Abstract
Objective
To provide guidelines for future trials, we reviewed the outcomes of children with synchronous bilateral Wilms tumors (BWT) treated on National Wilms Tumor Study-4 (NWTS-4).
Methods
NWTS-4 enrolled 3,335 patients (pts) including 188 pts with BWT (5.6%). Treatment and outcome data were collected.
Results
Among 188 BWT pts registered with NWTS-4, 195 kidneys in 123 patients had initial open biopsy, 44 kidneys in 31 pts had needle biopsies. Although pre-resection chemotherapy was recommended, 87 kidneys in 83 pts were managed with primary resection: Complete nephrectomy 48 in 48 pts, 31 partial/wedge nephrectomies in 27 pts, enucleations 8 in 8 pts. No initial surgery was performed in 45 kidneys in 43 pts, 5 kidneys in 3 pts not coded. Anaplasia was diagnosed after completion of the initial course of chemotherapy in 14 pts (initial surgical procedure: 9 open biopsies, 4 needle biopsies, 1 partial nephrectomy). The average number of days from the start of chemotherapy to diagnosis of anaplasia was 390 (range 44–1,925 days). Relapse or progression of disease occurred in 54 children. End stage renal failure occurred in 23 children, 6 of whom had bilateral nephrectomies. The 8 year event free survival (EFS) for BWT with favorable histology was 74%, and overall survival (OS) was 89%; while the EFS for BWT with unfavorable histology was 40%, OS was 45%.
Conclusion
The current analysis of patients with BWT treated on NWTS-4 shows that preservation of renal parenchyma is possible in many pts following initial preoperative chemotherapy. The incidence of end-stage renal disease remains significantly higher in children with BWT. Future studies are warranted to address the need for earlier biopsy in non-responsive tumors and earlier definitive surgery to recognize unfavorable histology in these high risk patients.
INTRODUCTION
Management of a child with bilateral Wilms tumor (BWT) is very challenging. Preservation of the maximum amount of renal parenchyma is needed to prevent renal failure, but complete resection is required to optimize the chances for cure of the malignancy. Synchronous BWT accounted for 5% of all patients registered to the National Wilms Tumor Study (NWTS) Group (NWTSG). [1] Prior to the initiation of the NWTS, ablative surgery was considered essential for cure, since these patients were thought to have a poor survival.[2] For some patients with synchronous bilateral tumors, this resulted in significant renal insufficiency or an anephric patient requiring renal transplantation.[3–5]
In 1977, Bishop et al [6] reviewed the early experience of the NWTSG with BWT and found that survival was comparable to other Wilms tumor patients. These authors and other later reports suggested that preoperative chemotherapy be considered for all children with synchronous BWT. These investigators showed that there was no difference in survival for patients managed initially with neoadjuvant chemotherapy after biopsy versus those undergoing primary surgical resection. The approach of using cytoreductive chemotherapy prior to surgical resection was advocated to preserve renal parenchyma and minimize the occurrence of renal insufficiency.
The NWTSG and the Société Internationale d’Oncologie Pediatrique (SIOP) have not performed randomized controlled studies of therapy for patients with bilateral disease. In the NWTSG, data have been collected on these patients and guidelines for their care provided, but they were not treated on study. We have reviewed the treatment and outcome of the patients enrolled on the fourth protocol from the NWTSG (NWTS-4) to provide guidelines for children treated on future protocols.
MATERIAL AND METHODS
NWTS-4 enrolled patients from August 1986 to September 1994. There were 188 patients registered as BWT or 5.6% of the total enrollment into the study. Patients with BWT were registered in a followed status and were not randomized on study. The NWTS-4 guidelines for children with BWT recommended initial biopsy followed by chemotherapy. The NWTS-4 staging system has been reported in detail [7]. Briefly, Stage II tumors penetrated the renal capsule but were completely excised. Biopsy was considered local spill and was designated Stage II. Stage III met one or more of the following criteria: positive lymph nodes, preoperative or intra-operative gross spillage of tumor cells, residual microscopic or gross disease. Stage IV had metastatic disease on presentation and Stage V had bilateral tumors. On NWTS-4 children with stage II disease did not receive flank radiation therapy (RT). Children with stage III and stage IV disease were to receive 10Gy to the flank or whole abdomen. The charts of these patients, maintained at the NWTS Data and Statistical Center in Seattle, Washington, were retrospectively reviewed including the operative reports, checklists completed by the operating surgeon, pathology reports and flow sheets detailing the patients’ treatment status. Specific information abstracted included the initial therapeutic approach, the extent of resection, and amount of renal parenchyma removed. The operations performed were categorized as follows: biopsy only, enucleation of tumor (no attempt to obtain a clear margin around the tumor), partial/wedge nephrectomy (removal of the tumor with the intent of having a clear margin of normal tissue and often positive microscopic margins), or radical nephrectomy. IRB approval for this existing data review (EDR) was obtained from the Fred Hutchinson Cancer Research Center.
