tool for identifying Hunter syndrome. The first iteration in which the ten signs and symptoms were given equal weight was not sufficiently sensitive and failed to yield a reliable threshold score that would identify Hunter syndrome without producing a large number of false negatives (and presumably false positives). The inclusion of patients with a family history of Hunter syndrome may have contributed to the failure of uniform weighting to correctly identify patients with Hunter syndrome because these patients may have had earlier diagnoses before the emergence of many signs and symptoms of Hunter syndrome than patients without a family history. The final iteration in which the Hunter syndrome-specific signs and symptoms were weighted 2 points (Table 2) appeared to correctly identify 95 % of Hunter patients in the study cohort with a HUNTER score of 6 or higher. Additionally, it seems unlikely that patients without Hunter syndrome or other MPS disorders would be likely to meet or exceed this threshold HUNTER score. It is important to note that exceeding this threshold is only suggestive of Hunter syndrome and biochemical and/or genetic confirmation is still necessary to confirm the presence of this disease and other forms of MPS.
We acknowledge that this tool requires validation in a clinical setting to assess the tool’s sensitivity, specificity, positive and negative predictive values, and patient variability across all types of pediatric patients. This study represents the first step in the development of tool based on hundreds of observations of children with Hunter syndrome, which would otherwise be impossible to develop without such a registry, given the rarity of this disorder. The tool at present is likely to generate false positive but may also help in the identification of other children with rare inherited disease, which will still be of great value. We hope this paper will serve as a first step toward a more refined tool with high predictive value.
Conclusions
The HUNTER mnemonic had a sensitivity of over 95 % to detect Hunter syndrome at a HUNTER score of 6 or greater and, given the overlap of clinical features, may also help in the identification of other MPSs. Validation of this tool in the clinical setting will be required to determine the sensitivity, specificity, as well as positive and negative predictive value of this tool, and to confirm its utility as a screen for Hunter syndrome in routine clinical practice. The approach described herein illustrates the utility of disease registries in the development of tools that may assist physicians in the diagnosis and management of rare, genetic disorders. As newborn screening for Hunter syndrome is presently unavailable, it is hoped that this simple mnemonic and associated scoring system will prove useful for pediatricians or other primary care clinicians when faced with a patient with Hunter syndrome or other MPS disease.
Acknowledgments
The Hunter Outcome Survey (HOS) is supported by Shire Human Genetic Therapies, Inc. (Shire HGT), which is responsible for maintaining the central database and for the conduct of statistical analyses. This manuscript was a Shire HGT initiative which was performed with the approval of the HOS advisory board. Editorial assistance was provided to the authors by Edward Weselcouch, PhD, of PharmaWrite, LLC (Princeton, NJ, USA) and Robin Smith, PhD, of The Curry Rockefeller Group, LLC (Tarrytown, NY, USA) and was paid for by Shire HGT. The authors would like to thank the Hunter syndrome patients and their families for their participation in HOS.
Disclosures
Gabriel M. Cohn, David A.H. Whiteman, and Isabelle Morin are employees of Shire Human Genetic Therapies, Inc.
