Abstract
Background
Overexpression of heat shock protein 70 (HSP70) has been observed in many types of cancer including gastric adenocarcinomas, although the exact role of HSP70 in carcinogenesis remains unclear.
Methods
The study analyzed a total of 458 radical gastrectomy specimens which were immunohistochemically stained with HSP70, p53, and Ki-67 antibodies.
Results
The study determined that the expression of HSP70 was significantly increased in early gastric cancer (EGC) compared to advanced gastric cancer (p<0.001). The HSP70 expression was correlated with well-differentiated tumor type, intestinal type of Lauren classification and the lower pT and pN stage. Negative expression of Ki-67 and p53 expression was associated with poor prognosis. The study did not find any correlation between HSP70 and p53 expression. The study determined that HSP70 expression in the EGC subgroup was associated with a poor prognosis (p=0.009), as well as negative Ki-67 expression (p=0.006), but was not associated with p53. Based on multivariate analysis, HSP70 expression (p=0.024), negative expression of Ki-67, invasion depth and lymph node metastasis were determined to be independent prognostic markers.
Conclusions
HSP70 is expressed in the early stages of gastric adenocarcinoma. In EGC, HSP70 is a poor independent prognostic marker and is correlated with a low proliferation index.
Keywords: HSP70 heat-shock proteins, Ki-67 antigen, Early gastric carcinoma
According to the National Cancer Information Center of Korea, gastric cancer was the most common cancer among Korean men.1,2 Although the incidence of gastric cancer has steadily decreased steadily over the last several years, gastric cancer-associated mortality is the third most common cause of mortality in Korean cancer patients. Gastric cancer is a biologically and genetically heterogeneous malignancy that involves several genetic mutations and epigenetic alterations, including CDH1, p53 and CTNNB1 mutations and hypermethylation of MLH1, MSH2, CDH1, CDKN2A, CDK2AP2, RASSF1A, and RUNX3.3 However, little is known about gastric carcinogenesis and many studies are working to understand the molecular mechanisms that are implicated in gastric adenocarcinoma.
Heat shock protein 70 (HSP70) is an ubiquitously expressed protein, and the expression of HSP70 is up-regulated by variable stresses such as heat, anticancer chemotherapy, oxidative stress, and chemical injury and the assistance of various protein folding and unfolding processes in the cell.4 HSP70 protein acts as a molecular chaperone in the development of malignant neoplasm, a stressful condition in which tumors are subjected to free radicals, a hypoxic environment and the accumulation of misfolded proteins, therefore the inhibition of HSP70 can be lethal to tumor cells.5 Overexpression of HSP70 has been reported in many types of cancer and has been correlated with poor differentiation, lymph node metastasis and a shorter patient survival prognosis.6-9 Some studies have reported that the role of HSP70 in human malignancies could be attributable to the interaction between p53 and HSP70 proteins, although other studies have reported contradictory results.10-12 Additional reports have discussed the possibility that HSP70 expression is correlated with the differentiation and low proliferation of tumor cells.10,13,14 The exact mechanism and function of HSP70 in cancer development remains unclear, although the expression of HSP70 is frequently found in malignant neoplasms.
A few studies that have been published investigated HSP70 expression in gastric adenocarcinoma but the studies have not revealed the prognostic impact of HSP70 expression in gastric cancer.14-16 The studies reported that HSP70 is overexpressed in gastric cancers, is involved in tumor differentiation, and that there is a correlation between HSP70 expression and lymph node metastasis and vascular invasion, although no correlation between p53 and HSP70 expression has been identified. The purpose of this study was to define the role of HSP70 in gastric adenocarcinoma; the investigation examined the immunohistochemical expression of HSP70, p53, and Ki-67 and analyzed correlations between the expression of these proteins with clinicopathologic characteristics and patient survival.
MATERIALS AND METHODS
Patients and samples
This study included a total of 458 gastric adenocarcinoma patients who underwent radical gastrectomy at Korea University Guro Hospital from 2002 to 2005. Each patient's records were reviewed to ensure that the study did not include any patient with a prior history of neo-adjuvant chemotherapy. Clinicopathologic data, including age, sex, distant metastasis, and survival data were obtained from the medical records. Archived formalin-fixed paraffin-embedded tissues were used for tissue microarray (TMA) and immunohistochemical staining.