Event-free survival was defined as the time from study entry to the first occurrence of progression, relapse after response or death from any cause. Survival was defined as the time from study entry to death from any cause. Patients without events were censored at their time of last follow-up. Estimates of time-to-event distributions were calculated using the Kaplan-Meier method, with confidence intervals calculated using Greenwood’s method. Comparisons of time-to-event distributions among patient subsets were made using the log-rank test.
RESULTS
The median age at diagnosis was 32 months (range 1–127 months). Median follow-up of non-failure patients is 13.9 years (range 0.014–19.8 years cut off date November 2009). There were 74 males and 114 females. Ethnicity was Asian 3 patients, African-American 31, Hispanic 19 and Caucasian 135.
Associated congenital anomalies were noted in 48 children. There were 10 children with Beckwith Wiedemann syndrome, 15 with hemi -hypertrophy and 6 with aniridia. Six males had hypospadias and 11 had cryptorchidism. No patients had Denys-Drash syndrome. One child had WAGR syndrome.
Local tumor stage was assigned both at initial exploration and after completion of all surgeries exclusive of surgery for tumor relapse. Stage I was assigned to 63 tumors at initial exploration and after completion of all surgery. Stage II was assigned to 249 tumors at initial surgery and 215 tumors after completion of all surgery. Stage III was assigned to 31 tumors at initial exploration and 73 tumors after completion of all surgery. Thirty three tumors were not assigned a local stage initially and three were not assigned after completion of all surgery. Nine tumors had unknown local stage after completion of all surgery.
Many children did not have a lymph node biopsy performed to adequately stage the tumor when the primary mass was biopsied resulting in an increase in the reported stage at surgical resection.
A staged nephron sparing approach based on response to chemotherapy creates many different permutations of surgical procedures for children with BWT. Although pre-resection chemotherapy was recommended, 87 kidneys in 83 pts were managed with primary resection: Forty-eight patients had complete nephrectomy of one kidney. Twenty-seven patients had 31 partial/wedge nephrectomies and eight patients underwent eight tumor enucleations. Table 1 summarizes the procedures performed in individual patients. Timing of surgical therapy was variable and many patients underwent several procedures due to multicentric disease. The total number of different procedures by kidney is listed in Table 2. Forty-five kidneys did not have initial biopsies and five were not coded. Two patients underwent biopsy of the tumor shortly after initiation of chemotherapy. Ultimately, there were 122 nephrectomies, 136 partial nephrectomies, 104 needle biopsies, and 14 kidneys had no surgery. Following surgical resection, six patients were anephric. 22 patients had 50% or less of both kidneys, 113 patients had less than 50% of one kidney and greater than 50% of the other kidney, and 47 patients had greater than 50% of both kidneys.
Table 1.
Summary of the surgical procedures performed on patients with BWT in NWTS-4
| Number of Cases | Kidney | Contralateral Kidney |
|---|---|---|
| 6 (3%) | nephrectomy | nephrectomy |
| 53 (28 %) | nephrectomy | partial/wedge nephrectomy |
| 51 (27%) | nephrectomy | needle biopsy |
| 6 (3%) | nephrectomy | no surgery |
| 35 (19%) | partial wedge/nephrectomy | partial wedge/nephrectomy |
| 10 (5%) | partial wedge/nephrectomy | needle biopsy |
| 3 (1%) | partial wedge/nephrectomy | no surgery |
| 19 (10%) | needle biopsy | needle biopsy |
| 5 (3%) | needle biopsy | no surgery |
Table 2.
NWTS 4 Bilateral Wilms Tumor Summary of Individual Procedures
| Surgical Procedures | Initial | Second | Third | Fourth |
|---|---|---|---|---|
| Complete Nephrectomy | 48(15%) | 56(26%) | 14(27%) | 2(25%) |
| Partial Nephrectomy/Wedge Resection | 31(10%) | 88(41%) | 18(35%) | - |
| Open Biopsy | 195(60%) | 56(26%) | 33(17%) | 6(75%) |
| Needle Biopsy | 44(13%) | 1(1%) | 0 | - |
| Enucleation | 8(2%) | 15(6%) | 3(2%) | - |
The pathology of the tumors pre and post- chemotherapy are described in Table 3. There were fewer blastemal predominant tumors after chemotherapy suggesting destruction of these chemosensitive cells by the therapy. Likewise, an increased prevalence of stromal predominant tumors was noted following chemotherapy. There were a number of lesions categorized as nephrogenic rests that were included in this study. This pathology review was conducted after the completion of NWTS-4. The definition of these lesions has evolved over time. Also, the diagnosis of a nephrogenic rest cannot be made with certainty unless the biopsy includes the interface between the rest and normal parenchyma.
Table 3.
