Abstract
Background/Aims
The aims of this study were (1) to identify the useful clinical parameters of noninvasive approach for distinguishing nonalcoholic steatohepatitis (NASH) from nonalcoholic fatty liver disease (NAFLD), and (2) to determine whether the levels of the identified parameters are correlated with the severity of liver injury in patients with NASH.
Methods
One hundred and eight consecutive patients with biopsy-proven NAFLD (age, 39.8±13.5 years, mean±SD; males, 67.6%) were prospectively enrolled from 10 participating centers across Korea.
Results
According to the original criteria for NAFLD subtypes, 67 patients (62.0%) had NASH (defined as steatosis with hepatocellular ballooning and/or Mallory-Denk bodies or fibrosis ≥2). Among those with NAFLD subtype 3 or 4, none had an NAFLD histologic activity score (NAS) below 3 points, 40.3% had a score of 3 or 4 points, and 59.7% had a score >4 points. Fragmented cytokeratin-18 (CK-18) levels were positively correlated with NAS (r=0.401), as well as NAS components such as lobular inflammation (r=0.387) and ballooning (r=0.231). Fragmented CK-18 was also correlated with aspartate aminotransferase (r=0.609), alanine aminotransferase (r=0.588), serum ferritin (r=0.432), and the fibrosis stage (r=0.314). A fragmented CK-18 cutoff level of 235.5 U/L yielded sensitivity, specificity, and positive and negative predictive values of 69.0%, 64.9%, 75.5% (95% CI 62.4-85.1), and 57.1% (95% CI 42.2-70.9), respectively, for the diagnosis of NASH.
Conclusions
Serum fragmented CK-18 levels can be used to distinguish between NASH and NAFL. Further evaluation is required to determine whether the combined measurement of serum CK-18 and ferritin levels improves the diagnostic performance of this distinction.
Keywords: Nonalcoholic fatty liver disease, Cytokeratin-18, Ferritin
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is recognized as one of the most common cause of chronic liver diseases in Western countries, as well as in Korea.1-3 NAFLD is considered liver manifestation of insulin resistance (IR) and metabolic syndrome since it is closely associated with obesity, hypertension and dyslipidemia.4-6 NAFLD is a condition of fat overaccumulation in the liver and clinicohistologic phenotype of NAFLD extends from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) and/or fibrosis.7 NASH can progress to cirrhosis8 or liver failure and increase the risk of hepatocellular carcinoma and induce liver related mortality.8-14 Although a liver biopsy is considered as a gold standard for the diagnosis and estimation of the activity of NAFLD, it is an invasive procedure with a considerable cost. Because fat accumulation and inflammation of NAFLD are more heterogeneous than those of chronic hepatitis C, it is more prone to the sampling errors.15 Steatohepatitis is not merely the presence of steatosis and inflammation but a specific histopathologic entity (macrovesicular steatosis, inflammation and ballooned hepatocyte and/or Mallory-Denk bodies).16 NAFLD activity assessment using a biopsied sample only may lead to inadequate evaluation. Therefore, various noninvasive laboratory tests or imaging studies for evaluating the extent of fat accumulation, the presence of necroinflammation and the stage of fibrosis has been studied to avoid unjustified liver biopsy.
Numerous biomarkers have been investigated in order to discriminate NASH from simple steatosis.17-27 However, there is no unique biomarker to meet the requirements sufficiently. Hepatocyte apoptosis is typically increased in subjects with NASH, not in those with NAFL.28 Cytokeratin 18 (CK-18) is the major intermediate filament protein in the liver resulting in the characteristic structural changes of apoptosis.29 Caspase generated CK-18 fragments were increased in patients with NAFLD compared with healthy age-matched controls, and plasma levels correlated with expression levels in the liver.30
The aims of this study are (1) to identify the useful clinical parameters of a noninvasive approach to distinguish NASH from NAFL; and (2) to determine whether these levels would be related to the severity of the liver injury in patients with NASH.
PATIENTS AND METHODS
Patients
All consecutive patients who underwent liver biopsy for suspected NAFLD between Jan. 2009 and Jul. 2011 were recruited prospectively at ten Korean university hospital: Soon Chun Hyang University Bucheon Hospital, Seoul St. Mary's Hospital of The Catholic University of Korea, Uijheongbu St. Mary's Hospital of The Catholic University of Korea, Yeouido St. Mary's Hospital of The Catholic University of Korea, Bucheon St. Mary's Hospital of The Catholic University of Korea, St. Paul's Hospital of The Catholic University of Korea, Bucheon St. Vincent's Hospital of The Catholic University of Korea, Incheon St. Mary's Hospital of The Catholic University of Korea, Soon Chun Hyang University Seoul Hospital, Chungbuk National University hospital of Chungbuk National University.
Elevation of aminotransferase levels for more than 3 months and/or fatty liver detected by ultrasonography were the main reasons for liver biopsy. Patients with history of significant alcoholic drinking (> 20 g/day) or hepatotoxic/herb medication were excluded. Patients with other causes of steatogenic drug (e.g. systemic steroids), viral, cholestatic, autoimmune, metabolic or hereditary disorder were also excluded. The patients who have undergone bariatric surgery with last 5 years were excluded.