Clinicopathologic data, including age, sex, distant metastasis, and survival data were obtained from medical records. All slide glasses and gross photos were reviewed and the histopathologic type, histologic grade, Lauren classification, invasion depth, regional lymph node metastasis, and lymphatic invasion were re-evaluated. All of the cases that were reviewed were reclassified according to the 7th American Joint Committee on Cancer/International Union against Cancer Classification (AJCC/UICC) TNM cancer classification system.17 In addition, the World Health Organization classification system was applied for adenocarcinoma grading.18 The follow-up period ranged from 2 to 2,514 days (mean, 50.5 months; median, 53.0 months). The mean age of the patients was 58 years (median, 61 years; range, 23 to 84 years) and the male to female ratio was 2:1. The study determined that early gastric carcinoma (EGC) was identified in 54% (n=246) of the patients, and advanced gastric carcinoma was found in 46% (n=212) of the patients. This study was approved by Institutional Review Board of the Korea University Guro Hospital (KUGGR-2010-033).
Tissue microarray and immunohistochemical staining
Hematoxylin and eosin-stained slide glasses from the selected patients were reviewed for TMA preparation. Portions from the leading edges of tumor infiltration were marked on the slides and used for the TMA construction. An additional 45 non-neoplastic mucosal tissues that were distant from the tumors were selected from the patient samples and used for non-neoplastic TMA control. The tissue core diameter used in TMA construction was 2.0 mm, and the TMA blocks were cut into 4 µm slices for immunohistochemical staining. The standard streptavidin-biotin peroxidase complex method was used for staining. After the deparaffinization and rehydration steps, the slides were heated in a microwave oven for 15 minutes in 10mM citrate buffer (pH 6.0) and treated with 3% hydrogen peroxide for 20 minutes. The antibodies that were used in this study included HSP70 (1:800, Abcam, Cambridge, UK), p53 (1:500, clone DO-7, Novocastra, Newcastle upon Tyne, UK), and Ki-67 (1:700, clone MIB-1, Dako, Glostrup, Denmark).
Immunohistochemical staining analysis
The immunohistochemical staining revealed that HSP70 expression was present in the cell cytoplasm. Based on these results, the study applied cytoplasmic staining intensity grading (0, no staining; 1, weak; 2, moderate; 3, strong) and determined the percent areas of positive tumor cells for analysis. The study considered positive expression to be present when more than 50% of tumor cells showed moderate or strong staining intensity.
In addition, the immunohistochemical staining of p53 and Ki-67 showed a nuclear staining pattern. The nuclear staining intensity (0, no staining; 1, weak; 2, moderate; 3, strong) and the percent areas of positive tumor cell nuclei were also graded. The study considered the staining to be positive when more than 10% of tumor cells showed moderate or strong staining intensity.
Statistical analysis
All statistical analyses were performed using SPSS ver. 12 (SPSS Inc., Chicago, IL, USA). The Kaplan-Meier plots and the log-rank test were used to analyze patient survival. Correlations between protein expression and the clinicopathological characteristics were analyzed using Pearson's chi-square (χ2) and linear-by-linear association tests. Finally, the Cox proportional hazard model was used for the multivariate analysis. In all of the statistical analyses, a p<0.05 was considered to be statistically significant.
RESULTS
Expression of HSP70, p53, and Ki-67 in gastric adenocarcinoma
The results indicated that for patients with non-neoplastic gastric mucosa, 6 (13%) out of 45 samples showed cytoplasmic expression of HSP70. Eighteen (40%) of the non-neoplastic samples showed weak HSP70 staining, although strong staining was not observed in non-neoplastic tissue. The study determined that for the cases of gastric adenocarcinoma, 155 (34%) of the samples indicated cytoplasmic expression of HSP70. In addition, from these HSP70 positive samples, the percentages of EGC and advanced gastric cancer (AGC) were determined to be 85 (46%) patients and 70 (26%) patients, respectively (Fig. 1).
