Abstract
Background
Familial hypercholesterolemia (FH) is an autosomal dominant disease caused by mutations in the genes coding for the low density lipoprotein receptor (LDLR), proprotein convertase subtilisin/kexin type-9 (PCSK9) or apo-lipoprotein B-100 (APOB). The aim of the present work was to determine the genetic basis of dyslipidemia in 11 unrelated Pakistani families.
Methods
High resolution melting (HRM), sequencing and restriction fragment length polymorphism (RFLP).
Results
Probands were screened for the promoter and all coding regions, including intron/exon boundaries, of LDLR and PCSK9 and part of exon 26 of APOB including p.(R3527Q). Two families were identified with previously unreported LDLR mutations (c.1019_1020delinsTG, p.(C340L) and c.1634G>A, p.(G545E)). Both probands had tendon xanthomas or xanthelasma and/or a history of cardiovascular disease. Co-segregation with hypercholesterolemia was demonstrated in both families. In silico studies predicted these variations to be damaging. In two families, novel PCSK9 variations were identified (exon2; c.314G > A, p.(R105Q) and exon3; c.464C>T, p.(P155L)). In silico studies suggested both were likely to be damaging, and family members carrying the p.(105Q) allele had lower total cholesterol levels, suggesting this is a loss-of-function mutation. For c.464C>T p.(P155L) the small number of relatives available precluded any strong inference.
Conclusion
This report brings to seven the number of different LDLR mutations reported in FH patients from Pakistan and, as expected in this heterogeneous population, no common LDLR mutation has been identified.
Keywords: Familial hypercholesterolemia, LDLR, PCSK9, Xanthomas, HRM, Consanguinity
Highlights
-
•
We examined the LDLR/PCSK9 genes in patients with FH from Pakistan.
-
•
Two novel LDLR mutations both showed co-segregation with hypercholesterolemia.
-
•
Two novel PCSK9 variations were found one of which was a loss of function mutation.
-
•
This brings to 7 the number of molecular causes of FH in patients from Pakistan.
1. Introduction
Familial hypercholesterolemia (FH) is a heterogeneous autosomal dominant disorder caused by mutations in the genes for the low density lipoprotein receptor (LDLR), apolipoprotein B (APOB) or proprotein convertase subtilase/kexin type 9 (PCSK9). Heterozygotes occur in the general population with a frequency of approximately 1 in 500, homozygotes at 1 in a million [1]. At present more than 1000 variants have been identified in the LDLR associated with FH and premature cardiovascular disease [2]. LDLR, predominantly expressed in hepatocytes, is processed through the rough endoplasmic reticulum (RER) and transported to the outer membrane. Here, low density lipoprotein (LDL) particles bind to the LDLR and undergo endocytosis. They are processed in the endosome generating free LDL, which is degraded, while the LDLR is recycled back to the membrane. Some of the LDLR is bound to PCSK9 before endocytosis which leads to the degradation of the receptor in the endosome, resulting in a decreased number of receptors available for membrane presentation. Mutations reported in the LDLR are associated with a decrease in LDLR function, resulting in an increase in plasma LDL and total cholesterol (TC), and greatly increased risk of premature coronary heart disease (CHD) which can be significantly reduced by statin treatment [3]. Mutations in APOB that disrupt binding of the LDL-C particle to the LDLR also cause FH [4], but such mutations (chiefly p.(R3527Q)) are commonly found only in subjects of European origin [5,6]. Mutations in PCSK9 that are gain-of-function increase LDLR degradation and cause a severe form of FH [7], but interestingly loss-of-function variants have also been reported that result in less LDLR degradation, and carriers of these variants have lower plasma LDL-C levels [8,9].
The spectrum of LDLR mutations causing FH has been studied in many different countries as this information is useful in devising the most efficient laboratory strategy for genetic testing. In genetically heterogeneous countries such as the UK, Italy or the Netherlands, there are many different mutations found [10,11]. Some mutations are more common, and may show geographical clustering [12]. By contrast, in countries with a strong “founder effect,” such as South Africa, Finland or even in Greece [13,14], only a few mutations explain the majority of FH patients making genetic testing much easier. To date there have been few studies examining the spectrum of LDLR mutations in FH families from Pakistan or the Indian subcontinent, although some patients examined in UK studies originate from the subcontinent [15]. We have previously reported the identification of mutations in four different patients from Pakistan, two nonsense mutations, p.(Val806GlyfsX11) [16] and p.(C296X) [17] and three missense mutations p.(R88S) [17], p.(V639G) and p.(T404I)/p.(N405T), which are on the same allele [18]. Here we extend the study to 11 additional probands and their extended families. Of these, six had LDL-C levels above the UK cut-off for a clinical diagnosis of FH of 4.9 mmol/l but, because there are no validated LDL-C cut-offs for the clinical diagnosis of FH in Pakistan, we included 4 probands with LDL-C above 3.9 mmol/l, and one with premature CAD but no elevation of plasma lipid levels.