Pathologic Findings of Specimens Obtained Before and After Chemotherapy
| Pre-Chemotherapy Pattern | Post-Chemotherapy Pattern | |
|---|---|---|
| Inadequate Specimen | 4 (1%) | 4 (1%) |
| Blastemal Predominant | 60 (16%) | 17 (7%) |
| Epithelial Predominant | 64 (17%) | 17 (7%) |
| Stromal Predominant | 6 (2%) | 12 (4%) |
| Mixed Cell Wilms Tumor | 133 (35%) | 36 (14%) |
| Teratoid | 5 (1%) | 7 (3%) |
| Diffuse Anaplasia | 7 (2%) | 16 (6%) |
| Focal Anaplasia | 2 (.05%) | 3 (0.5%) |
| Not Coded | 37(10%) | 137 (52%) |
| Intralobar Nephrogenic Rests | 50 (13%) | 3 (0.5%) |
| Perilobar | 6 (2%) | 9 (3%) |
| Rest (Other) | 2 (.05%) | 5 (2%) |
Anaplasia was identified at initial diagnosis in nine patients (eight unilateral, one bilateral). For the children with anaplasia, the initial chemotherapy regimen was EE-4A (vincristine, dactinomycin) in five and DD-4A (vincristine, dactinomycin and doxorubicin) in four. The initial chemotherapy regimen was not changed for any of these patients due to progression of disease. One kidney did not respond to initial therapy and the regimen was altered. None of these patients relapsed in the kidney or renal bed.
Anaplasia was diagnosed after completion of the initial course of chemotherapy in 14 pts. Nine of these children had open biopsies at diagnosis, four had needle biopsies, and one had a partial nephrectomy. No patient had anaplasia identified by a needle biopsy. The median number of days from the start of first chemotherapy to establishing the diagnosis of anaplasia in these patients was 177 days (mean 390 range 44–1,945 days). For these kidneys with discordant pathology post- chemotherapy, one child changed regimens due to lack of response to chemotherapy and six changed regimens due to progression of disease. None of these children had local relapse in the kidney or the renal bed.
For the children with favorable histology tumors, the initial chemotherapy regimen was based on the highest local tumor stage.
EE-4A was administered to: nine (4.8%) Stage I, 101 (54%) Stage II, 13 (6.9%) Stage III and six (3.2%) stage IV patients, not explored two (1%); regimen DD-4A was administered to: 40 (21%) Stage II, 13 (6.9%) Stage III patients, not explored two (1%). A second chemotherapy regimen was initiated in 87 children. The most common reasons for the switch were inadequate tumor response (37), disease progression (17) and unfavorable histology (15). Twenty four children received a third chemotherapy regimen (including ten for inadequate response and seven for disease progression). (Table 4)
Table 4.
Reasons for Change in Chemotherapy
| Reasons for change in therapy | 87 Cases to Regimen 2 | 61 Cases changed from 2 drugs to 3 drugs | 12 Cases changed from 3 drugs to something else | 24 cases changed to chemo Regimen 3 |
|---|---|---|---|---|
| Tumor Stage | 9 (10%) | 8 (13%) | 1 (8%) | 2(8%) |
| Inadequate Response | 37 (42%) | 27(44%) | 4(33%) | 10 (42%) |
| Unfavorable Histology | 15(17%) | 9(15%) | 4(33%) | 3(13%) |
| Toxicity | 2(2%) | 1(1.5%) | 1(8%) | 1(4%) |
| Progression | 17(20%) | 11(18%) | 1(8%) | 7 (29%) |
| Unknown | 4(5%) | 4(7%) | - | 1(4%) |
| Not Coded | 3(4%) | 1(1.5%) | 1(8%) | - |
| Total | 87 | 61 | 12 | 24 |
The duration of chemotherapy was quite variable as was the number of different chemotherapy regimens received. The minimum number of days elapsed from initiation of chemotherapy to surgical resection was two and the maximum number of days was 1,574 (median - 159 days). The mean duration of chemotherapy after needle biopsy was 20 weeks and the mean duration of chemotherapy after open wedge biopsies was 39 months.
Sixteen children had metastatic disease at diagnosis including 14 children with pulmonary metastases (11 bilateral) and one child with multiple hepatic lesions. The initial chemotherapy regimen was DD-4A in 13 children. However, three children were under treated initially with EE-4A.
Radiation therapy was administered to 64 children. No child received radiation therapy prior to initial surgery. Radiation therapy was given to 101 kidneys. Indications for radiation therapy included: local tumor control - 31 kidneys, tumor histology - five kidneys, inadequate response to chemotherapy - 45 kidneys, and unknown - 20 kidneys.
Relapse or progression was identified in 54 children: 29 in the residual kidney, two in the local tumor bed, nine at distant sites (eight lung, one liver), 11 to multiple sites, and in three the site was not coded. Sixteen of the children with relapse in the kidney had a partial/wedge nephrectomy prior to relapse, two children had enucleations and eleven patients had only undergone an open biopsy.
Surgical treatment of local relapse included: 11 complete nephrectomies, 18 partial/wedge nephrectomies, five open biopsies and one needle biopsy.
Twenty-three patients developed end stage renal disease (ESRD). Of these only six had aniridia and one had WAGR syndrome, and none had Denys-Drash syndrome. The range of days post diagnosis to ESRD was 170 to 6,736 days (median -1,057 days). The initial surgery for eight of the 23 was complete nephrectomy combined with three contralateral partial nephrectomies and four contralateral biopsies. The remaining 15 patients had initial bilateral open biopsies. Ultimately six children had bilateral nephrectomies. Only six of the 23 patients who developed ESRD received radiation therapy. Eight children received only a single chemotherapy regimen (6 EE-4A, 2 DD-4A); five children received a second chemotherapy regimen (1 DD-4A x2, 4 EE-4A+DD-4A), and nine children received a third chemotherapy regimen. (5 EE-4A+DD-4A+Ifosfomide+VP16; 2 EE-4A+DD-4A+Ifosfomide+VP16, 1 DD4-AX3, 1 EE-4A+ DD-4A+EE-4A).