The following physicians were managing patients enrolled in HOS at the time of this study:
Austria—Graz: Barbara Plecko; Salzburg: Olaf Bodamer
Belgium—Brussels: Linda De Meirleir
Brazil—Fortaleza: Erlane Ribeiro; Porto Alegre: Roberto Giugliani; Rio de Janeiro: Raquel Taveres Boy da Silva; Salvador: Angelina Acosta; São Paulo: Ana Martins
Bulgaria—Sofia: Radka Tincheva
Canada—Toronto: Joe Clarke; Vancouver: Lorne Clarke
Croatia—Zagreb: Ingeborg Barišić, Ivo Barić
Czech Republic—Prague: Jiri Zeman
Denmark—Copenhagen: Allan Meldgaard Lund
France—Lyon: Nathalie Guffon; Paris: Vassili Valayannopoulos, Bénédicte Héron
Germany—Mainz: Michael Beck, Gudrun Schulze Frenking; Hamburg: Nicole Muschol; Magdeburg: Silke Klose; Berlin: Julia Hennermann
Greece—Thessaloniki: Dimitrios Zafeiriou
Hungary—Budapest: Zsuzsanna Almássy
Ireland—Dublin: Eileen Treacy
Italy—Ancona: Orazio Gabrielli; Bari: Francesco Papadia; Bologna: Alessandro Cicognani; Genova: Maja Di Rocco; Monza: Rossella Parini; Padova: Maurizio Scarpa; Rome: Claudio Feliciani
Poland—Warsaw: Anna Tyliki-Szymanska
Portugal—Porto: Elisa Leao Teles, Esmeralda Martins; Lisbon: Ana Gaspar
Russia—Moscow: Peter Novikov
Spain—Badalona: Guillem Pintos; Badajoz: Enrique Galán; Barcelona: Mireia del Toro, Merce Pined; Bilbao: Luis Aldámiz; Las Palmas: Milagros Marti; Linares: Pilar Munguira; Madrid: Luis González; Murcia: Rosario Domingo; Ourense: Gemma Novoa; Palma de Mallorca: Begoña de Azua; Salamanca: Aránzazu Hernández; Seville: Dolores Lluach; Valencia: Jaime Dalmau; Valladolid: José Manuel Muro; Zaragoza: Carlos Alcalde, Antonio Baldellou, Juan Pérez
Sweden—Stockholm: Gunilla Malm, Ingrid Dahlman; Lund: Dominiki Papadopoulou; Halmstad: Nils Nilsson; Gothenburg: Niklas Darin
Taiwan—Taipei: Shuan-Pei Lin
The Netherlands—Rotterdam: Ans van der Ploeg
UK—Amersham: UK PMS Society; Birmingham: Chris Hendriksz; Cambridge: Uma Ramaswami; London: Ashok Vellodi; Manchester: Simon Jones, James Edmond Wraith, Stephen Waldek
USA—Atlanta, GA: Paul Fernhoff; Baltimore, MD: Ada Hamosh; Boston, MA: Katherine Sims; Chapel Hill, NC: Joseph Muenzer; Charlottesville, VA: William Wilson; Chicago, IL: Barbara Burton; Cincinnati, OH: Nancy Leslie; Columbus, OH: Kim McBride; Denver, CO: Janet Thomas; Greenville, SC: Curtis Rogers; Hartford, CT: Robert Greenstein; Houston, TX: Christine Eng; Iowa City, IA: Sara Copeland; Jackson, MS: Omar Abdul-Rahman; Kansas City, MO: Laurie Smith; Lebanon, NH: John Moeschler; Miami, FL: Parul Jayaker; Minneapolis, MN: Nancy Medelsohn, Chet Whitley; New York, NY: Greg Pastores; Norfolk, VA: Virginia Proud; Oakland, CA: Paul Harmatz; Omaha, NE: William Rizzo; Paterson, NJ: Jennifer Ibrahim; Phoenix, AZ: Kirk Aleck; Portland, OR: Robert Steiner; Salt Lake City, UT: David Viskochil; Seattle, WA: Ronald Scott; Sioux Falls, SD: Laura Keppen; St. Louis, MO: Dorothy Grange; Washington, DC: Cynthia Tifft
References
- 1.Bach G, Eisenberg F, Jr, Cantz M, Neufeld EF. The defect in the Hunter syndrome: deficiency of sulfoiduronate sulfatase. Proc Natl Acad Sci USA. 1973;70:2134–2138. doi: 10.1073/pnas.70.7.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baugher KM, Mattu A. Ten rules to assess and manage the acutely deteriorating patient: a practical mnemonic. Patient Saf Surg. 2011;5:29. doi: 10.1186/1754-9493-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton BK, Giugliani R. Diagnosing Hunter syndrome in pediatric practice: practical considerations and common pitfalls. Eur J Pediatr. 2012;171:631–639. doi: 10.1007/s00431-012-1703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandes CJ, Speer ME. Using mnemonics and visual imagery to teach the new neonatal resuscitation program. J Perinatol. 2002;22:411–413. doi: 10.1038/sj.jp.7210739. [DOI] [PubMed] [Google Scholar]