2. Methods
2.1. Blood sampling and DNA extraction
The current study was approved by the Ethics Committee and Institutional Review Board of Shifa College of Medicine, Shifa International Hospital, Islamabad. Six patients were identified based on the UK Simon Broome criteria, including an elevated level of TC (> 4.9 mmol/l) and development of xanthomas or xanthelasma. Four probands had LDL-C levels below this cut off (≥ 3.9 mmol/l) and one was included on the basis of early cardiovascular diseases (HC29-1) although their lipid levels were not elevated (Table 1). Blood was obtained from the subjects by venipuncture after obtaining informed written consent from the patient or their representative. DNA was extracted from the lymphocytes using a standard organic method as described elsewhere [19].
Table 1.
Characteristics of probands.
| ID | Age (years) | Gender | Total-C mmol/l | LDL mmol/l | TG mmol/l | HDL mmol/l | Clinical status |
|---|---|---|---|---|---|---|---|
| HC23-1a | 45 | M | 9.78 | 5.74 | 3.04 | 1.47 | |
| HC26-2 | 52 | F | 6.31 | 3.90 | 0.90 | 1.84 | |
| HC27-1a | 36 | M | 7.34 | 5.20 | 2.62 | 1.22 | |
| HC29-1 | 54 | M | 3.23 | 2.15 | 1.02 | 0.78 | CABG |
| HC30-2a | 23 | M | 6.54 | 5.84 | 1.97 | 1.19 | |
| HC32-2 | 65 | M | 6.34 | 4.34 | 1.43 | 1.16 | MI |
| HC33-4 | 34 | M | 6.62 | 4.53 | 2.21 | 0.98 | |
| HC34-13a | 35 | F | 8.84 | 6.00 | 2.31 | 1.76 | |
| HC35-1a | 41 | M | 8.53 | 6.72 | 1.56 | 1.03 | Xanthelasma |
| HC36-5 | 30 | M | 6.34 | 4.27 | 2.25 | 1.03 | |
| HC39-7a | 2 | M | 24.18 | 22.08 | 2.31 | 1.03 | Tendon xanthoma |
Subjects with a Simon Broome clinical diagnosis of possible or definite FH.
2.2. High resolution melting (HRM)
All the coding regions including the intron–exon boundaries and the promoter (to c.-298) of the LDLR, exon 2 to 12 of PCSK9 and part of exon 26 of APOB including p.R3527, were screened by HRM and those samples with a shift in melting temperature were sequenced. Exon 1 and the promoter region of PCSK9 were screened by sequencing alone; this region contains several common polymorphisms making HRM results difficult to analyze.
HRM was performed in the Rotorgene 6000 using AccuMelt HRM SuperMix (Quanta Biosciences, Gaithersburg, Maryland, USA) with 60 ng of gDNA and 8 pmol of each primer in a final volume of 20 μl or the LightScanner High Sensitivity Master Mix (Idaho Technology Inc. Utah, USA) with 30 ng of genomic DNA and 4 pmol of each primer in a final volume of 10 μl, according to the manufacturer's instructions. The primers and PCR conditions used for the LDLR, APOB and PCSK9 genes are given in Supplementary Table 1.
2.3. Restriction fragment length polymorphism (RFLP)
The LDLR has several common polymorphisms (Supplementary Table 2), which result in an HRM shift which is similar to that caused by a mutation. To overcome this, samples were genotyped for common polymorphisms by RFLP and their genotypes compared with the HRM result. Those with shifts due to the polymorphism were not examined further. However, variants of samples without the polymorphism but with a shift were sequenced to find the cause of the shift.
RFLP was also used to screen the families of probands with potential mutations to determine segregation of the variant with the FH phenotype (Supplementary Table 2). Polymerase chain reaction (PCR) amplification was carried out using 60 ng of gDNA, 2 mM Mg2 +, 8 pmol of each primer, and 0.4U of Taq DNA polymerase (Invitrogen) with the appropriate 1 × buffer in a final volume of 20 μl. The PCR was performed on a G-Storm thermal cycler (GRI) at 95 °C/3 min followed by 30 cycles of 95 °C/30 s; 60 °C/30 s (except for primers of exon 2 of LDLR which were annealed at 57 °C); 72 °C/30 s and a final step of 72 °C/5 min. Primers used for amplification were the same as those used for HRM (Supplementary Table 1). The amplified products were then digested with the appropriate restriction enzyme (NEB (UK) Ltd., Hitchin, Herts) using 8 μl of PCR product and 3U of the appropriate enzyme (Supplementary Table 2) in a total volume of 13 μl and run on a 2% agarose gel in 1 × TBE buffer.