Surgical complications were identified in 28 patients. These included bowel obstruction in 14 children, extensive hemorrhage in one, vascular injury in one, and visceral injury in two patients. Only three children had complications related to the renal collecting system. There were two patients with a urine leak and one child with urinary obstruction.
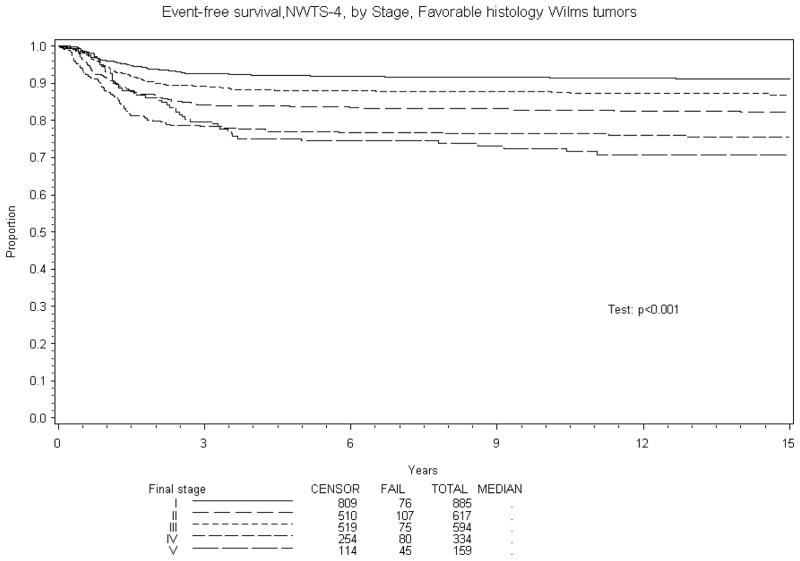
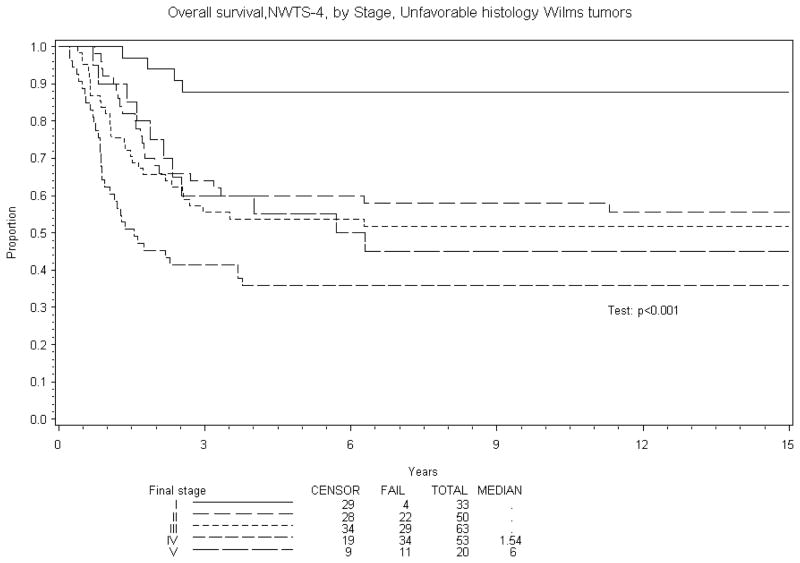
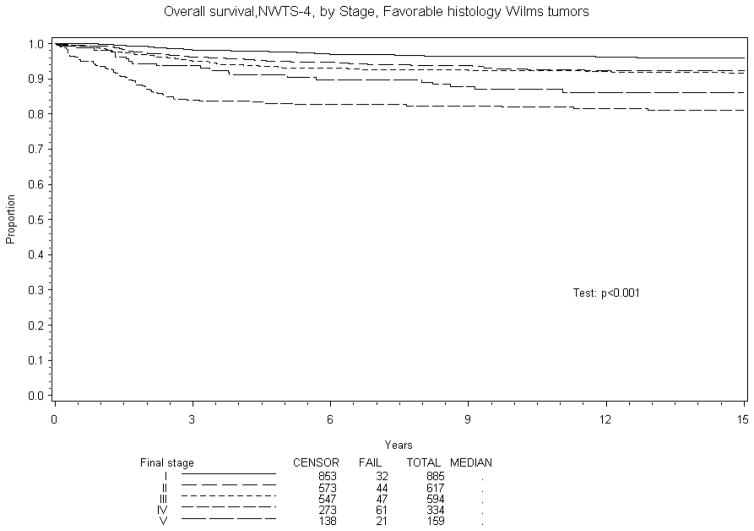
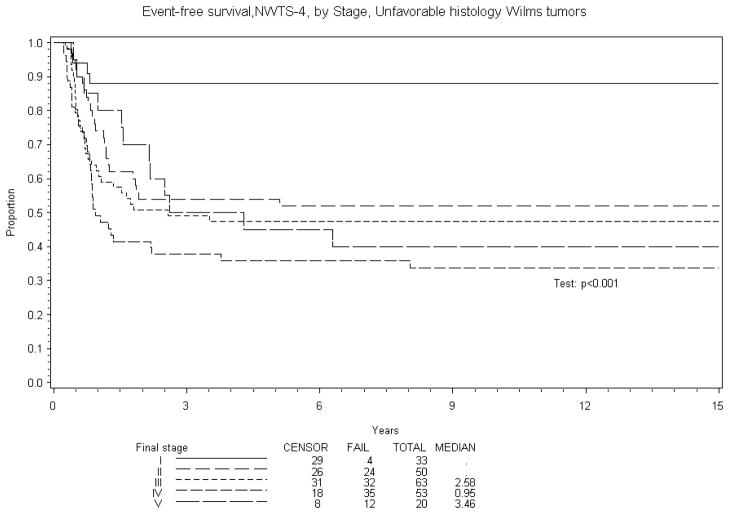
Eight year EFS was 70% and OS 84% for BWT patients treated in NWTS-4. A comparison of EFS and OS with other stages of Wilms tumor treated on NWTS-4 is reported in Table 5. The results are subdivided into in three groups: overall, favorable and unfavorable histology. Figures 1–4 show the Kaplan-Meier estimates all with p values <0.001. BWT patients consistently had lower EFS and OS except for patients with stage 4 disease where OS was higher in BWT. There appeared to be a higher retrieval rate (long term survival following treatment failure) for patients with bilateral disease, as compared to those with stage IV disease.
Table 5.
8 -year EFS and OS estimates for all Wilms tumor patients by histology and Stage on NWTS-4
| All Patients | Favorable histology | Unfavorable histology | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage | N of cases | 8-year EFS (95% confidence interval) | 8-year OAS (95% confidence interval) | N of cases | 8-year EFS (95% confidence interval) | 8-year OAS (95% confidence interval) | N of cases | 8-year EFS (95% confidence interval) | 8-year OAS (95% confidence interval) |
| I | 918 | 91% (89%, 93%) | 96% (95%, 97%) | 885 | 92% (90%, 93%) | 97% (95%, 98%) | 33 | 88% (71%, 95%) | 88% (71%, 95%) |
| II | 617 | 81% (77%, 84%) | 91% (89%, 93%) | 617 | 83% (80%, 86%) | 94% (92%, 95%) | 50 | 52% (37%, 65%) | 58% (43%, 70%) |
| III | 594 | 84% (81%, 87%) | 89% (86%, 91%) | 594 | 88% (85%, 90%) | 93% (90%, 94%) | 63 | 47% (35%, 59%) | 52% (38%, 63%) |
| IV | 334 | 71% (66%, 75%) | 76% (71%, 79%) | 334 | 76% (71%, 81%) | 82% (78%, 86%) | 53 | 36% (23%, 49%) | 36% (23%, 49%) |
