Abstract
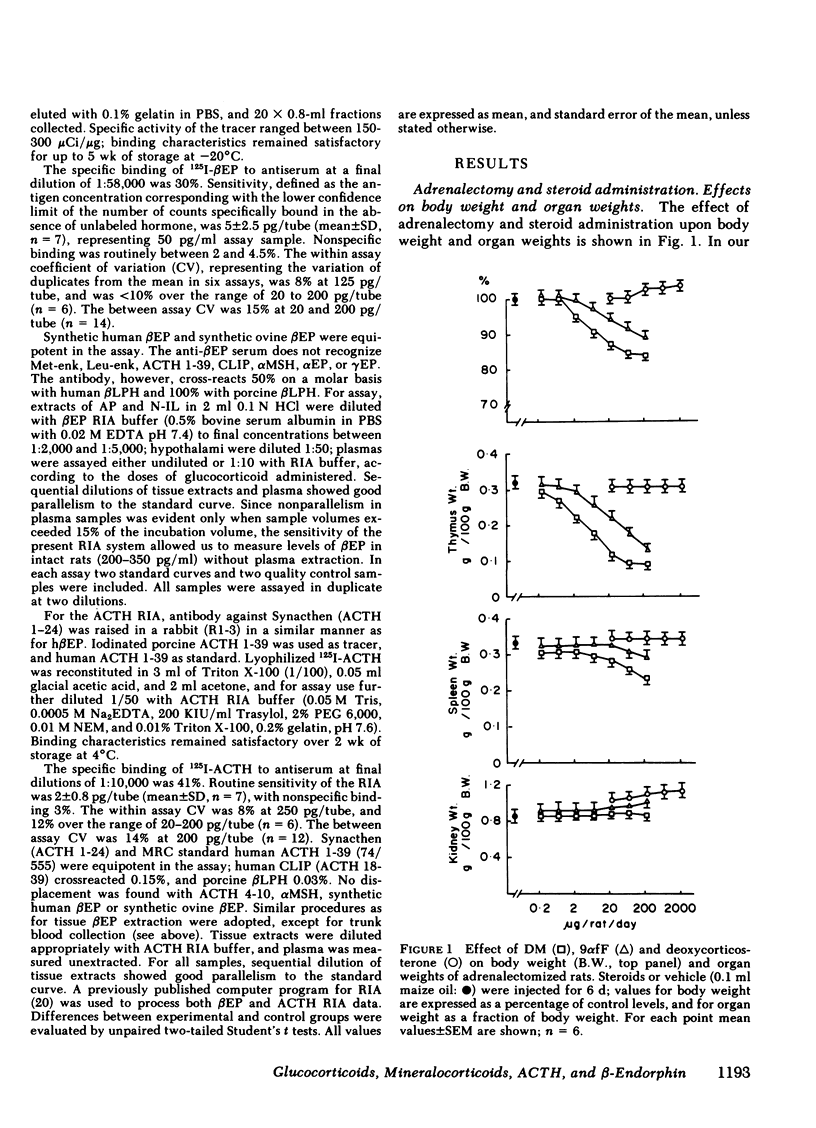
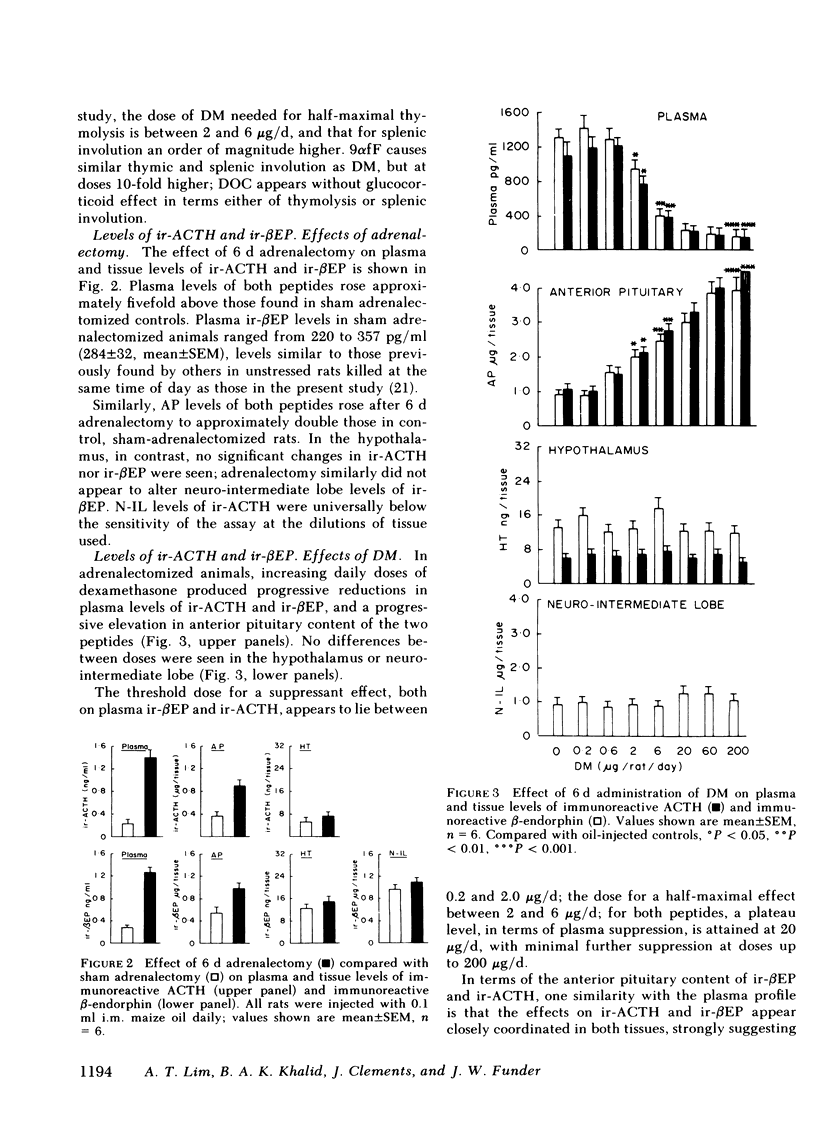
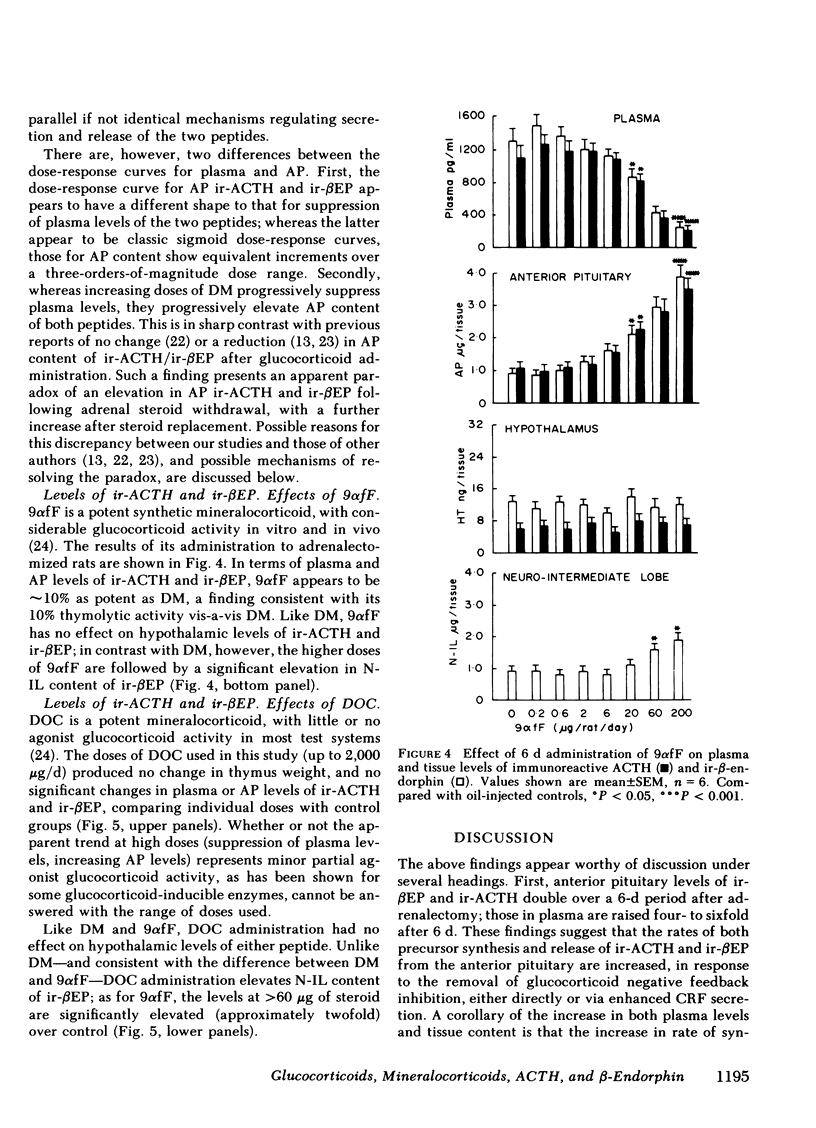
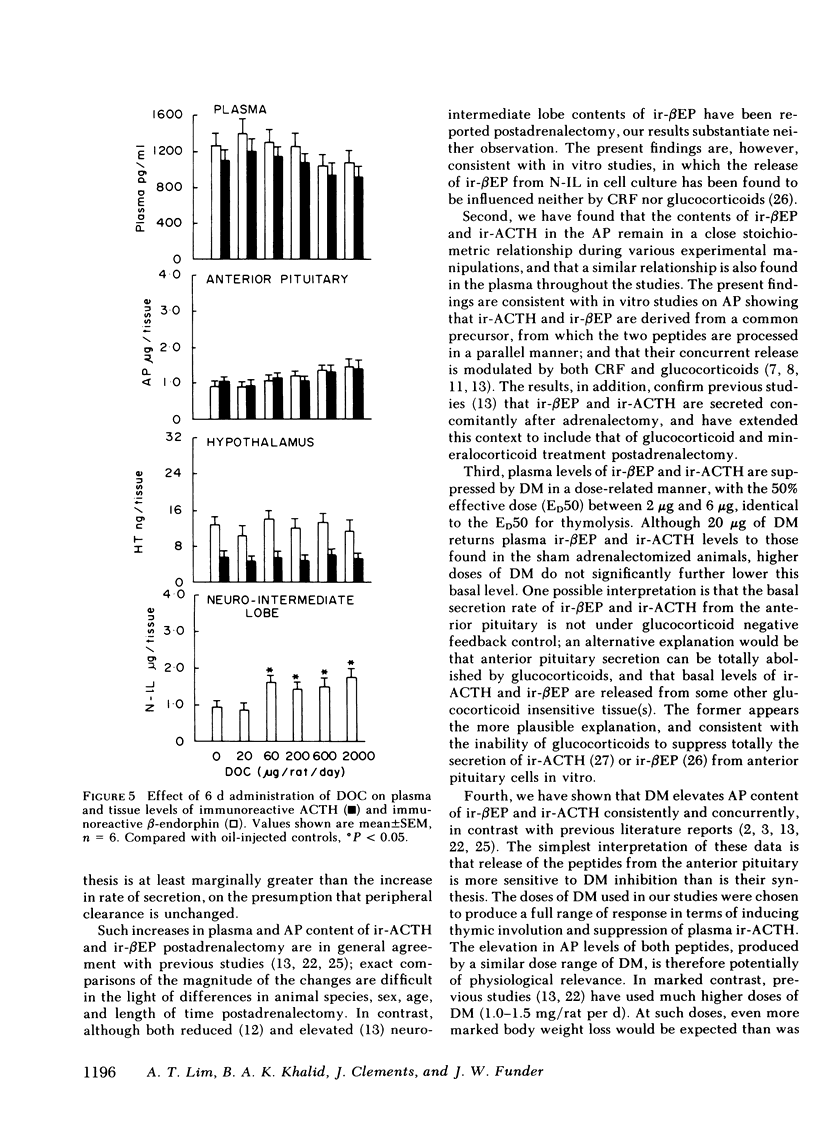
Immunoreactive ACTH (ir-ACTH) and immunoreactive β-endorphin (ir-βEP) were determined in plasma, anterior pituitary, neuro-intermediate lobe, and hypothalamus of sham-adrenalectomized rats, and adrenalectomized rats given six daily injections of vehicle (oil), dexamethasone, 9α-fluorocortisol or deoxycorticosterone. 6 d after adrenalectomy, anterior pituitary ir-ACTH and ir-βEP were double, and plasma levels approximately fivefold those in controls. Adrenalectomy did not alter hypothalamic levels of either peptide, or ir-βEP in neuro-intermediate lobe, in which tissue ir-ACTH was below detection limit at routine dilutions. Dexamethasone (0.2-200 μg/d) concurrently suppressed plasma ir-ACTH and ir-βEP, with a near maximal effect at 20 μg, and a half-maximal effect between 2 and 6 μg; similar dose-response characteristics were found for thymolysis. Step-wise increases in anterior pituitary content of both peptides were found, with no change in hypothalamic levels of either peptide, or neuro-intermediate lobe ir-βEP. 9α-fluorocortisol (0.2-200 μg/d) produced plasma, anterior pituitary, and hypothalamic effects equivalent to dexamethasone, but with one-tenth the potency. Unlike dexamethasone, higher doses of 9α-fluorocortisol significantly elevated neuro-intermediate lobe ir-βEP. Deoxycorticosterone (2-2,000 μg/d) produced no significant changes in plasma, anterior pituitary or hypothalamic levels of either peptide; like 9α-fluorocortisol, doses of >60 μg/d significantly elevated neuro-intermediate lobe ir-βEP. Whereas ir-ACTH and ir-βEP synthesis in and release from the anterior pituitary are under complex negative feedback glucocorticoid control, there exists a mineralocorticoid-specific effect on neuro-intermediate lobe content of ir-βEP.