All enrolled patients were Koreans, and the study protocol was approved by the review board at each participating institution. All subjects gave consents prior to the participation.
Clinical assessment
The medical history, including co-morbid illness (such as hypertension or diabetes) and drug/herb intake, anthropometric, laboratory and clinical data were collected from all patients at the same day of liver biopsy.
Body mass index (BMI) was calculated as body weight in kilograms divided by body height in square meters (kg/m2). Waist circumference was measured in a standing position at a level of the umbilicus with the tape all around the body in the horizontal position.
Venous blood samples were taken in the morning after a 12 hours overnight fasting on the day of liver biopsy. The laboratory evaluation in all patients included a blood cell count and the measurement of aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), total cholesterol, triglyceride (TG), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, albumin, glucose, C-reactive protein (CRP), immunoreactive insulin, ferritin, and fragmented CK-18. IR was evaluated according to homeostatic model assessment (HOMA),31 as fasting serum insulin (in µIU/mL) multiplied by fasting serum glucose (in mg/dL), divided by 405.
The diagnosis of metabolic syndrome32 was carried out according to the joint statement of the International Diabetes Federation, National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, and International Association for the Study of Obesity, and based on the presence of three or more the following criteria: (1) central obesity (waist circumference ≥90 cm in men and ≥80 cm in women), (2) TG >150 mg/dL, (3) reduced HDL-cholesterol (<40 mg/dL in men and <50 mg/dL in women), (4) blood pressure ≥130/85 mmHg and (5) fasting plasma glucose ≥100 mg/dL, or drug treatment for the above metabolic abnormalities.
Histologic evaluation
All the patients underwent an ultrasonic guided percutaneous liver biopsy using a 16-gauge needle (Acecut®; TSK Laboratory, Tochigi, Japan) under local anesthesia. Liver biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin and stained with hematoxylin and eosin, Masson-trichrome, and/or reticulin stain. The slide were reviewed in conference by both experienced hepatopathologists (ESJ and HKK) who were blinded to all clinical, demographic and laboratory information, the diagnosis of NASH were made by consensus.
Histological grading and staging of NAFLD were scored semiquantitatively according to the original criteria for NAFLD subtypes,9,33-35 and NAFLD histologic activity score (NAS) system.16 According to the original criteria, the NAFLD was histologically categorized into four subtypes9: (1) steatosis alone (NAFLD type 1), (2) steatosis with lobular inflammation only (NAFLD type 2), (3) steatosis with hepatocellular ballooning (NAFLD type 3), or (4) steatosis with Mallory-Denk bodies or fibrosis (NAFLD type 4). NAFLD subtypes 3 and 4 were considered to represent NASH.34 Histologic finding with stage 2 or above fibrosis were also defined as NASH.21 The NAS identified the degree of steatosis (0-3), lobular inflammation (0-3), and hepatocellular ballooning (0-2).16 The NAS was the sum of above numerical pathologic scores and ranged from 0 to 8. The stage of fibrosis was scored on a five-point scale, as follows: stage 0=no fibrosis, stage 1=perisinusoidal or periportal fibrosis, stage 2=perisinusoidal and portal/periportal fibrosis, stage 3=bridging fibrosis, and stage 4=cirrhosis.16
Caspase generated CK-18 fragment
Serum samples were obtained from the patients on the same day of the liver biopsies and stored at -80℃ until just before analysis. The levels of the apoptosis-associated CK-18 in sera the were measured by the M30-Apoptosense enzyme-linked immunosorbent assay (ELISA) kit (PEVIVA AB, Bromma, Sweden).19,36 All assays were performed in duplicate and the absorbance was determined by using a microplate reader (Molecular Devices M2, Sunnyvale, CA).
Statistical analysis
Continuous variables were expressed as means±standard deviation. Categorical data analysis was performed using the Chi-square test and Fisher's exact test, as appropriate. Quantitative data analysis was performed using independent t-test and oneway analysis of variance for normal distributional data, or Mann-Whitney U test and Kruskal-Wallis test for non-parametric data. Spearman's correlation analysis or pairwised correlation analysis was used to assess relationship between CK-18/ferritin and hepatic steatosis, lobular activity, ballooning, and fibrosis grading, as appropriate. The predictive value of a variable for the detection of hepatic fibrosis was evaluated using a receiver-operating characteristic curve analysis. P value of <0.05 was considered as statistically significant.
RESULTS
Patients' characteristics
There were 108 patients recruited in this study. The baseline clinical and laboratory characteristics of the patients are described in Table 1. Seventy-two (67.6%) were male. Patients mean age was 39.0±13.5 years, ranging from 19 to 80 years. BMI was 28.7±3.8 kg/m2 and 93 (86.1%) patients were overweight with a BMI or more than 25 kg/m2. Fifty-two (48.1%) patients have metabolic syndrome (Table 1).
Table 1.