2.4. Sequence analysis
The samples for sequencing were amplified using the same primers used for HRM (Supplementary Table 1) using the conditions above. The amplified products were purified using GFX columns (GE Healthcare) and sent to Source BioScience LifeSciences for sequencing.
2.5. Multiplex ligation-dependent probe amplification (MLPA)
For screening gross rearrangements within the LDLR, Multiplex Ligation-dependent Probe Amplification (MLPA; Kit P062, MRC-Holland, Amsterdam, The Netherlands) was used. Analysis was performed on all probands following the manufacturer's instructions.
2.6. In silico analysis
In order to predict the possible impact of a variation on the function of LDLR or PCSK9 the online tools Polyphen 2 (http://genetics.bwh.harvard.edu/pph2/) [20] and SIFT (http://sift.jcvi.org/www/SIFT_enst_submit.html) [21] were used.
3. Results
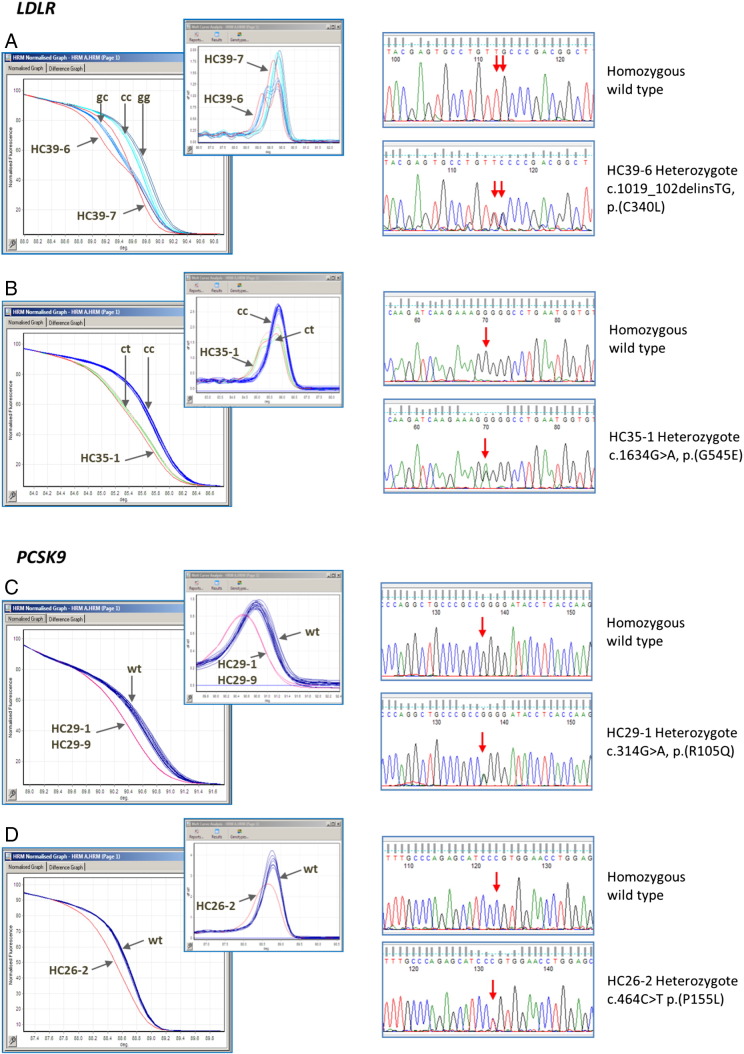
In the current study 11 families with FH and/or myocardial infarction (MI) were screened for possible mutations in LDLR, APOB and PCSK9 causing hyper- or hypo-cholesterolemia. The mean TC of the probands was 8.36 ± 1.4 mmol/l, triglycerides 2.71 ± 0.47 mmol/l, high density lipoprotein-cholesterol (HDL-C) 2.19 ± 0.52 mmol/l and LDL-C 5.07 ± 1.6 mmol/l. The sequencing of selected individuals identified by HRM resulted in the identification of two novel variants in LDLR and two novel variants in PCSK9 in four different families (Fig. 1).