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura A., Bowers C. Y., Schally A. V., Saito M., Miller M. C., 3rd Effect of corticotropin-releasing factor, dexamethasone and actinomycin D on the release of ACTH from rat pituitaries in vivo and in vitro. Endocrinology. 1969 Aug;85(2):300–311. doi: 10.1210/endo-85-2-300. [DOI] [PubMed] [Google Scholar]
- Barnea A., Cho G., Pilotte N. S., Porter J. C. Regional differences in the molecular weight profiles of corticotropin and alpha-melanotropin the hypothalamus. Endocrinology. 1981 Jan;108(1):150–156. doi: 10.1210/endo-108-1-150. [DOI] [PubMed] [Google Scholar]
- Buckingham J. C., Hodges J. R. Interrelationships of pituitary and plasma corticotrophin and plasma corticosterone in adrenalectomized and stressed, adrenalectomized rats. J Endocrinol. 1974 Oct;63(1):213–222. doi: 10.1677/joe.0.0630213. [DOI] [PubMed] [Google Scholar]
- Burger H. G., Lee V. W., Rennie G. C. A generalized computer program for the treatment of data from competitive protein-binding assays including radioimmunoassays. J Lab Clin Med. 1972 Aug;80(2):302–312. [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Existence of a common precursor to ACTH and endorphin in the anterior and intermediate lobes of the rat pituitary. J Supramol Struct. 1978;8(3):247–262. doi: 10.1002/jss.400080304. [DOI] [PubMed] [Google Scholar]
- FORTIER C. Effect of hydrocortisone on pituitary ACTH and adrenal weight in the rat. Proc Soc Exp Biol Med. 1959 Jan;100(1):16–19. doi: 10.3181/00379727-100-24507. [DOI] [PubMed] [Google Scholar]