| V | 159 | 70% (63%, 76%) | 84%, 75%, 87%) | 159 | 74% (66%, 80%) | 89% (84%, 93%) | 20 | 40% (19%, 60%) | 45% (23%, 65%) |
Figure 1.
Kaplan-Meier estimates of event-free survival for favorable histology (FH) Wilms tumor patients by Stage
Figure 4.
Overall Survival NWTS-4. Unfavorable histology Wilms tumors
DISCUSSION
The impetus for initial treatment with neoadjuvant chemotherapy for BWT is to avoid renal failure by maximal preservation of renal parenchyma. Numerous studies document that the majority of children presenting with synchronous BWT will have a dramatic response to preoperative chemotherapy with reduction of the tumor burden. [1, 8–10] Bishop et al noted a significant difference in the incidence of renal failure in NWTS 1 patients with BWT (9% synchronous, 18% metachronous) versus unilateral involvement (1% incidence). [6] The primary cause of renal failure was bilateral nephrectomy (74%) for persistent or recurrent tumor. Breslow et al more recently reported that the long term risk of renal failure with BWT in children treated on NWTS 1–4 approached 15% at 15 years post treatment. [11]
For the past two decades, the NWTSG has recommended that all children with BWT receive preoperative chemotherapy in an attempt to avoid initial total nephrectomy. In this NWTS-4 cohort, 23 of 188 children (12%) developed ESRD. Six cases of renal failure (23%) were the result of bilateral nephrectomy. Eight of twenty-three patients (35%) who ultimately developed ESRD did not have a nephron sparing initial surgical approach. 15 out of 123 patients (12%) who had initial bilateral open biopsies went on to develop ESRD. Although an implicit selection bias is present given the lack of a standardized protocol, this finding may be the strongest argument we can make with this cohort for a nephron sparing surgical approach. The second leading cause for renal failure was treatment related factors such as radiation, chemotherapy or surgical complications. [12] The incidence of renal failure was not surprising given that 22 of our 188 patients had < 50% of renal of parenchyma remaining in both kidneys postcompletion of all procedures.