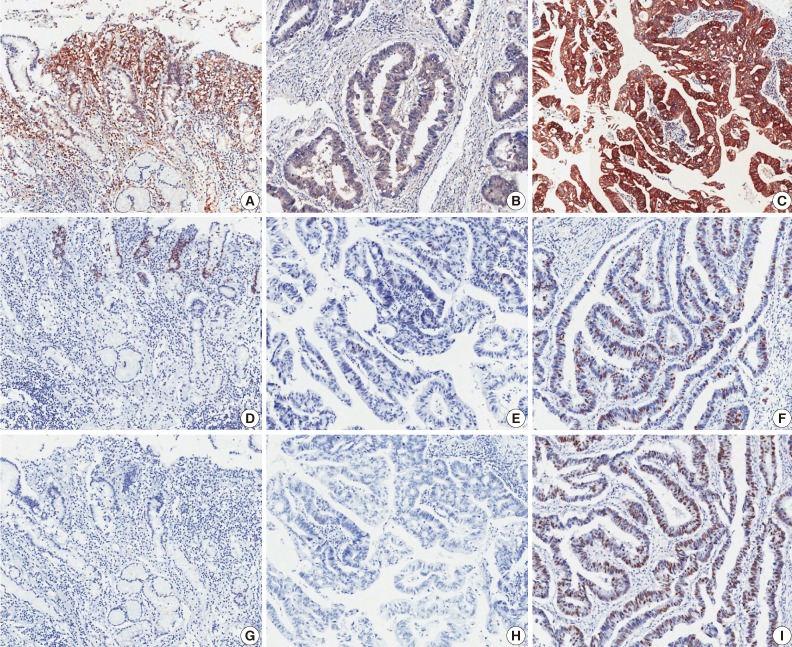
Fig. 1.
Microscopic images of immunohistochemical staining. Heat shock protein 70 (A-C), Ki-67 (D-F), and p53 (G-I). Left panels are from non-neoplastic gastric mucosa (A, D, G), middle panels are negative expression samples from gastric adenocarcinoma (B, E, H), and right panels are positive expression samples from gastric adenocarcinoma (C, F, I).
Immunohistochemical p53 expression was not observed in patients with non-neoplastic gastric mucosa. The analysis showed that only a small percentage of scattered cells (<10%) showed weak to moderate nuclear staining of p53 in non-neoplastic samples. In the gastric adenocarcinoma patients, p53 positivity was observed in 163 (36%) samples, which included 59 EGC (32%) and 104 AGC (38%) samples. The research indicated that Ki-67 positivity was observed in 63 (38%) of the total gastric adenocarcinoma samples, which included a total of 100 (54%) EGC and 65 (24%) AGC cases.
HSP70 expression and clinicopathologic characteristic correlations
HSP70 expression was found to be significantly associated with lower histologic grade (p=0.007), intestinal type Lauren classification (p<0.001), lower pathologic T category (p<0.001), lower pathologic N category (p<0.001), and lower TNM stage (p<0.001). No significant correlation between sex, age, lymphatic invasion, p53 and Ki-67 expression and HSP70 expression was observed (p>0.05) in this study. The expression of p53 was associated with the pT category (p=0.014) and the pN category (p=0.042). In addition, negative Ki-67 expression was also found to be associated with the pT category (p<0.001) and the pN category (p<0.001).
The gastric adenocarcinomas were divided into two sub-groups, EGC and AGC, and the analysis investigated the correlation between HSP70 expression and clinicopathologic characteristics. The study determined that EGC was significantly associated with positive expression of HSP70 (p<0.001). For the AGC subgroup, HSP70 expression was determined to have correlations between male (p=0.007) and higher histologic grades (p=0.046), intestinal type Lauren classification (p=0.001), and Ki-67 expression (p=0.002). Negative Ki-67 expression was the only clinicopathologic characteristic that was directly correlated with HSP70 expression in the EGC subgroup (Table 1).
Table 1.