- FORTIER C. Pituitary ACTH and plasma free corticosteroids following bilateral adrenalectomy in the rat. Proc Soc Exp Biol Med. 1959 Jan;100(1):13–16. doi: 10.3181/00379727-100-24506. [DOI] [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- Guillemin R., Vargo T., Rossier J., Minick S., Ling N., Rivier C., Vale W., Bloom F. beta-Endorphin and adrenocorticotropin are selected concomitantly by the pituitary gland. Science. 1977 Sep 30;197(4311):1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- HODGES J. R., VERNIKOS J. The effects of hydrocortisone on the level of corticotrophin in the blood and pituitary glands of adrenalectomized and of stressed adrenalectomized rats. J Physiol. 1960 Mar;150:683–693. doi: 10.1113/jphysiol.1960.sp006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höllt V., Przewłocki R., Herz A. beta-Endorphin-like immunoreactivity in plasma, pituitaries and hypothalamus of rats following treatment with opiates. Life Sci. 1978 Sep 11;23(10):1057–1065. doi: 10.1016/0024-3205(78)90667-7. [DOI] [PubMed] [Google Scholar]
- Jones M. T., Hillhouse E. W. Structure-activity relationship and the mode of action of corticosteroid feedback on the secretion of corticotrophin-releasing factor (corticoliberin). J Steroid Biochem. 1976 Nov-Dec;7(11-12):1189–1202. doi: 10.1016/0022-4731(76)90054-6. [DOI] [PubMed] [Google Scholar]
- Jones M. T., Tiptaft E. M., Brush F. R., Fergusson D. A., Neame R. L. Evidence for dual corticosteroid-receptor mechanisms in the feedback control of adrenocorticotrophin secretion. J Endocrinol. 1974 Feb;60(2):223–233. doi: 10.1677/joe.0.0600223. [DOI] [PubMed] [Google Scholar]
- Kalin N. H., Risch S. C., Cohen R. M., Insel T., Murphy D. L. Dexamethasone fails to suppress beta-endorphin plasma concentrations in humans and rhesus monkeys. Science. 1980 Aug 15;209(4458):827–828. doi: 10.1126/science.6250217. [DOI] [PubMed] [Google Scholar]
- Kraicer J., Conrad R. G. Circulating adrenocorticotropin (ACTH) as a controlled variable in the regulation of ACTH secretion. Can J Physiol Pharmacol. 1971 Aug;49(8):744–751. doi: 10.1139/y71-101. [DOI] [PubMed] [Google Scholar]