While the treatment strategy for patients with BWT has focused on renal preservation, survival is the most important endpoint in treating children with cancer. Recent data suggest that survival is lower for patients with BWT than unilateral Wilms tumor. [13] It is difficult to know if the approach of preoperative chemotherapy to preserve renal parenchyma is the reason for this poorer outcome. Incomplete resection of the tumor using renal conserving surgery or under treatment of patients due to understaging could lead to reduced survival compared to primary surgical resection for BWT. The adverse outcome could also be related to some intrinsic difference in the biology of the tumors. Prior NWTSG reports have noted good outcomes with a nephron sparing approach for synchronous BWT. [14,15] Blute et al reported on 145 patients enrolled in NWTS-2 and -3 and found complete excision of all gross disease was possible in only 38% of patients, following one or more operations. They found no statistical difference in outcome between patients undergoing an initial definitive surgery (complete or partial nephrectomy) compared to those undergoing biopsy alone at diagnosis (82% vs. 57%, 3 yr. survival; P ≥. 10). [15] In NWTS-4, complete removal of all gross tumor was successful in 118 of 134 (88%) kidneys following renal parenchymal sparing surgery (either partial nephrectomy or enucleation), but local recurrence of tumor occurred in 8.2% of the remaining kidneys.
Outcomes for BWT patients have improved with the adoption of a nephron sparing approach to initial treatment as one key component along with modifications in adjuvant therapy. Retrospective review of 185 patients registered with the NWTSG from January 1974 to July 1986 with stage V tumors[1] reported that overall survival at that time was 83%, 73% and 70% at 2, 5 and 10 years, respectively. Unfavorable histology, age at diagnosis and the most advanced stage of the individual tumors were the most important prognostic variables. Overall survival for patients with BWT appears to have improved for patients treated in NWTS-4, compared to the period from 1974–1986; 8-year overall survival for NWTS-4 patients with Stage V disease was estimated to be 84%. Unfavorable histology remains an adverse prognostic factor.
Similar findings are reported by SIOP. A 10-year OS of 69% was achieved for patients with synchronous BWT treated with either preoperative radiotherapy and/or chemotherapy. A number of deaths from disease recurrence occurred more than 3 years after diagnosis. [9]
Factors that may have contributed to the poor outcomes are: under-staging and/or under treatment, delay in local disease control and an increased incidence of anaplasia. The ability to determine the local tumor stage at diagnosis in patients with synchronous BWT is somewhat limited. The size of the tumor often precludes access to the hilar area and great vessels to examine lymph nodes. In many cases, a very small incision is made with no attempts to sample nodes. In this cohort from NWTS-4, no patient with BWT undergoing initial biopsy alone had positive lymph nodes identified. Shamberger et al demonstrated that failure to sample lymph nodes to evaluate for the presence of microscopic extra renal disease can lead to under-staging and an increased risk for abdominal recurrence, presumably due to under treatment.[16] We found a much higher number of stage III disease after final resection compared to the initial stage. This reflects the inability to stage patients at diagnosis and also the lack of lymph node biopsy. This may still underestimate, however, the true stage as the pre-operative chemotherapy may have obliterated evidence of metastatic disease in lymph nodes and elsewhere. [17] These findings have led to the suggestion in future studies to intensify the chemotherapy upfront for all children with BWT to avoid under treatment and to avoid delays to definitive surgery which may be contributing to their lower survival.
When there is minimal reduction in tumor size after initial chemotherapy, efforts should be made to biopsy instead of prolonging the duration or changing the chemotherapy with the hope of eliciting a better response. Of the 188 patients on NWTS-4, 38 had progressive or non-responsive disease. [18] These patients received a median of 7 months (range 2–29 months) of chemotherapy prior to definitive surgery. Pathology review of the resected tumors found that 15 had either rhabdomyomatous differentiation, complete necrosis or stromal differentiation. These patients did not require prolonged/intensive courses of chemotherapy or changes in regimen leading to delays in definitive surgery.
Twenty-seven of 188 (14.4%) NWTS-4 patients with BWT had diffuse anaplasia. This was identified in none of the seven patients who underwent initial needle biopsy, three of the nine who underwent wedge biopsy and seven of the nine who underwent partial or complete nephrectomies. [19] The mean duration of chemotherapy after needle biopsy was 20 weeks and the mean duration of chemotherapy after open wedge biopsies was 39 months highlighting the potential problems of continued therapy without interval biopsies. Based on these two findings future protocols should require earlier biopsy or resection of tumors that do not have a radiographic response to therapy. This will avoid prolonged ineffective therapies for patients with diffuse anaplasia or mature tumors.
Preoperative chemotherapy has been used extensively in trials conducted by the SIOP. In SIOP-9, conducted from 1987 to 1991, patients with unilateral tumors were randomized to receive either 4 or 8 weeks of dactinomycin and vincristine preoperatively. There was a 48% reduction in tumor volume after four weeks that increased to 62% after eight weeks of preoperative chemotherapy. [17,20] A review by the German Pediatric Hematology Group (GPOH) of their patients with BWT reported maximum tumor shrinkage in the first 12 weeks of chemotherapy.[21] These studies suggest that continuing pre-operative chemotherapy longer than 12 weeks is unlikely to facilitate resection and also support recommended resection after four cycles (12 weeks) of intensified neoadjuvant therapy.