Fig. 1.
HRM profiles and sequencing of the variations found in LDLR and PCSK9.
- HRM. Exon7 has a common polymorphism (c.1060 + 10G>C). The shifts for the different genotypes are indicated together with HC39-6; heterozygous for the mutation, and HC39-7 who was found to be homozygous for the mutation.
- Sequencing. The wild type sequence is shown in the upper panel and the heterozygous (HC39-6) sequence for the double base change in the bottom panel. The relevant bases are arrowed.
- HRM. Exon11 has a polymorphism (c.1617C>T). The shifts for the different genotypes are indicated together with HC35-1; heterozygous for the mutation.
- Sequencing. The wild type sequence is shown in the upper panel and the heterozygous (HC35-1) sequence in the bottom panel. The relevant bases are arrowed.
- HRM. The wild type and the shift in the two HC29 individuals are indicated, both are heterozygous for the mutation.
- Sequencing. The wild type sequence is shown in the upper panel and the heterozygous (HC29-1) sequence in the bottom panel. The relevant bases are arrowed.
- HRM. The wild type and the shift in the individual HC26-2; heterozygous for the mutation, are indicated.
- Sequencing. The wild type sequence is shown in the upper panel and the heterozygous (HC26-2) sequence in the bottom panel. The relevant bases are arrowed.
4. LDLR variations
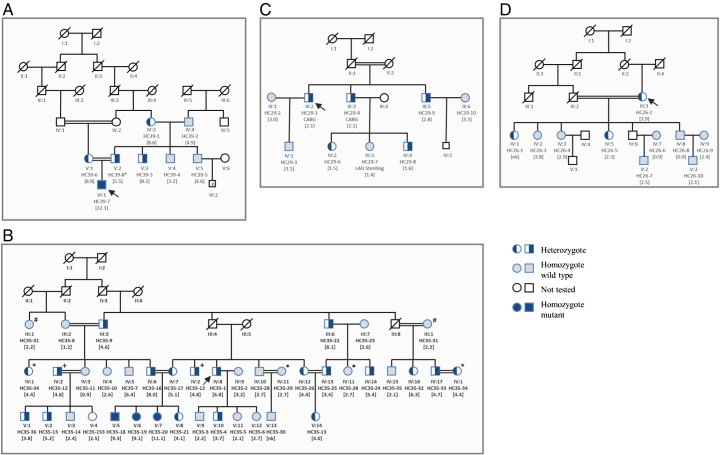
In family HC39 the proband (VI-1) was found to be homozygous for the mutation c.1019_1020delinsTG, p.(C340L) and had an extremely high total cholesterol of 24.2 mmol/l. His mother and father were heterozygous for this mutation and the mother had a correspondingly lower total cholesterol (10.6 mmol/l). The father's cholesterol level was unavailable. Both the proband's paternal grandmother and paternal uncle also had significantly elevated total cholesterol levels and were tested (by RFLP, data not shown) and found to be carriers of the mutation (Fig. 2A). As shown in Fig. 3A cysteine at amino acid position 340 is highly conserved among vertebrates, from zebra fish to mammals. Both SIFT and Polyphen2 predict this variant as probably damaging.
Fig. 2.
Co-segregation of LDL-C levels with the mutations found in the families' of the probands. A) Family HC39 with LDLR Exon 7 c.1019_1020delinsTG, p.(C340L). B) Family HC35 with LDLR Exon11 c.1634G>A, p.(G545E) where each paired symbol (*, +, #, ) represents the same person. C) Family HC29 with PCSK9 Exon 2 c.314G>A p.(R105Q). D) Family HC26 with PCSK9 Exon 3 c.464C>T p.(P155L). A double line between individuals indicates consanguinity. Probands are indicated by arrows, nk; not known.
Fig. 3.
Conservation of amino acid residues in different species. A) LDLR Exon 7 c.1019_1020delinsTG, (p.C340L) found in family HC39. B) LDLR Exon 11 c.1634G>A (p.G545E) found in family HC35. C) PCSK9 Exon 2 c.314G>A (p.R105Q) found in family HC29 D) PCSK9 Exon 3 c.464C>T (p.P155L) found in family HC26.