- Krieger D. T., Liotta A. S., Hauser H., Brownstein M. J. Effect of stress, adrenocorticotropin or corticosteroid treatment, adrenalectomy, or hypophysectomy on hypothalamic immunoreactive adrenocorticotropin concentrations. Endocrinology. 1979 Sep;105(3):737–742. doi: 10.1210/endo-105-3-737. [DOI] [PubMed] [Google Scholar]
- Krozowski Z., Funder J. W. Mineralocorticoid receptors in rat anterior pituitary: toward a redefinition of "mineralocorticoid hormone". Endocrinology. 1981 Oct;109(4):1221–1224. doi: 10.1210/endo-109-4-1221. [DOI] [PubMed] [Google Scholar]
- Liotta A. S., Gildersleeve D., Brownstein M. J., Krieger D. T. Biosynthesis in vitro of immunoreactive 31,000-dalton corticotropin/beta-endorphin-like material by bovine hypothalamus. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1448–1452. doi: 10.1073/pnas.76.3.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka H., Mulrow P. J., Li C. H. Beta-lipotropin: a new aldosterone-stimulating factor. Science. 1980 Jul 11;209(4453):307–308. doi: 10.1126/science.6247763. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: cell-free synthesis of the precursor and identification of corticotropin peptides in the molecule. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4826–4830. doi: 10.1073/pnas.74.11.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Phillips M., Rosa P. A., Herbert E. Steps involved in the processing of common precursor forms of adrenocorticotropin and endorphin in cultures of mouse pituitary cells. Biochemistry. 1978 Aug 22;17(17):3609–3618. doi: 10.1021/bi00610a030. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Schmit J. P. Structure-activity relationships for glucocorticoids-I. Determination of receptor binding and biological activity. J Steroid Biochem. 1977 Sep;8(9):911–919. doi: 10.1016/0022-4731(77)90187-x. [DOI] [PubMed] [Google Scholar]