Correlations between HSP70 expression and clinicopathologic characteristics
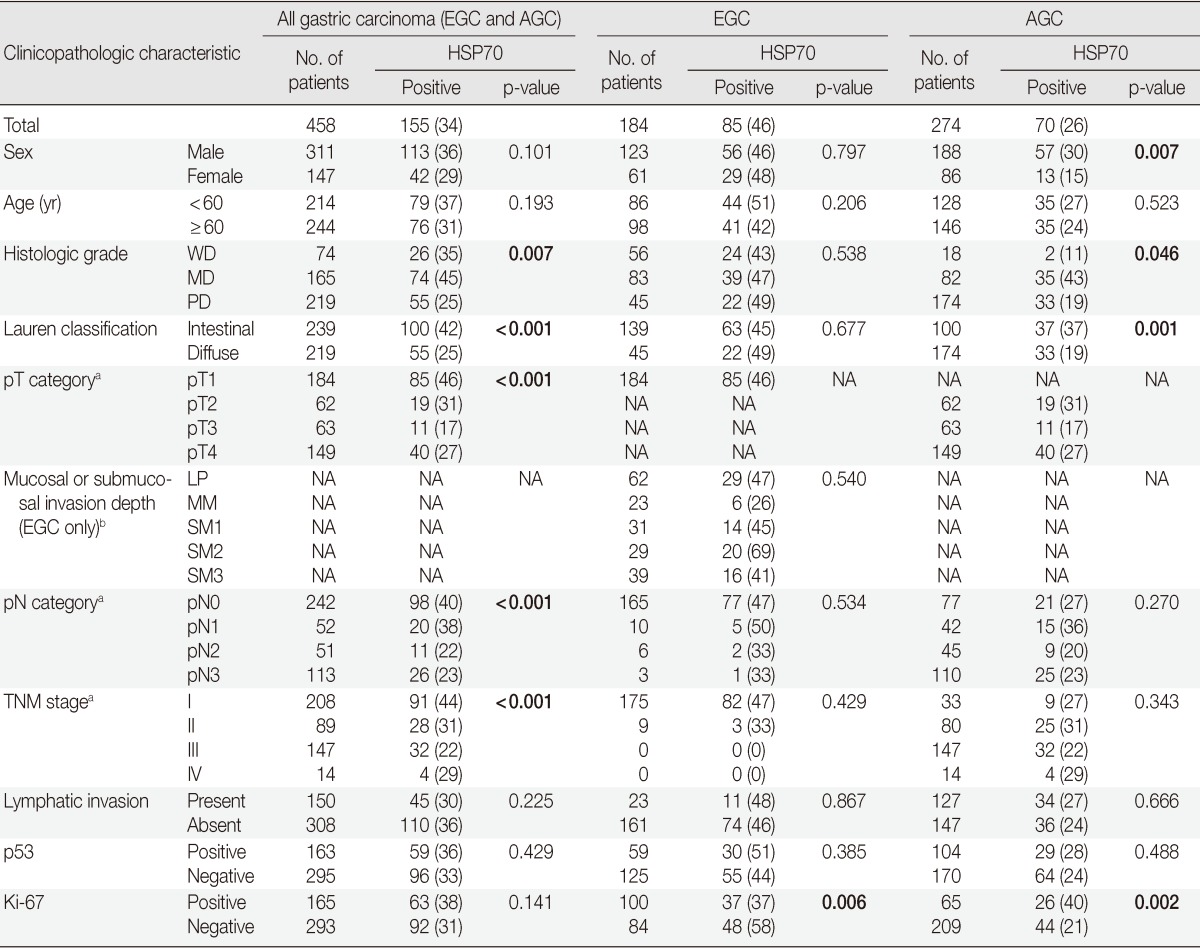
Values are presented as number (%).
HSP70, heat shock protein 70; EGC, early gastric cancer; AGC, advanced gastric cancer; NA, not applicable; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated; LP, lamina propria; MM, muscularis mucosa; SM, submucosa.
aAJCC Cancer Staging Manual, 7th edition; bJapanese Classification of Gastric Carcinoma, 2nd edition.