Baseline characteristics of Korean patients with nonalcoholic fatty liver disease, relative to nonalcoholic fatty liver disease subtype
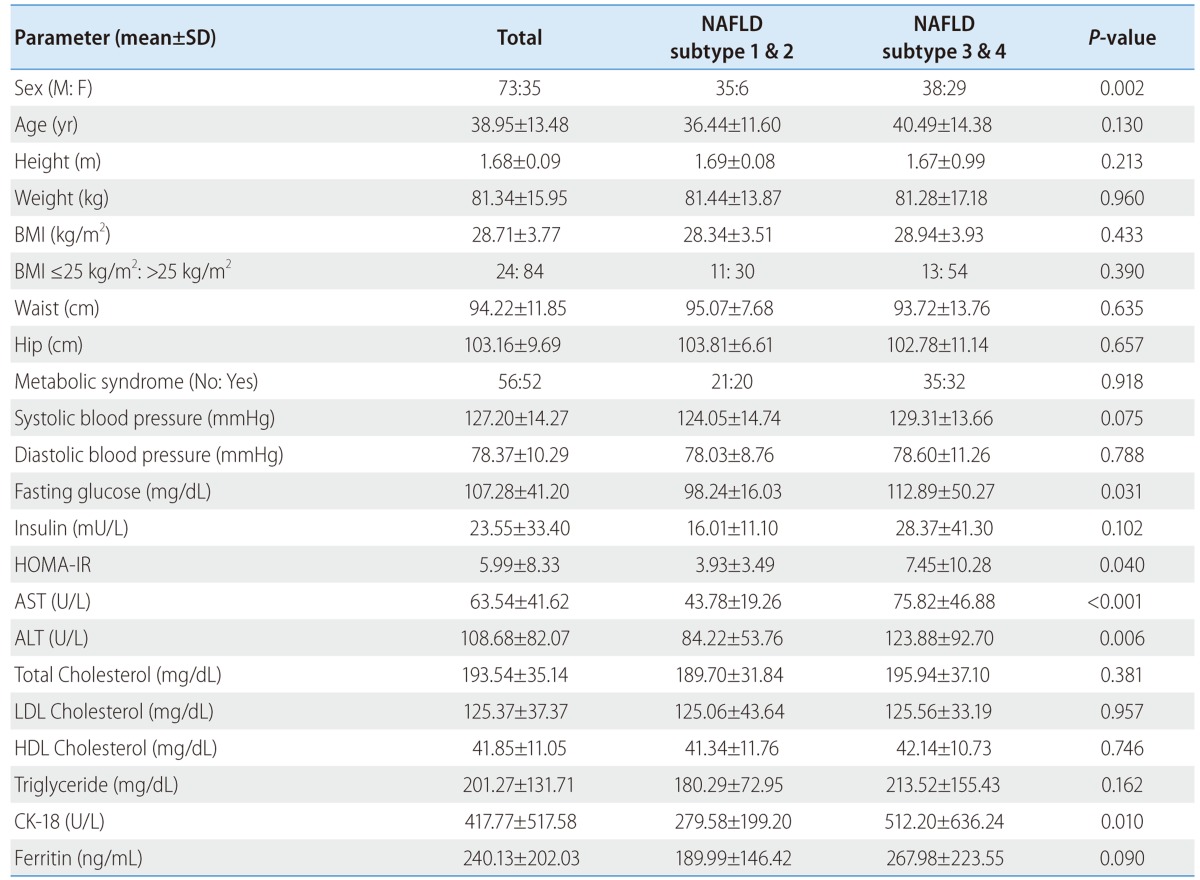
NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; HOMA-IR, homeostatic model assessment-insulin resistance; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CK-18, cytokeratin-18.
The levels of fasting glucose, HOMA-IR, AST, ALT and portion of female are higher in subtype 3 and 4 of original criteria for NAFLD than subtype 1 and 2 (P<0.05). Presence of metabolic syndrome, BMI, levels of cholesterol and TG are not different significantly between above two groups (Table 1).
Comparison between NAFLD subtype and NAS in Korean patients with NAFLD
According to original criteria for NAFLD subtypes, the patients were categorized into 1 patient (1.0%) of NAFLD type 1, 40 (37.0%) of NAFLD type 2, 39 (36.1%) of NAFLD type 3 and 28 (25.9%) of NAFLD type 4. Therefore, with original criteria for NAFLD subtypes, 67 (62.0%) had NASH (steatosis with hepatocellular ballooning and/or Mallory-Denk bodies or fibrosis≥2).
With NAS system, there were 9 patients (8.3%) with "NAS ≤2", 54 (50%) with "NAS: 3-4", and 45 (41.7%) with "NAS≥5", respectively (Table 2). The numbers of patients with fibrosis 0 or 1 are 73. Thirty-six (49.3%) in this group are NAFLD subtype 1 or 2 (Table 2).
Table 2.
Histologic features of Korean patients with nonalcoholic fatty liver disease

NAFLD: nonalcoholic fatty liver disease; NAS: nonalcoholic fatty liver histologic activity score.