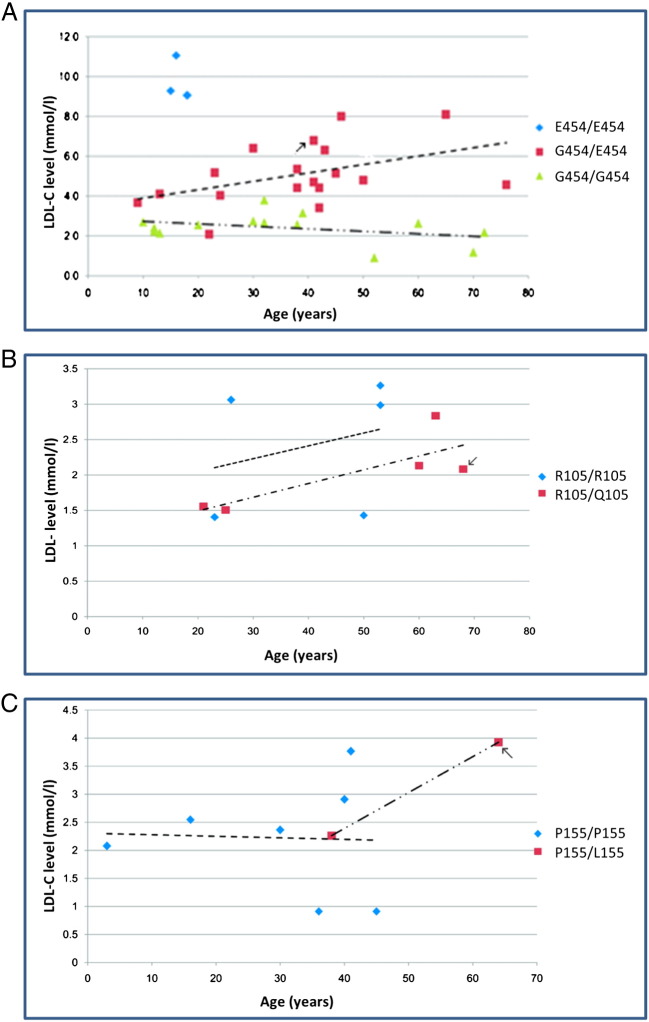
In family HC35, the proband (HC35-1; IV-8) was heterozygous for the variation c.1634G>A, p.(G545E) and had a total cholesterol level of 8.5 mmol/l, while the three family members homozygous for this mutation had average total cholesterol levels of 11.4 mmol/l. Most of the patients from family HC35 were not on regular statin treatment (Fig. 2B). The increase in LDL-C levels of the family members with or without the mutation and age are shown in Fig. 4A. While there was essentially no increase with age in non-carriers there was a significant increase in carriers. Overall mean total cholesterol levels were significantly higher in carriers compared to non-carriers (6.9 mmol/l vs. 4.1 mmol/l p = 2.6E-07). As shown in Fig. 3B, the glycine residue at this position is highly conserved in different species except zebra fish, which has a serine at this position. Both SIFT and Polyphen2 predict this variant as probably damaging.
Fig. 4.
The increase in LDL-C levels of family members with age with or without a mutation. A) Family HC35 LDL-C levels with or without LDLR mutation p.(G454E). B) Family HC29 LDL-C levels with or without PCSK9 mutation p.(R105Q). C) Family HC26 LDL-C levels with or without PCSK9 mutation p.(P155L). Probands are indicated by arrows.
5. PCSK9 variations
In family HC29, the proband (III-2) (Fig. 2C) was heterozygous for c.314G>A, p.(R105Q) with a total cholesterol level of 3.21 mmol/l, but with premature CHD. Individuals heterozygous for this variant had a mean total cholesterol level of 3.19 mmol/l as compared to the non-carriers with an average total cholesterol of 3.93 mmol/l (p = 0.8). The proband (III-2) and his brother (III-3) had undergone coronary artery bypass grafting (CABG) at the age of 50 and 40 years respectively, while one of their offspring (IV-3) had left anterior descending artery (LAD) stenting at the age of 21 years. As shown in Fig. 4B cholesterol levels rise with age, in both carriers and non-carriers, but the average level in carriers appears lower although this does not reach statistical significance. As shown in Fig. 3C, the arginine residue at this position is not highly conserved in different species although it is only substituted by lysine which is a similarly charged residue. Polyphen predicts this variant to be damaging whereas SIFT predicts that this variation will be tolerated.
In the family HC26, the proband (III-3) (Fig. 2D) was found to be heterozygous for c.464C>T p.(P155L) with a total cholesterol level of 6.34 mmol/l. Mean total cholesterol levels were only available for two of the three carriers in this family; mean (SD) in carriers is 5.20 mmol/l (1.56) compared to 4.77 mmol/l (1.34) in seven non-carriers (p = 0.71). As shown in Fig. 3D, the proline residue at this position is conserved in all species, and both SIFT and Polyphen2 predict this variant as damaging. However this variant did not show strong evidence for co-segregation with lipid levels in this small family (Fig. 4C).