The relative proportions of histologic subtypes of Wilms tumor (WT) differ following preoperative chemotherapy when compared to those reported following primary surgical resection. (22–24) All of the previous data reported is from patients with unilateral Wilms tumor. Stromal and epithelial predominant tumors are found more often after chemotherapy. These histologic subtypes may demonstrate a poor clinical response to therapy, but have an excellent prognosis if the tumor is completely excised. (22) The proportion of blastemal predominant tumors is decreased after chemotherapy, indicating some response of this tumor type to the preoperative chemotherapy. However, patients with blastemal predominant tumors after chemotherapy had a 31% relapse rate in SIOP-9. (21)
Based on the above data, the current Children’s Oncology Group (COG) BWT protocol includes intensification of initial chemotherapy, requires second look surgery at 6 weeks for non-responders (less than 50% reduction in the size of the tumor) and definitive surgery at 12 weeks. Chemotherapy will be modified based on pathologic findings (using the SIOP classification) after second look and definitive surgery. The use of earlier second look surgery may address the issues of possible under-treatment of anaplastic tumors and prolonged treatment for differentiated or necrotic tumors.
Partial nephrectomy or wedge excision of the tumor is preferred, but only if it will not compromise tumor resection and negative margins are established. Tumor enucleation may be considered for large central tumors, particularly those with mature elements, where more extensive resection may impair blood supply to the remaining kidney. Davidoff et al reported a single institution series where 10 patients with BWT and favorable histology all had successful bilateral nephron sparing surgery. [25] Many of these children had very large tumors even after pre-operative chemotherapy, but were able to undergo renal sparing surgery. This emphasizes that it is easy to underestimate the amount of renal parenchyma that can be salvaged when compressed by a large tumor and a nephron sparing approach is advocated in all patients.
The current analysis of children with BWT treated on NWTS-4 shows that preservation of renal parenchyma is possible following initial preoperative chemotherapy. The incidence of ESRD (12%) remains significantly higher in children with BWT and event free and overall survival are lower than patients with unilateral disease for all stages except stage IV. Future studies are warranted to: address the need for earlier biopsy in non-responsive tumors; earlier definitive surgery to recognize unfavorable histology, to determine if a systematic nephron-sparing approach can decrease the incidence of ESRD in these high risk patients and to assess if intensified chemotherapy will increase the survival in children with BWT.
Figure 2.
Below displays the Kaplan-Meier estimates of overall survival for FH Wilms tumor patients by Stage
Figure 3.
The Kaplan-Meier estimates of event-free survival for unfavorable histology (focal and diffuse anaplasia) Wilms tumor patients by Stage.
Acknowledgments
From the National Wilms Tumor Study Group, supported in part by USPHS Grant CA-42326. Principal investigators at participating institutions also receive independent support from the National Cancer Institute. The authors thank the many pediatric oncologists, pathologists, surgeons, radiation therapists and other health professionals of the Pediatric Oncology Group and Children’s Cancer Study Group who managed these children, without whom this study would have been impossible.
References
- 1.Montgomery BT, Kelalis PP, Blute ML, et al. Extended follow-up of bilateral Wilms tumor: Results of National Wilms Tumor Study. J Urol. 1991;146:514–518. doi: 10.1016/s0022-5347(17)37840-0. [DOI] [PubMed] [Google Scholar]
- 2.Swenson O, Brennan RP. Aggressive approach to the treatment of Wilms’ tumors. Am J Surg. 1967;166:657–667. doi: 10.1097/00000658-196710000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Penn I. Renal transplantation for Wilms’ tumor: Report of 20 cases. J Urol. 1979;122:793–794. doi: 10.1016/s0022-5347(17)56607-0. [DOI] [PubMed] [Google Scholar]
- 4.DeMaria JE, Hardy BE, Brzezinski A, et al. Renal transplantation in patients with bilateral Wilms’ tumor. J Pediatr Surg. 1979;14:577–579. doi: 10.1016/s0022-3468(79)80143-8. [DOI] [PubMed] [Google Scholar]
- 5.de Lorimier AA, Belzer F, Kountz S, et al. Treatment of bilateral Wilms’ tumor. Am J Surg. 1971;122:275–281. doi: 10.1016/0002-9610(71)90330-8. [DOI] [PubMed] [Google Scholar]