In NASH group (NAFLD subtype 3 or 4), none had below 3 points of NAS, 27 (40.3%) had 3-4 points of NAS, and 40 (59.7%) had over 4 points of NAS. In non-NASH group (NAFLD subtype 1 or 2), 9 (22.0%) had below 3 points of NAS, 27 (65.9%) had 3-4 points of NAS, and 5 (12.2%) had over 4 points of NAS (Fig. 1). And also, 27 (42.9%) of patients below 5 points of NAS are in group of NAFLD subtype 3 or 4 and 5 (11.1%) of patients above 4 points of NAS are in group of NAFLD subtype 1 or 2, respectively (Fig. 1).
Figure 1.
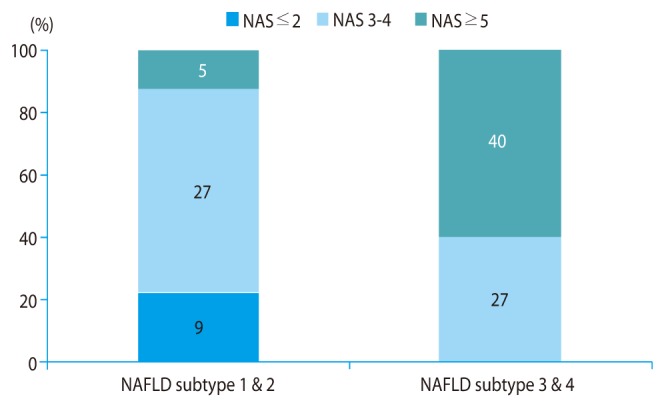
Correlation between nonalcoholic fatty liver disease subtype and nonalcoholic fatty liver disease histology activity score. NAFLD, nonalcoholic fatty liver disease; NAS, nonalcoholic fatty liver histologic activity score.
Diagnosis of NASH using serum biomarkers in NAFLD patients
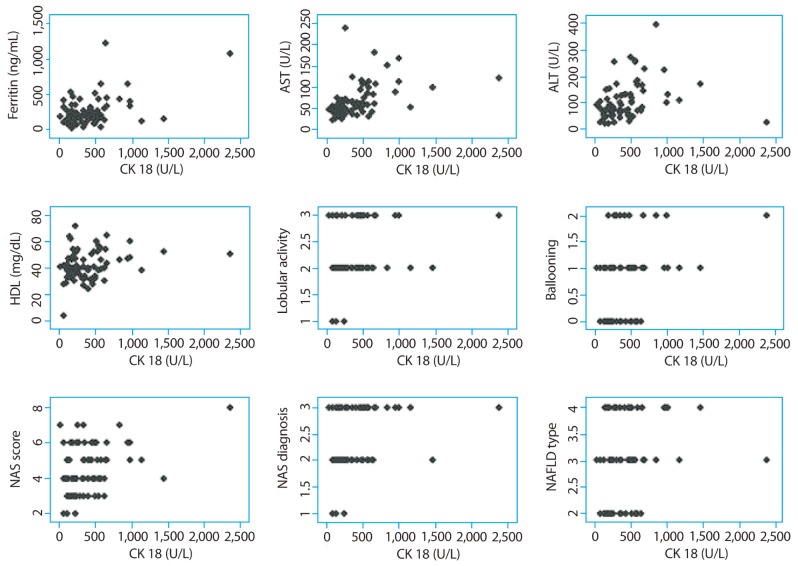
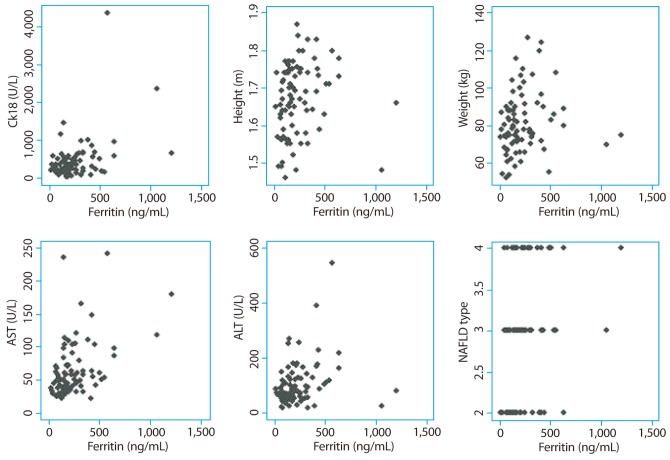
CK-18 level had positive correlation with systolic blood pressure, AST, ALT, HDL-cholesterol, ferritin, lobular inflammation, ballooning, fibrosis, NAS and NAFLD subtype (Table 3 and Fig. 2). Serum ferritin level had positive correlation with insulin, HOMA-IR, AST, ALT, fibrosis and NAS (Table 3 and Fig. 3). Serum ferritin levels showed weaker positive correlation with histopathologic characteristics including NAS (r=0.258) and stage of fibrosis (r=0.272) than fragmented CK-18, respectively.
Table 3.