Patient survival analysis
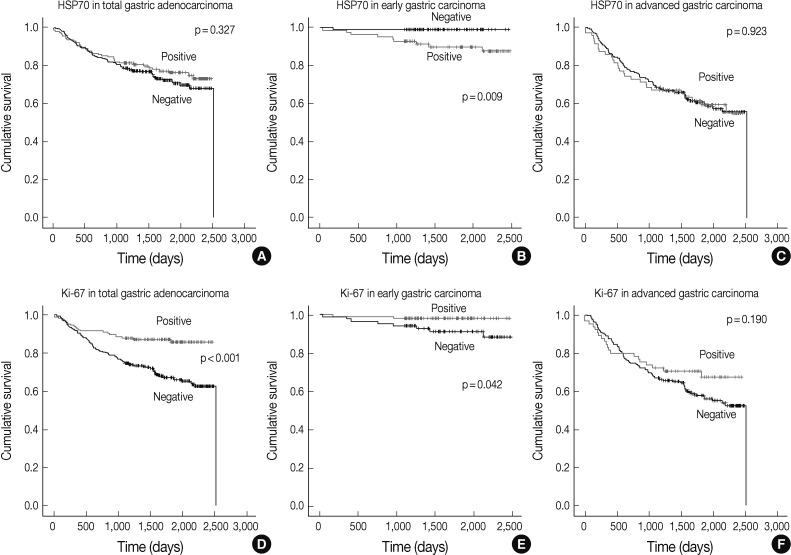
In all cases including the EGC and AGC subgroups, poor patient prognoses were associated with old age (p=0.016), higher histologic grade (p=0.001), diffuse type Lauren classification (p<0.001), higher pathologic T category (p<0.001), higher pathologic N category (p<0.001), the presence of lymphatic invasion (p<0.001), p53 expression (p=0.022), and negative expression of Ki-67 (p<0.001). The study did not find a correlation between HSP70 expression and the rate of patient survival (p=0.327).
The survival analysis of the EGC subgroup revealed that lymph node metastasis (p=0.021), HSP70 expression (p=0.009), and negative Ki-67 expression (p=0.042) were parameters that correlated with poor patient survival (Fig. 2). Sex (p=0.108), age (p=0.070), histologic grade (p=0.743), Lauren classification (p=0.707), depth of invasion (p=0.099), lymphatic invasion (p=0.477), and p53 expression (p=0.179) were not found to be associated with patient survival.
Fig. 2.
Analysis of gastric adenocarcinoma patient survival. Kaplan-Meier plots with heat shock protein 70 (HSP70) expression data in all cases of gastric adenocarcinoma including the early and advanced gastric carcinoma subgroups (A), early gastric carcinoma (B), and advanced gastric carcinoma (C). Lower graphs show Kaplan-Meier plots of Ki-67 expression in all gastric adenocarcinoma cases (D), early gastric carcinoma (E), and advanced gastric carcinoma (F). p-values obtained from log-rank tests are shown in each graph.
The analysis of the AGC subgroup survival revealed that old age (p=0.042), higher pathologic T category (p<0.001), higher pathologic N category (p<0.001), and the presence of lymphatic invasion (p<0.001) were all associated with poor survival rates. The study did not identify any significant correlation between HSP70 expression (p=0.923), p53 expression (p=0.151), sex (p=0.517), histologic grade (p=0.726), Lauren classification (p=0.474), Ki-67 expression (p=0.190), and patient survival.
Multivariate analysis
Multivariate analysis was performed with data from the EGC subgroup. The analysis considered parameters that included lymph node metastasis, depth of invasion, histologic grade, Ki-67 expression and HSP70 expression. The multivariate analysis indicated that HSP70 expression was an independent predictor of shorter survival (p=0.024). Other factors that contributed to survival included lymph node metastasis (p=0.003), submucosal invasion (p=0.015), and negative expression of Ki-67 (p=0.039) (Table 2).
Table 2.
Multivariate analysis of early gastric cancer
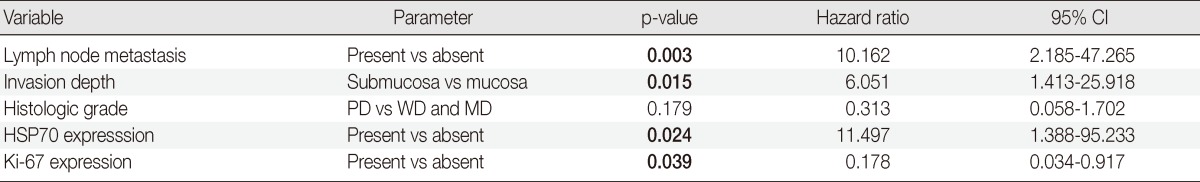
CI, confidence interval; PD, poorly differentiated; WD, well differentiated; MD, moderately differentiated; HSP70, heat shock protein 70.