- 6.Bishop HC, Teft M, Evans A, et al. Survival in bilateral Wilms’ tumor - Review of 30 National Wilms’ Tumor Study Cases. J Pediatr Surg. 1977;12:631–638. doi: 10.1016/0022-3468(77)90385-2. [DOI] [PubMed] [Google Scholar]
- 7.D’Angio GJ, Evans AE, Breslow NE, et al. The treatment of Wilms tumor: Results of the Second National Wilms Tumor Study. Cancer. 1976;47:2302–11. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Blute ML, Kelalis PP, Offord KP, et al. Bilateral Wilms’ tumor. J Urol. 1987;138:968. doi: 10.1016/s0022-5347(17)43474-4. [DOI] [PubMed] [Google Scholar]
- 9.Coppes MJ, de Kraker J, van Kijken HJM, et al. Bilateral Wilms’ tumor: Long-term survival and some epidemiological features. J Clin Oncol. 1989;7:310–315. doi: 10.1200/JCO.1989.7.3.310. [DOI] [PubMed] [Google Scholar]
- 10.Laberge J, Nguyen LT, Homsy YL, Doody DP. Bilateral Wilms’ tumors: Changing concepts in management. J Pediatr Surg. 1987;22:730–735. doi: 10.1016/s0022-3468(87)80615-2. [DOI] [PubMed] [Google Scholar]
- 11.Breslow NE, Collins AJ, Ritchey ML, et al. End stage renal disease in patients with Wilms Tumor: result from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol. 2005;174:1972–5. doi: 10.1097/01.ju.0000176800.00994.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritchey ML, Green DM, Thomas P, et al. Renal failure in Wilms tumor. Med Pediatr Oncol. 1996;26:75–80. doi: 10.1002/(SICI)1096-911X(199602)26:2<75::AID-MPO1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 13.Dome J, Cotton C, Perlman E, et al. Treatment of Anaplastic Histology Wilms’ Tumor: Results from the fifth national wilms tumor study. J Clin Oncol. 2006;24:15:2352–2358. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 14.Bishop HC, Teft M, Evans A, et al. Survival in Bilateral Wilms’ tumor- Review of 30 National Wilms’ Tumor Study Cases. J Pediatr Surg. 1977;12:631–638. doi: 10.1016/0022-3468(77)90385-2. [DOI] [PubMed] [Google Scholar]
- 15.Blute ML, Kelalis PP, Offord KP, et al. Bilateral Wilms’ Tumor. J Urol. 1987;138:968–973. doi: 10.1016/s0022-5347(17)43474-4. [DOI] [PubMed] [Google Scholar]
- 16.Shamberger RC, Guthrie KA, Ritchey ML, et al. Surgery-related factors and local recurrences of Wilms tumor in National Wilms Tumor Study 4. Ann Surg. 1999;229:292–297. doi: 10.1097/00000658-199902000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graf N, Tournade MF, de Kraker J. The role of preoperative chemotherapy in the management of Wilms’ tumor. The SIOP studies. Urol Clin North Am. 2000;27:443–454. doi: 10.1016/s0094-0143(05)70092-6. [DOI] [PubMed] [Google Scholar]
- 18.Shamberger RC, Haase GM, Argani P, et al. Bilateral Wilms tumors with progressive or nonresponsive disease. J Pediatr Surg. 2006;41:652–657. doi: 10.1016/j.jpedsurg.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton TE, Green DM, Perlman EJ, et al. Bilateral Wilms Tumor with Anaplasia: Lessons from the National Wilms Tumor Study Group. J Pediatr Surg. 2006;41:1641–4. doi: 10.1016/j.jpedsurg.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 20.Tournade MF, Com-Nougue C, de Kraker J, et al. Optimal duration of preoperative therapy in unilateral and nonmetastatic Wilms’ tumor in children older than 6 months: results of the Ninth International Society of Paediatric Oncology Wilms’ Tumor Trial and Study. J Clin Oncol. 2001;19:488–500. doi: 10.1200/JCO.2001.19.2.488. [DOI] [PubMed] [Google Scholar]
- 21.Weirich A, Leuschner I, Harms D, et al. Clinical impact of histologic subtypes in localized non-anaplastic nephroblastoma treated according to the trial and study SIOP-9/GPOH. Ann Oncol. 2001;12:311–319. doi: 10.1023/a:1011167924230. [DOI] [PubMed] [Google Scholar]
- 22.Boccon-Gibod L, Rey A, Sandstedt B, et al. Complete necrosis induced by preoperative chemotherapy in Wilms tumor as an indicator of low risk: Report of the International Society of Paediatric Oncology (SIOP) Nephroblastoma Trial and Study 9. Med Pediatr Oncol. 2000;34:183–190. doi: 10.1002/(sici)1096-911x(200003)34:3<183::aid-mpo4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Zuppan CW, Beckwith JB, Weeks DA, et al. The effect of preoperative therapy on the histologic features of Wilms’ tumor. An analysis of cases from the Third National Wilms’ Tumor Study. Cancer. 1991;68:385–394. doi: 10.1002/1097-0142(19910715)68:2<385::aid-cncr2820680229>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Green DM, Beckwith JB, Breslow NE, et al. Treatment of children with stages II to IV anaplastic Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1994;12:2126–2131. doi: 10.1200/JCO.1994.12.10.2126. [DOI] [PubMed] [Google Scholar]
- 25.Davidoff A, Giel D, Jones D, et al. The Feasibility and Outcome of Nephron sparing Surgery for Children with Bilateral Wilms Tumor. Cancer. 2008;112:2060–70. doi: 10.1002/cncr.23406. [DOI] [PubMed] [Google Scholar]