Correlation coefficient of cytokeratin-18 and serum ferritin levels in patients with nonalcoholic fatty liver disease
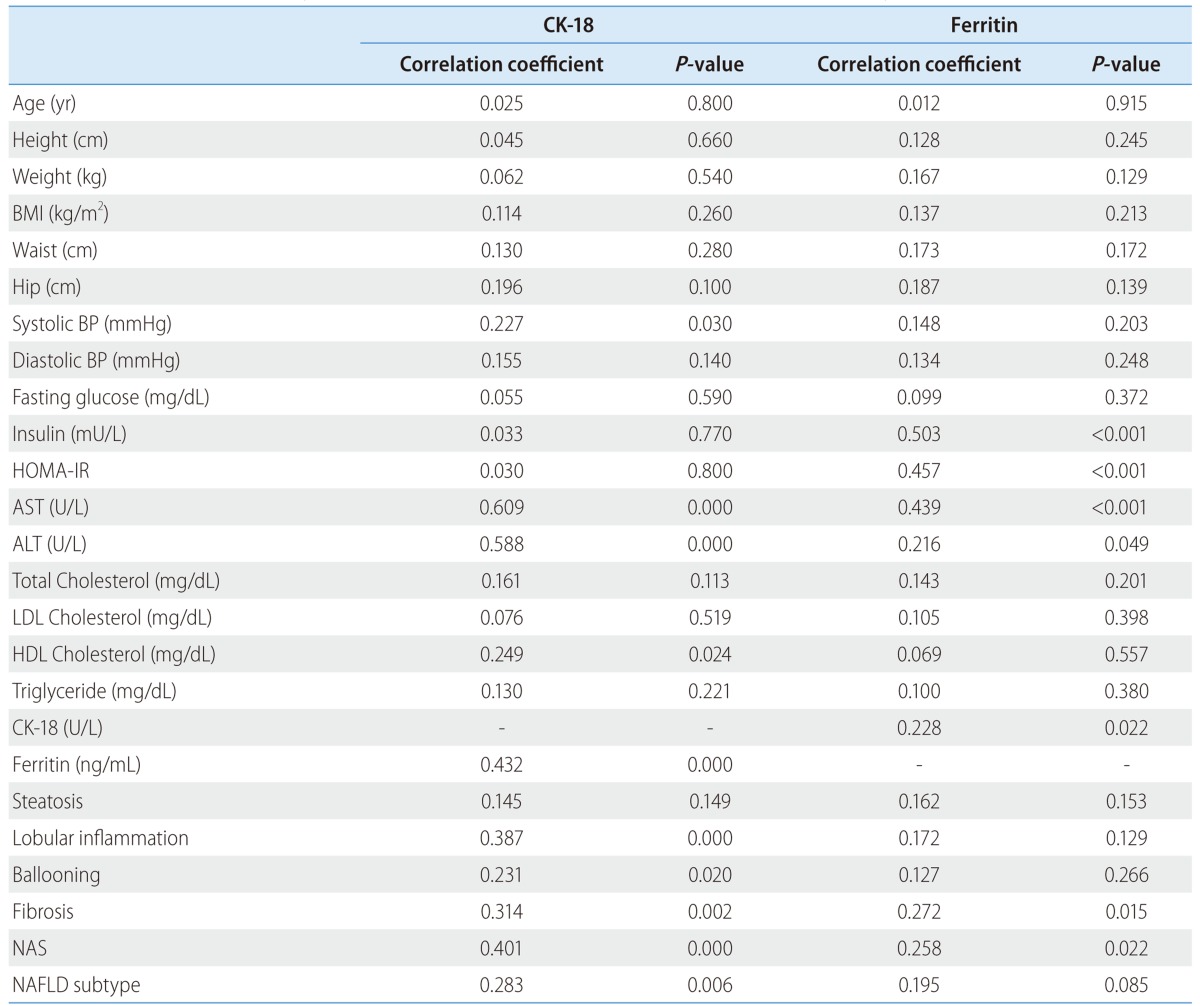
BMI, body mass index; HOMA-IR, homeostatic model assessment-insulin resistance; BP, blood pressure; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CK-18, cytokeratin-18; NAS, NAFLD histologic activity score, NAFLD, nonalcoholic fatty liver disease.
Figure 2.
Correlation scattergrams for cytokeratin-18 levels. CK-18, cytokeratin-18; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL, high-density lipoprotein; NAS, nonalcoholic fatty liver disease activity score; NAFLD, nonalcoholic fatty liver disease.
Figure 3.
Correlation scattergrams for ferritin levels. CK-18, cytokeratin-18; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL, high-density lipoprotein; NAFLD, nonalcoholic fatty liver disease.
The serum CK-18 level was significantly higher in NAFLD subtype 3 or 4 group than that of NAFLD subtype 1 or 2. However, the ferritin level was not significantly elevated in this group (Table 1).