6. Discussion
In order to develop the most efficient genetic testing approach for patients with FH, there is a need to define all the pathogenic variants in the LDLR gene in different populations. Pakistan is a multi-racial nation and therefore this heterogeneous population is expected to carry a number of novel LDLR mutations. To date five different mutations in Pakistani FH patients have been reported [16–18]. Among the families in the current study two additional novel mutations were identified in two different families. All except one of the probands screened here had hypercholesterolemia (mean total cholesterol in Pakistan reported to be 4.5 mmol/l [22]), and although only six had LDL-C levels above the UK cut-off for a clinical diagnosis of FH (> 4.9 mmol/l), because there are no validated LDL-C cut-offs for the clinical diagnosis of FH in Pakistan, we included 4 probands with LDL-C above 3.9 mmol/l, and one with premature CAD but no elevation of plasma lipid levels. After excluding this subject, the mutation detection rate of 2/10 (20%) in this sample of patients is lower than previously reported for definite FH patients but is similar to the 20-30% reported in possible FH patients in the UK [14]. The presence of tendon xanthomas is the major diagnostic criterion for definite as opposed to possible FH [1] and only one of the probands had tendon xanthomas (HC39-7).
In family HC39, the LDLR mutation p.(C340L) was found in a homozygous 2 year old child with xanthomas, while his mother was heterozygous for this change. This change lies in exon 7 of the LDLR which encodes part of the epidermal growth factor (EGF) like region of the LDLR. This cysteine residue is highly conserved among vertebrates, from zebra fish to mammals, indicating its important role in the function (Fig. 3A) of this region. Although this cysteine residue is not in the seven cysteine repeats important for the binding of LDL-C to the receptor, it is a part of the EGF like 1 domain involved in the displacement of LDL-C in the acidic environment of the endosome [23]. In family HC35, the mutation p.(G545E) was observed in the heterozygous proband with LDL-C level of 6.8 mmol/l, while three individuals of family HC35 were homozygous for the mutant allele. The glycine residue at this position is highly conserved in different species except zebra fish, which has a serine at this position (Fig. 3B). Both SIFT and Polyphen2 predict this variant as damaging.
In Family HC35, there were enough carriers and non-carriers to compare the rate of increase in LDL-C levels with increasing age. Compared to the non-carriers, where there was no evidence of an age effect, mean levels were higher in older compared to younger carriers. While this cross-sectional data has limitations, it suggests that the impact of the LDLR defect on LDL clearance may be seen most clearly in individuals where age-related effects on plasma lipid metabolism places the system under metabolic stress.
Two potentially pathogenic variants in PCSK9 were identified, p.(R105Q) and p.(P155L), located in the pro-domain and autocatalytic domain of PCSK9 respectively. Interestingly, carriers of p.(R105Q) had 19% lower total cholesterol levels compared to non-carriers in family HC29, with the lower cholesterol levels being apparent in individuals of different ages. This suggest p.(R105Q) is a loss-of-function variant not a gain-of-function FH-causing mutation. This variant is in the pro-domain of PCSK9, the autocatalytic domain does not start until aa153, so it is not clear what the molecular mechanism of this variant would be. More than an 80% decrease in autocatalytic activity has been reported due to mutations in the pro-segment of PCSK9 [24,25] and, if this is the mechanism, the number of LDLR on hepatocytes would be higher, which would increase the uptake of circulating LDL-C and result in the lower plasma cholesterol levels seen. Cameron et al. [23] reported 32% more LDLR on the cell surface in p.(G106R) mutants of PCSK9 compared to the wild-type PCSK9, with 71% increased LDL-C clearance through internalization leading to lowering of LDL-C in the serum. The in silico predictions that this variant would be probably damaging support this.
The effect of the p.(P155L) PCSK9 variant detected is less clear cut. Mayne et al. [26] reported a loss of function mutation p.(Q152H) in PCSK9, in a French Canadian family. This variant is near the autocatalytic site which is cleaved by the enzyme SIP at p.Q152, with the variant allele, p.(152H), not undergoing efficient autocatalytic cleavage, resulting in a 79% decrease in serum levels of PCSK9 and up to 48% lowering of TC among the carriers. Benjannet et al. [24] reported that the proline residue at position 155 in the catalytic site is important for autocatalytic activity, presumably because the β-turn following this residue is important for the cleavage and subsequent release of mature PCSK9 from the endoplasmic reticulum. In support of this the in silico analysis suggests that this variant will be damaging, but this cannot be used to infer whether this would be a loss or gain of function effect. Unfortunately the family HC26 is too small to give a clear answer. LDL-C levels were not significantly different in the carriers compared to non-carriers, and extending the family by tracing further relatives would be useful.