DISCUSSION
HSP70 is a highly conserved protein that is overexpressed in many types of malignancies.11 HSP70 is an important protein because of its involvement in diverse roles in carcinogenesis and numerous studies have reported the value of identifying HSP70 expression in the diagnosis, prognosis, and prediction of many cancers.10,12,13,19,20 Several studies have investigated the role of HSP70 in gastric adenocarcinomas, although direct correlations between HSP70 expression and clinicopathologic factors and the prognostic implications have not previously been determined.14-16,21
We examined HSP70 expression in gastric adenocarcinomas and analyzed the correlation between expression, clinicopathologic factors and patient survival. The study determined that some aspects of HSP70 expression including negative correlation with pT category and pN category, and no correlation with lymphatic invasion were discordant with previously published data. However, some of the results were concordant with previous studies, including a positive correlation with differentiated type and intestinal type of Lauren classification, no correlation with p53 expression and patient survival rates.14-16
In addition, some previous studies that have addressed HSP70 expression in malignant tumors of other organs reported that HSP70 expression is increased in malignancies, especially in early stage tumors, but that HSP70 expression does not increase with tumor progression.13,22,23 Based on these reports, we hypothesized that an increase of HSP70 expression could be found in early stages of gastric carcinogenesis and that the clinical implications of the increased expression could be limited to the EGC stage. Therefore, we divided patient samples into two subgroups, EGC and AGC, and analyzed the HSP70 expression in both of these subgroups. The study determined that HSP70 expression was more frequently observed in EGC and was correlated with poor patient survival in the EGC subgroup. No significant correlation between HSP70 expression and patient survival were determined for the AGC subgroup. These results suggest that HSP70 expression in gastric adenocarcinoma may be associated with carcinogenesis and the early stages of tumor progression.
The study did not observe any association between low pT category, low pN category and HSP70 expression in the EGC and AGC subgroup analyses. This result indicates that there was no direct correlation between HSP70 expression and tumor progression or lymph node metastasis. However, the analysis indicated that HSP70 expression increases in early stages of gastric adenocarcinoma, but its role in gastric carcinogenesis does not promote tumor invasion or lymph node metastasis.
Several previous studies suggested that the role of HSP70 in malignant neoplasm is related to p53 function, but this study did not identify any correlation between HSP70 and p53 expression.24,25 Additionally, another study that examined the roles of HSP70 and p53 expression in gastric cancer was concordant with our data.14 The results indicated that in cases of gastric adenocarcinoma, the role of HSP70 does not appear to act through interaction with p53.
The survival analysis indicated that negative expression of Ki-67 was correlated with poor patient survival in the cases gastric adenocarcinoma and in the EGC subgroup. Negative Ki-67 expression was found to be correlated with the pT and pN stages in all adenocarcinoma cases and with HSP70 expression in the EGC subgroup. Furthermore, HSP70 expression and negative Ki-67 expression were determined to be independent poor prognostic markers of EGC. Currently, there are conflicting reports about the correlation between the Ki-67 index and poor prognostic factors such as invasion depth and lymph node metastasis. A previous study reported an association between negative expression of Ki-67 and tumor invasion depth in gastric adenocarcinoma.26 Another EGC study also reported that Ki-67 was expressed in mucosal cancer, and was more commonly expressed than in submucosal cancer, although the findings in this study were not determined to be statistically significant.27 These conflicting results that address the expression of Ki-67 in gastric adenocarcinoma may be due to intratumoral heterogeneity of Ki-67 expression. Therefore, in order to avoid random tissue selection effect in tumors that showed heterogeneous Ki-67 expression, we selected tumor portions from invasive borders which were used to represent the depth of tumor invasion.
Additional studies have reported that HSP70 expression is correlated with differentiated tumor type and low proliferation of tumor cells.4,14 Because HSPs could be pre-differentiation markers which are induced during tumor differentiation, which occurs with a decrease in cell proliferation, the role of HSPs in carcinogenesis may be correlated with tumor differentiation and low proliferative activity of tumor cells.21,28-30 In this study, we also observed that HSP70 expression is correlated with tumor differentiation and negative Ki-67 expression. In addition, the expression of HSP70 in gastric adenocarcinoma may be correlated with proliferative activity and tumor cell differentiation in the early stage of gastric adenocarcinoma which may contribute to the prognostic impact of HSP70 in early gastric adenocarcinoma.