A fragmented CK-18 cutoff value of 235.5 U/L calculated using the receiver operating characteristic curve showed a sensitivity of 69.0%, a specificity of 64.9%, and positive and negative predict values (PPV and NPV) of 75.5% (95% confidence interval [CI] 62.4-85.1) and 57.1% (95% CI 42.2-70.9), respectively, for the diagnosis of NASH. Additional measurement of ferritin to CK-18 improved a specificity of 85.2% and a PPV of 92.6% (95% CI 82.4-97.1), respectively (Table 4).
Table 4.
The use of cytokeratin-18 and serum ferritin levels for the diagnosis of significant fibrosis and nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease, using original criteria
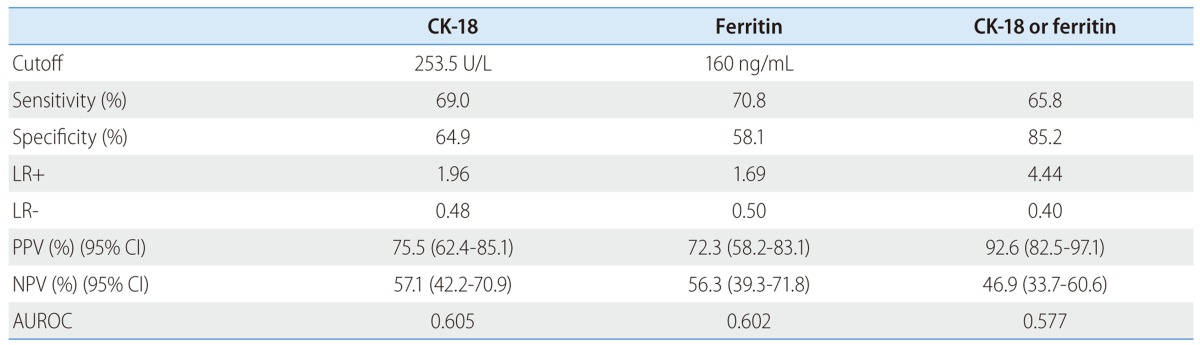
AUROC, area under receiver operating characteristics; LR+, positive likelihood ratio; LR-, negative likelihood ratio; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval; CK-18, cytokeratin-18.
DISCUSSION
Since the progression is very different depending on NAFLD subtype, the diagnosis of NASH is important to predict prognosis and to identify candidates who require treatment. Although there has been several previous studies investigating CK-18 as a biomarker to replace biopsy, this research provides an evidence that CK-18 actually helps in the diagnosis of NASH in Koreans.
Nowadays, the activity of NAFLD is assessed by typical histologic findings with original criteria9,33-35 or Brunt criteria7 and also NAS designated by the NASH Clinical Research Network (NASH CRN).16 The NAS could provide a disease activity score for patients who most likely have NASH.16 The NAS has reasonable inter-rater reproducibility and represent changes more sensitively allowing evaluating of therapeutic response or natural course possible. However, this system has not been validated to see if the system could predict progression to cirrhosis or liver related mortality.16,35 Even though several new diagnostic criteria for NASH has been developed, the original criteria for NAFLD subtypes demonstrated the best predictability for liver-related mortality in patients with NAFLD the most effectively.33 Thus we decided to use the original diagnostic criteria in order to diagnose NASH and to take NAS system as an auxiliary test.
In previous study, 16% of biopsies did not meet NASH criteria yet had a NAS ≥5,37 and a NAS cut-off of 5 points significantly underestimated the diagnosis of NASH compared with the global assessment.21 Five (11.1%) of 45 patients with NAS greater than 5 had are NAFDL subtype 1 or 2 in our cohort. Furthermore, when 67 patients (67%) showed NAFLD subtype 3 or 4, only 45 patients (41.7%) scored greater than 5 under NAS system. This result indicates that the NAS cannot replace a pathologist's diagnostic determination of NASH.16
In the present study, we assessed histology activity with original criteria along with NAS. There is no patient scored below NAS 3 in group of NAFLD subtype 3 or 4. On the other hand, thirty two patients from NAFLD subtype 1 or 2 scored 3 or greater points under NAS system. The discordance rate between the original criteria and NAS in the diagnosis of NASH was 29.6%.
Elevation of aminotransferase level can be a clue for diagnosis of NAFLD. However, it has some of its own weaknesses in the diagnosis of NAFLD. 1) It does not assess the degree of lipid accumulation, 2) It does not provide a cause of liver disease and 3) It does not discriminate between NASH and NAFL.6
Apoptosis of hepatocytes plays an important role in the progression of the NASH and the liver injury. Hepatocytes containing Mallory bodies are likely to undergo apoptosis. The major components of Mallory body include CK-8 and 18, ubiquitin and heat shock proteins 70 and 90.38 Accumulation of fatty acids and lipid peroxides in hepatocytes may activate caspase 3 and promote cleavage of CK-18.39 Determination of CK-18 fragments in the blood correlates with the magnitude of hepatocyte apoptosis, predicting the presence of NASH and reflecting the severity of histologic activity in patients with NAFLD19,30,40,41 more sensitively than serum alanine aminotransferase levels.36 In our study, Fragmented CK-18 levels showed a positive correlation with NAS (r=0.401), as well as the NAS component such as lobular inflammation (r=0.387) and ballooning(r=0.231) and also the stage of fibrosis (r=0.314) (Table 4).