In conclusion, we have identified two additional novel mutations in LDLR in FH patients from Pakistan, bringing the total number identified to date to seven. All of these mutations occur in only one family, although the sample of patients examined to date is too small to be able to say whether or not any may be common. None of these mutations have been identified in FH patients from the Indian subcontinent living in the UK [14], suggesting that the genetic heterogeneity in the subcontinent is likely to be considerable. It is unclear whether the patients where no mutation could be found have a mutation in a region of the LDLR gene not examined here, or that they may have a mutation in a yet to be discovered gene or, perhaps most likely, that they do not have monogenic FH. Further family studies may help to distinguish these difficulties. The current study highlights the benefits of a definitive DNA-based diagnosis for FH families where, based upon this genetic data, better counselling and a better choice of future treatments is available. For the mode of action of the PCSK9 variants in decreasing LDL-C levels, further functional studies will enhance our knowledge and may be helpful in devising novel therapeutics.
Acknowledgement
This work was financially supported by grant no. 934 from the Higher Education Commission of Pakistan, awarded to R.Q. Part of this work was supported by Shifa College of Medicine through a core grant to R.Q. W.A. was supported by an IRSIP grant from HEC for his studies at UCL. We would like to thank the FH families and healthy individuals for donating their blood. S.E.H., R.W. and W.P. are supported by the British Heart Foundation (RG008/08).
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cca.2013.03.017.
Appendix A. Supplementary data
Supplementary materials.
References
- 1.Marks D., Thorogood M., Neil H.A., Humphries S.E. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168:1–14. doi: 10.1016/s0021-9150(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 2.Leigh S.E., Foster A.H., Whittall R.A., Hubbart C.S., Humphries S.E. Update and analysis of the University College London low density lipoprotein receptor familial hypercholesterolemia database. Ann Hum Genet. 2008;72:485–498. doi: 10.1111/j.1469-1809.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 3.Neil A., Cooper J., Betteridge J., Capps N., McDowell I., Durrington P. Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Eur Heart J. 2008;29:2625–2633. doi: 10.1093/eurheartj/ehn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soutar A.K., Naoumova R.P. Mechanisms of disease: genetic causes of familial hypercholesterolemia. Nat Clin Pract Cardiovasc Med. 2007;4:214–225. doi: 10.1038/ncpcardio0836. [DOI] [PubMed] [Google Scholar]
- 5.Dušková L., Kopečková L., Jansová E., Tichý L., Freiberger T., Zapletalová P. An APEX-based genotyping microarray for the screening of 168 mutations associated with familial hypercholesterolemia. Atherosclerosis. 2011;216:139–145. doi: 10.1016/j.atherosclerosis.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Liyanage K.E., Burnett J.R., Hooper A.J., van Bockxmeer F.M. Familial hypercholesterolemia: epidemiology, Neolithic origins and modern geographic distribution. Crit Rev Clin Lab Sci. 2011;48:1–18. doi: 10.3109/10408363.2011.565585. [DOI] [PubMed] [Google Scholar]
- 7.Naoumova R.P., Tosi I., Patel D., Neuwirth C., Horswell S.D., Marais A.D. Severe hypercholesterolemia in four British families with the D374Y mutation in the PCSK9 gene: long-term follow-up and treatment response. Arterioscler Thromb Vasc Biol. 2005;25:2654–2660. doi: 10.1161/01.ATV.0000190668.94752.ab. [DOI] [PubMed] [Google Scholar]
- 8.Cohen J., Pertsemlidis A., Kotowski I.K., Graham R., Garcia C.K., Hobbs H.H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 9.Scartezini M., Hubbart C., Whittall R.A., Cooper J.A., Neil A.H., Humphries S.E. The PCSK9 gene R46L variant is associated with lower plasma lipid levels and cardiovascular risk in healthy U.K. men. Clin Sci (Lond) 2007;113:435–441. doi: 10.1042/CS20070150. [DOI] [PubMed] [Google Scholar]