The exact mechanism of HSP70 in carcinogenesis and tumor progression has not yet been determined. However, research has indicated that HSP70 expression is essential to tumor cell survival and its ability to avoid apoptosis, which reflects the diverse roles of HSP70 expression in tumorigenesis, tumor progression and tumor treatment.4,11 The overexpression of HSP70 is often reported in malignancies such as gastric adenocarcinomas.15-17,21 The results of this experiment suggest that HSP70 is especially overexpressed in the early stage of gastric adenocarcinoma and that the expression is inversely correlated with poor differentiation or diffuse types of tumor cells. In addition, especially in EGC cases, HSP70 expression is an independent poor prognostic marker that, along with negative Ki-67 expression, may be correlated with low tumor cell proliferation and tumor cell differentiation. Additional studies are essential to examine and determine the precise role of HSP70 in gastric adenocarcinoma.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Rüschoff J, Hanna W, Bilous M, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–650. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 2.Cancer incidence statistics in Korea [Internet] Goyang: National Cancer Information Center; 2012. [cited 2012 Jan 2]. Available from: http://www.cancer.go.kr/ncic/cics_f/01/012/index.html. [Google Scholar]
- 3.Jang BG, Kim WH. Molecular pathology of gastric carcinoma. Pathobiology. 2011;78:302–310. doi: 10.1159/000321703. [DOI] [PubMed] [Google Scholar]
- 4.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 5.Nylandsted J, Rohde M, Brand K, Bastholm L, Elling F, Jäättelä M. Selective depletion of heat shock protein 70 (Hsp70) activates a tumor-specific death program that is independent of caspases and bypasses Bcl-2. Proc Natl Acad Sci U S A. 2000;97:7871–7876. doi: 10.1073/pnas.97.14.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur J, Das SN, Srivastava A, Ralhan R. Cell surface expression of 70 kDa heat shock protein in human oral dysplasia and squamous cell carcinoma: correlation with clinicopathological features. Oral Oncol. 1998;34:93–98. doi: 10.1016/s1368-8375(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 7.Lazaris AC, Theodoropoulos GE, Davaris PS, et al. Heat shock protein 70 and HLA-DR molecules tissue expression: prognostic implications in colorectal cancer. Dis Colon Rectum. 1995;38:739–745. doi: 10.1007/BF02048033. [DOI] [PubMed] [Google Scholar]
- 8.Ciocca DR, Stati AO, Amprino de Castro MM. Colocalization of estrogen and progesterone receptors with an estrogen-regulated heat shock protein in paraffin sections of human breast and endometrial cancer tissue. Breast Cancer Res Treat. 1990;16:243–251. doi: 10.1007/BF01806332. [DOI] [PubMed] [Google Scholar]
- 9.Elpek GO, Karaveli S, Simşek T, Keles N, Aksoy NH. Expression of heat-shock proteins hsp27, hsp70 and hsp90 in malignant epithelial tumour of the ovaries. APMIS. 2003;111:523–530. doi: 10.1034/j.1600-0463.2003.1110411.x. [DOI] [PubMed] [Google Scholar]
- 10.Nanbu K, Konishi I, Komatsu T, et al. Expression of heat shock proteins HSP70 and HSP90 in endometrial carcinomas: correlation with clinicopathology, sex steroid receptor status, and p53 protein expression. Cancer. 1996;77:330–338. doi: 10.1002/(SICI)1097-0142(19960115)77:2<330::AID-CNCR16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993;85:570–574. doi: 10.1093/jnci/85.7.570. [DOI] [PubMed] [Google Scholar]
- 13.Chuma M, Sakamoto M, Yamazaki K, et al. Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology. 2003;37:198–207. doi: 10.1053/jhep.2003.50022. [DOI] [PubMed] [Google Scholar]
- 14.Maehara Y, Oki E, Abe T, et al. Overexpression of the heat shock protein HSP70 family and p53 protein and prognosis for patients with gastric cancer. Oncology. 2000;58:144–151. doi: 10.1159/000012091. [DOI] [PubMed] [Google Scholar]