- 5.Guffon N, Bertrand Y, Forest I, Fouilhoux A, Froissart R. Bone marrow transplantation in children with Hunter syndrome: outcome after 7 to 17 years. J Pediatr. 2009;154:733–737. doi: 10.1016/j.jpeds.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 6.Hall B. Mongolism in newborn infants. An examination of the criteria for recognition and some speculations on the pathogenic activity of the chromosomal abnormality. Clin Pediatr. 1966;5:4–12. doi: 10.1177/000992286600500102. [DOI] [PubMed] [Google Scholar]
- 7.Jones SA, Almassy Z, Beck M, Burt K, Clarke JT, Giugliani R, Hendriksz C, Kroepfl T, Lavery L, Lin SP, Malm G, Ramaswami U, Tincheva R, Wraith JE. Mortality and cause of death in mucopolysaccharidosis type II-a historical review based on data from the Hunter Outcome Survey (HOS) J Inherit Metab Dis. 2009;32:534–543. doi: 10.1007/s10545-009-1119-7. [DOI] [PubMed] [Google Scholar]
- 8.Martin R, Beck M, Eng C, Giugliani R, Harmatz P, Munoz V, Muenzer J. Recognition and diagnosis of mucopolysaccharidosis II (Hunter syndrome) Pediatrics. 2008;121:e377–e386. doi: 10.1542/peds.2007-1350. [DOI] [PubMed] [Google Scholar]
- 9.Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- 10.Muenzer J, Wraith JE, Beck M, Giugliani R, Harmatz P, Eng CM, Vellodi A, Martin R, Ramaswami U, Gucsavas-Calikoglu M, Vijayaraghavan S, Wendt S, Puga AC, Ulbrich B, Shinawi M, Cleary M, Piper D, Conway AM, Kimura A. A phase II/III clinical study of enzyme replacement therapy with idursulfase in mucopolysaccharidosis II (Hunter syndrome) Genet Med. 2006;8:465–473. doi: 10.1097/01.gim.0000232477.37660.fb. [DOI] [PubMed] [Google Scholar]
- 11.Muenzer J, Beck M, Eng CM, Escolar ML, Giugliani R, Guffon NH, Harmatz P, Kamin W, Kampmann C, Koseoglu ST, Link B, Martin RA, Molter DW, Munoz Rojas MV, Ogilvie JW, Parini R, Ramaswami U, Scarpa M, Schwartz IV, Wood RE, Wraith E. Multidisciplinary management of Hunter syndrome. Pediatrics. 2009;124:e1228–e1239. doi: 10.1542/peds.2008-0999. [DOI] [PubMed] [Google Scholar]
- 12.Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, editor. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 3421–3452. [Google Scholar]
- 13.Samala RV, Navas V, Saluke E, Ciocon JO. Heart failure in frail, older patients: we can do 'MORE'. Cleve Clin J Med. 2011;78:837–845. doi: 10.3949/ccjm.78a.11085. [DOI] [PubMed] [Google Scholar]
- 14.Warnica JW. Canadian mnemonics for heart sounds. CMAJ. 2007;176:69. doi: 10.1503/cmaj.1060180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wraith JE, Beck M, Giugliani R, Clarke J, Martin R, Muenzer J. Initial report from the Hunter Outcome Survey. Genet Med. 2008;10:508–516. doi: 10.1097/GIM.0b013e31817701e6. [DOI] [PubMed] [Google Scholar]
- 16.Young ID, Harper PS. Mild form of Hunter's syndrome: clinical delineation based on 31 cases. Arch Dis Child. 1982;57:828–836. doi: 10.1136/adc.57.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young ID, Harper PS. The natural history of the severe form of Hunter's syndrome: a study based on 52 cases. Dev Med Child Neurol. 1983;25:481–489. doi: 10.1111/j.1469-8749.1983.tb13794.x. [DOI] [PubMed] [Google Scholar]