- Schleiffer R., Mialhe C., Briaud B., Lutz-Bucher B., Koch B. Effects of adrenalectomy and hypercorticism on the ACTH content of the anterior and posterior pituitary in rats with inherited diabetes insipidus (Brattleboro strain). Horm Metab Res. 1979 Feb;11(2):130–135. doi: 10.1055/s-0028-1092694. [DOI] [PubMed] [Google Scholar]
- Simantov R. Glucocorticoids inhibit endorphin synthesis by pituitary cells. Nature. 1979 Aug 23;280(5724):684–685. doi: 10.1038/280684a0. [DOI] [PubMed] [Google Scholar]
- Tseng L. F., O'Rourke M. A., Li C. H., Loh H. H. Reduction of beta-endorphin content in the rat pituitary after dehydration and adrenalectomy. Int J Pept Protein Res. 1979;14(3):213–215. doi: 10.1111/j.1399-3011.1979.tb01927.x. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Yang L., Minick S., Guillemin R. Effects of purified hypothalamic corticotropin-releasing factor and other substances on the secretion of adrenocorticotropin and beta-endorphin-like immunoactivities in vitro. Endocrinology. 1978 Nov;103(5):1910–1915. doi: 10.1210/endo-103-5-1910. [DOI] [PubMed] [Google Scholar]
- Vinson G. P., Whitehouse B. J., Dell A., Etienne T., Morris H. R. Characterisation of an adrenal zona glomerulosa-stimulating component of posterior pituitary extracts as alpha-MSH. Nature. 1980 Apr 3;284(5755):464–467. doi: 10.1038/284464a0. [DOI] [PubMed] [Google Scholar]
- Watson S. J., Akil H. alpha-MSH in rat brain: occurrence within and outside of beta-endorphin neurons. Brain Res. 1980 Jan 20;182(1):217–223. doi: 10.1016/0006-8993(80)90849-5. [DOI] [PubMed] [Google Scholar]
- Zakarian S., Smyth D. Distribution of active and inactive forms of endorphins in rat pituitary and brain. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5972–5976. doi: 10.1073/pnas.76.11.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]