- 10.Humphries S.E., Whittall R.A., Hubbart C.S., Maplebeck S., Cooper J.A., Soutar A.K. Genetic causes of familial hypercholesterolaemia in patients in the UK: relation to plasma lipid levels and coronary heart disease risk. J Med Genet. 2006;43:943–949. doi: 10.1136/jmg.2006.038356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romano M., Di Taranto M.D., D'Agostino M.N., Marotta G., Gentile M., Abate G. Identification and functional characterization of LDLR mutations in familial hypercholesterolemia patients from Southern Italy. Atherosclerosis. 2010;210:493–496. doi: 10.1016/j.atherosclerosis.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 12.Kusters D.M., Huijgen R., Defesche J.C., Vissers M.N., Kindt I., Hutten B.A. Founder mutations in the Netherlands: geographical distribution of the most prevalent mutations in the low-density lipoprotein receptor and apolipoprotein B genes. Neth Heart J. 2011;19:175–182. doi: 10.1007/s12471-011-0076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traeger-Synodinos J., Mavroidis N., Kanavakis E., Drogari E., Humphries S.E., Day I.N. Analysis of low density lipoprotein receptor gene mutations and microsatellite haplotypes in Greek FH heterozygous children: six independent ancestors account for 60% of probands. Hum Genet. 1998;102:343–347. doi: 10.1007/s004390050703. [DOI] [PubMed] [Google Scholar]
- 14.Miltiadous G., Elisaf M., Bairaktari H., Xenophontos S.L., Manoli P., Cariolou M.A. Characterization and geographic distribution of the low density lipoprotein receptor (LDLR) gene mutations in northwestern Greece. Hum Mutat. 2001;17:432–433. doi: 10.1002/humu.1121. [DOI] [PubMed] [Google Scholar]
- 15.Taylor A., Wang D., Patel K., Whittall R., Wood G., Farrer M. Mutation detection rate and spectrum in familial hypercholesterolaemia patients in the UK pilot cascade project. Clin Genet. 2010;77:572–580. doi: 10.1111/j.1399-0004.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 16.Ajmal M., Ahmed W., Sadeque A. Identification of a recurrent insertion mutation in the LDLR gene in a Pakistani family with autosomal dominant hypercholesterolemia. Mol Biol Rep. 2010;37:3869–3875. doi: 10.1007/s11033-010-0043-0. [DOI] [PubMed] [Google Scholar]
- 17.Ajmal M., Ahmed W., Akhtar N., Sadeque A., Khalid A., Benish Ali S.H. A novel pathogenic nonsense triple-nucleotide mutation in the low-density lipoprotein receptor gene and its clinical correlation with familial hypercholesterolemia. Genet Test Mol Biomarkers. 2011;15:601–606. doi: 10.1089/gtmb.2010.0184. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed W., Ajmal M., Sadeque A., Whittall R.A., Rafiq S., Putt W. Novel and recurrent LDLR gene mutations in Pakistani hypercholesterolemia patients. Mol Biol Rep. 2012;39:7365–7372. doi: 10.1007/s11033-012-1568-1. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J. 3rd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2001. Molecular cloning: a laboratory manual. [Google Scholar]
- 20.Adzhubei I.A., Schmidt S., Peshkin L., Ramensky V.E., Gerasimova A., Bork P. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar P., Henikoff S., Ng P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed W., Malik M., Saeed I., Khan A.A., Sadeque A., Kaleem U. Role of tissue plasminogen activator and plasminogen activator inhibitor polymorphism in myocardial infarction. Mol Biol Rep. 2011;38:2541–2548. doi: 10.1007/s11033-010-0392-8. [DOI] [PubMed] [Google Scholar]
- 23.Innerarity T.L. Structural biology. LDL receptor's beta-propeller displaces LDL. Science. 2002;298:2337–2339. doi: 10.1126/science.1080669. [DOI] [PubMed] [Google Scholar]
- 24.Benjannet S., Rhainds D., Essalmani R., Mayne J., Wickham L., Jin W. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279:48865–48875. doi: 10.1074/jbc.M409699200. [DOI] [PubMed] [Google Scholar]
- 25.Cameron J., Holla O.L., Ranheim T., Kulseth M.A., Berge K.E., Leren T.P. Effect of mutations in the PCSK9 gene on the cell surface LDL receptors. Hum Mol Genet. 2006;15:1551–1558. doi: 10.1093/hmg/ddl077. [DOI] [PubMed] [Google Scholar]
- 26.Mayne J., Dewpura T., Raymond A., Bernier L., Cousins M., Ooi T.C. Novel loss-of-function PCSK9 variant is associated with low plasma LDL cholesterol in a French–Canadian family and with impaired processing and secretion in cell culture. Clin Chem. 2011;57:1415–1423. doi: 10.1373/clinchem.2011.165191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.