Markedly increased level of plasma CK-18 fragments was noted in the patients with NASH compared with the patients with simple steatosis as well as that of the normal biopsies (median [interquartile range]: 765.7 U/L [479.6-991.1], 202.4 U/L [160.4-258.2], 215.5 U/L [150.2-296.2], respectively; P<0.001).30 CK-18 fragment levels independently predicted NASH (odd ratio 1.95; 95% CI 1.18-3.22; P=0.009 for every 50 U/L increase).30 Furthermore, in meta-analysis of diagnostic accuracy, pooled area under the receiver operating characteristic curve, sensitivity and specificity of CK-18 for NASH are 0.82 (0.78-0.88), 0.78 (0.64-0.92), and 0.87 (0.77-0.98), respectively.40
The main reason for the results being different from our studies is that they classified the patients according to the consensus of the NASH CRN Pathology Committee Criteria, while we used the original NAFLD criteria. Furthermore, by discerning NAFLD subtype 1 & 2 and NAFLD subtype 3 & 4, we were able to demonstrate some different qualities from the existing studies which only distinguished simple steatosis and NASH. Also, patients with morbid obesity were included in some studies used in the meta-analysis. Though CK-18 is a very promising biomarker for the determination of NASH, cutoff level to diagnose NASH has not been confirmed and assay for CK-18 is not commercially available as yet. Therefore, there are some obstacles to be overcome in order to apply CK-18 in the clinical practice.42
An elevation of serum ferritin concentrations in the absence of iron overload, can be resulted from inflammation, liver necrosis and alcohol abuse.43 As for NAFLD, increased serum ferritin levels are noted in patients with diabetes mellitus44 and NASH.45 Elevated ferritin levels is considered to be a representation of the metabolic syndrome and of hepatic damage due to inflammatory cytokine activation.46 Even though, there is no solid conclusion whether increased ferritin levels are associated with fibrosis or presence of NASH in the patients with NAFLD, serum ferritin is a discriminant marker for both fibrosis and inflammation, and an independent factor associated with NASH in histologically proven NAFLD patients.45,47 Furthermore, serum ferritin can be useful for selecting patients that should undergo liver biopsy among the patients with NAFLD.45
This study has some limitations. First, histologic findings of the liver biopsy were used as the gold standard. Sampling variability or interpretation error could be present. Nevertheless, liver biopsy is currently the only reference standard, and the slide were reviewed in conference by two experienced hepatopathologists. Second, we could not compare our cohort with non-NAFLD control cohort. Using the viral hepatitis cohort as the control is not recommended due to the variety of confounding factors that may complex the viral hepatitis pathology. Moreover, obtaining normal liver tissue without any overt problems is next to impossible and also unethical. Third, the patients were recruited in a tertiary academic hospital and all patients had liver biopsy performed. This meant patients with more severe disease activity than NAFLD patients in the general population may have been included in our cohort. Unfortunately, we didn't get HFE genotyping to exclude primary hemochromatosis and we could check fasting transferrin saturation in only a limited number of patients. Despite several limitations, as far as we know, this is the first prospective study recruiting to biopsy proven NAFLD in large scale in Korea, we are planning to have a further observational study with this cohort.
In this prospective cohort study, measurement of serum CK-18 and ferritin levels helped to distinguish NASH from simple steatosis, and to assess the fibrosis in Korean patients with biopsy proven NAFLD. We need further evaluation on whether the combined measurement of serum CK-18 and ferritin levels improves the diagnostic performance of NASH. To confirm these results, larger validation analyses and longitudinal prospective studies are needed.
Acknowledgement
The writing group would like to appreciate Dr. Seul Ki Min for providing us with statistical advice.
This study was supported by the Research Fund of the Korean Association for the Study of the Liver (KASL), Korea.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUROC
area under receiver operating characteristics
- BMI
body mass index
- BP
blood pressure
- CK-18
cytokeratin-18
- CRP
C-reactive protein
- ELISA
enzyme-linked immunosorbent assay
- GGT
gamma-glutamyl transpeptidase
- HDL
high-density lipoprotein
- HOMA-IR
homeostatic model assessment-insulin resistance
- IR
insulin resistance
- KASL
Korean Association for the Study of the Liver
- LDL
low-density lipoprotein
- LR+
positive likelihood ratio
- LR-
negative likelihood ratio
- CI
confidence interval
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD histologic activity score
- NASH
nonalcoholic steatohepatitis
- NASH CRN
NASH Clinical Research Network
- NPV
negative predictive value
- PPV
positive predictive value
- TG
triglyceride
Footnotes
The authors have no conflicts to disclose.