- 15.Canoz O, Belenli O, Patiroglu TE. General features of gastric carcinomas and comparison of HSP70 and NK cell immunoreactivity with prognostic factors. Pathol Oncol Res. 2002;8:262–269. doi: 10.1007/BF03036742. [DOI] [PubMed] [Google Scholar]
- 16.Isomoto H, Oka M, Yano Y, et al. Expression of heat shock protein (Hsp) 70 and Hsp 40 in gastric cancer. Cancer Lett. 2003;198:219–228. doi: 10.1016/s0304-3835(03)00305-7. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Greeene FL, Trotti A. AJCC cancer staging handbook: from the AJCC cancer staging manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 18.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th ed. Lyon: IARC Press; 2010. [Google Scholar]
- 19.Piura B, Rabinovich A, Yavelsky V, Wolfson M. Heat shock proteins and malignancies of the female genital tract. Harefuah. 2002;141:969–972. 1010, 1009. [PubMed] [Google Scholar]
- 20.Ciupitu AM, Petersson M, Kono K, Charo J, Kiessling R. Immunization with heat shock protein 70 from methylcholanthrene-induced sarcomas induces tumor protection correlating with in vitro T cell responses. Cancer Immunol Immunother. 2002;51:163–170. doi: 10.1007/s00262-002-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao ZG, Shen WL. Heat shock protein 70 antisense oligonucleotide inhibits cell growth and induces apoptosis in human gastric cancer cell line SGC-7901. World J Gastroenterol. 2005;11:73–78. doi: 10.3748/wjg.v11.i1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park CS, Joo IS, Song SY, Kim DS, Bae DS, Lee JH. An immunohistochemical analysis of heat shock protein 70, p53, and estrogen receptor status in carcinoma of the uterine cervix. Gynecol Oncol. 1999;74:53–60. doi: 10.1006/gyno.1999.5429. [DOI] [PubMed] [Google Scholar]
- 23.Cornford PA, Dodson AR, Parsons KF, et al. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099–7105. [PubMed] [Google Scholar]
- 24.Fourie AM, Hupp TR, Lane DP, et al. HSP70 binding sites in the tumor suppressor protein p53. J Biol Chem. 1997;272:19471–19479. doi: 10.1074/jbc.272.31.19471. [DOI] [PubMed] [Google Scholar]
- 25.Rohde M, Daugaard M, Jensen MH, Helin K, Nylandsted J, Jäättelä M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005;19:570–582. doi: 10.1101/gad.305405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramires M, David L, Leitão D, Seixas M, Sansonetty F, Sobrinho-Simões M. Ki67 labelling index in gastric carcinomas. An immunohistochemical study using double staining for the evaluation of the proliferative activity of diffuse-type carcinomas. J Pathol. 1997;182:62–67. doi: 10.1002/(SICI)1096-9896(199705)182:1<62::AID-PATH849>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Lim MS, Lee HW, Im H, et al. Predictable factors for lymph node metastasis in early gastric cancer-analysis of single institutional experience. J Gastrointest Surg. 2011;15:1783–1788. doi: 10.1007/s11605-011-1624-5. [DOI] [PubMed] [Google Scholar]
- 28.Shakoori AR, Oberdorf AM, Owen TA, et al. Expression of heat shock genes during differentiation of mammalian osteoblasts and promyelocytic leukemia cells. J Cell Biochem. 1992;48:277–287. doi: 10.1002/jcb.240480308. [DOI] [PubMed] [Google Scholar]
- 29.Spector NL, Ryan C, Samson W, Levine H, Nadler LM, Arrigo AP. Heat shock protein is a unique marker of growth arrest during macrophage differentiation of HL-60 cells. J Cell Physiol. 1993;156:619–625. doi: 10.1002/jcp.1041560322. [DOI] [PubMed] [Google Scholar]
- 30.Spector NL, Mehlen P, Ryan C, et al. Regulation of the 28 kDa heat shock protein by retinoic acid during differentiation of human leukemic HL-60 cells. FEBS Lett. 1994;337:184–188. doi: 10.1016/0014-5793(94)80270-x. [DOI] [PubMed] [Google Scholar]