References
- 1.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 2.Park YJ, Lim JH, Kwon ER, Kim HK, Jung MC, Seol KH, et al. Development and validation of a simple index system to predict nonalcoholic fatty liver disease. Korean J Hepatol. 2011;17:19–26. doi: 10.3350/kjhep.2011.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park SH, Jeon WK, Kim SH, Kim HJ, Park DI, Cho YK, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21:138–143. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- 4.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal AJ American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 7.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 8.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 9.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 10.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 12.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 13.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 14.Fontana RJ, Sanyal AJ, Ghany MG, Lee WM, Reid AE, Naishadham D, et al. Factors that determine the development and progression of gastroesophageal varices in patients with chronic hepatitis C. Gastroenterology. 2010;138:2321–2331. doi: 10.1053/j.gastro.2010.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 17.Kim CW, Chang Y, Sung E, Shin H, Ryu S. Serum ferritin levels predict incident non-alcoholic fatty liver disease in healthy Korean men. Metabolism. 2012;61:1182–1188. doi: 10.1016/j.metabol.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 18.Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2012;55:77–85. doi: 10.1002/hep.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milner KL, van der Poorten D, Xu A, Bugianesi E, Kench JG, Lam KS, et al. Adipocyte fatty acid binding protein levels relate to inflammation and fibrosis in nonalcoholic fatty liver disease. Hepatology. 2009;49:1926–1934. doi: 10.1002/hep.22896. [DOI] [PubMed] [Google Scholar]
- 21.Shen J, Chan HL, Wong GL, Choi PC, Chan AW, Chan HY, et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J Hepatol. 2012;56:1363–1370. doi: 10.1016/j.jhep.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Zhang Y, Wu K, Fan D. Serum cytokeratin-18 fragment level: a noninvasive biomarker for not only nonalcoholic steatohepatitis, but also alcoholic steatohepatitis. Hepatology. 2010;51:1865–1866. doi: 10.1002/hep.23433. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Fang Q, Gao F, Fan J, Zhou J, Wang X, et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol. 2010;53:934–940. doi: 10.1016/j.jhep.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Assy N, Schlesinger S, Hussein O. Elevated plasma protein C levels correlate with the presence of fatty liver (NASH and NAFLD) Gut. 2005;54:729. doi: 10.1136/gut.2004.060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnefont-Rousselot D, Ratziu V, Giral P, Charlotte F, Beucler I, Poynard T. Blood oxidative stress markers are unreliable markers of hepatic steatosis. Aliment Pharmacol Ther. 2006;23:91–98. doi: 10.1111/j.1365-2036.2006.02719.x. [DOI] [PubMed] [Google Scholar]
- 26.Shimada M, Kawahara H, Ozaki K, Fukura M, Yano H, Tsuchishima M, et al. Usefulness of a combined evaluation of the serum adiponectin level, HOMA-IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. Am J Gastroenterol. 2007;102:1931–1938. doi: 10.1111/j.1572-0241.2007.01322.x. [DOI] [PubMed] [Google Scholar]
- 27.Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257–268. doi: 10.1007/s00535-010-0305-6. [DOI] [PubMed] [Google Scholar]
- 28.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 29.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 30.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 31.Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care. 1997;20:1087–1092. doi: 10.2337/diacare.20.7.1087. [DOI] [PubMed] [Google Scholar]
- 32.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 33.Younossi ZM, Stepanova M, Rafiq N, Makhlouf H, Younoszai Z, Agrawal R, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53:1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- 34.Rafiq N, Bai C, Fang Y, Srishord M, McCullough A, Gramlich T, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol. 2009;7:234–238. doi: 10.1016/j.cgh.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–353. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsutsui M, Tanaka N, Kawakubo M, Sheena Y, Horiuchi A, Komatsu M, et al. Serum fragmented cytokeratin 18 levels reflect the histologic activity score of nonalcoholic fatty liver disease more accurately than serum alanine aminotransferase levels. J Clin Gastroenterol. 2010;44:440–447. doi: 10.1097/MCG.0b013e3181bdefe2. [DOI] [PubMed] [Google Scholar]
- 37.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, et al. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res. 2007;313:2033–2049. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 40.Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 41.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 42.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The Diagnosis and Management of Non-alcoholic Fatty Liver Disease: Practice Guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Bell H, Skinningsrud A, Raknerud N, Try K. Serum ferritin and transferrin saturation in patients with chronic alcoholic and non-alcoholic liver diseases. J Intern Med. 1994;236:315–322. doi: 10.1111/j.1365-2796.1994.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 44.Turlin B, Mendler MH, Moirand R, Guyader D, Guillygomarc'h A, Deugnier Y. Histologic features of the liver in insulin resistance-associated iron overload. A study of 139 patients. Am J Clin Pathol. 2001;116:263–270. doi: 10.1309/WWNE-KW2C-4KTW-PTJ5. [DOI] [PubMed] [Google Scholar]
- 45.Manousou P, Kalambokis G, Grillo F, Watkins J, Xirouchakis E, Pleguezuelo M, et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int. 2011;31:730–739. doi: 10.1111/j.1478-3231.2011.02488.x. [DOI] [PubMed] [Google Scholar]
- 46.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. NAFLD and hyperinsulinemia are major determinants of serum ferritin levels. J Hepatol. 2007;46:700–707. doi: 10.1016/j.jhep.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 47.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]