Abstract
Background
Activity-dependent neuroprotector (ADNP) is a neuroprotective molecule containing an 8-amino acid peptide, NAPVSIPQ (NAP), that is sufficient for its neuroprotective effects.
Objective
To assess the expression of ADNP in the human immune system in normal subjects and multiple sclerosis patients.
Materials and Methods
ADNP expression was assessed in peripheral blood mononuclear cells (PBMCs) from healthy donors and multiple sclerosis (MS) patients using staining with anti-ADNP (NAP) antibodies and markers for T cells, B cells, monocytes and natural killer cells. ADNP mRNA was determined in peripheral blood from MS patients (n = 24) and matched controls (n = 21). Expression of activation markers CD69 and CD154 and of IFN-γ was assessed by flow cytometry in stimulated PBMCs. Effects of NAP on immune cell proliferation was assessed by tritiated thymidine incorporation.
Results
Monocytes, B cells and T cells, but not regulatory (CD4+CD25+) T cells expressed ADNP. NAP peptide decreased the expression of CD69, CD154 and IFN-γ in PBMC and caused suppressed anti-CD3-/anti-CD28-stimulated PBMC proliferation. ADNP mRNA was reduced in MS compared to control peripheral blood.
Conclusion
ADNP is expressed in many immune system cells. ADNP mRNA is reduced in PBMCs in MS. The peptide NAP, which plays an important role in neuroprotection, has potential immunomodulatory properties.
Key Words: Multiple sclerosis, Activity-dependent neuroprotective protein, NAP, Cytokines
Introduction
Activity-dependent neuroprotector (ADNP) was first detected as a protein induced by vasoactive intestinal polypeptide (VIP) in astrocytes [1]. VIP protects neurons against damage through a variety of mechanisms [2]. Treatment with VIP also reduces the severity of experimental autoimmune encephalomyelitis, the animal model of multiple sclerosis (MS), by suppressing central nervous system inflammation [3]. Although ADNP mediates neuroprotective effects of VIP, it has also been shown to be neuroprotective in a variety of other experimental models [4]. In humans, ADNP is expressed in the spleen, peripheral blood leukocytes, cerebellum, hippocampus, cerebral cortex [5] and astrocytes [6]. Quintana et al. [7] also showed ADNP expression in macrophages.
Human ADNP contains 9 zinc fingers, a homeobox domain, a nuclear localization signal, a cellular export and import signal [5], and a signal peptide that may indicate a secreted protein [8].
ADNP contains an 8-amino acid sequence, NAPVSIPQ (termed NAP), which provides neuroprotection in vitro and in vivo in a variety of neuronal injury models [9]. This short peptide elicits neuroprotection by interacting with neurons [6], binding to tubulin and stabilizing microtubules [10], and its cellular uptake may not require surface receptors [6]. In addition to the neuroprotective effect, ADNP (NAP) also has immunomodulatory effects. Quintana et al. [7] showed that NAP suppressed production of pro-inflammatory cytokines TNF-α, IL-6 and IL-12 from murine macrophages. We recently observed a reduced expression of ADNP in peripheral blood mononuclear cells (PBMCs) of MS patients as part of our microarray studies of gene expression. This led us, in the studies presented here, to validate these results by quantifying the expression of ADNP mRNA in peripheral blood from patients with MS and healthy volunteers, and to further analyze the expression of ADNP in cells of the immune system, utilizing markers for T cells (CD4, CD25, CD3), B cells (CD19), natural killer (NK) cells (CD3–CD56+) and the monocyte-macrophage lineage (CD14). We investigated the effect of NAP on T cell activation by measuring surface expression of CD69 and CD154 and its effect on the expression of the pro-inflammatory cytokine, IFN-γ. As downregulation of ADNP has effects on cell proliferation [5], we also studied the effects of NAP on the proliferation of PBMCs.
Materials and Methods
Surface and Intracellular Staining of PBMCs
These studies were approved by the Nottingham Research Ethics Committee (patients) and the University of Nottingham Ethics Committee (healthy controls). PBMCs were isolated from healthy volunteers and patients with MS by gradient centrifugation with Histopaque 1077 (Sigma Aldrich, Dorset, UK). One million cells were surface-stained with 5 μl antiCD4 (ECD), 5 μl CD25 (PC5), 3 μl CD3 (PC7), 5 μl CD19 (PE; all from Beckman Coulter, Fullerton, Calif., USA) and the relevant isotypes. PBMCs were left to stand in the dark on ice for 30 min. Cells were incubated in 500 μl of 2% formaldehyde at room temperature for a further 5 min. Cells were washed in 1 ml PBA (phosphate-buffered saline, 0.5% bovine serum, 1% sodium azide) and resuspended in 1 ml saponin buffer (PBA, 0.1% Saponin) for permeabilization. Cells were washed twice in saponin buffer containing 10% FCS. Cells were then stained with 1 μg (5 μl) of the primary ADNP antibody (Abcam, Cambridge, Mass., USA), incubated for 30 min, and washed in saponin buffer. Cells were then incubated with 5 μl of the secondary antibody conjugated to FITC (Abcam), washed with saponin buffer, and fixed with 0.5% formaldehyde. Preliminary blocking experiments showed that incubation of the anti-ADNP (NAP) antibody with excess NAP peptide consistently reduced fluorescence staining by >80%.
For studies of the effect of NAP on lymphocyte proliferation and IFN-γ production, one million PBMCs were stimulated in vitro with 1 μg/ml anti-CD3 and anti-CD28 (Ab Beckman Coulter, Roissy, France) or 20 ng/ml PDB and 5 μg Ionomycin (Sigma Aldrich) with or without different concentrations of the synthetic peptide NAP (0, 0.25, 0.5 and 1 μg/ml); 10 μg/ml of brefeldin was added 8 h prior to intracellular staining with anti-IFN-γ (Beckman Coulter, Fullerton, Calif., USA). NAP was synthesized in the polymer synthesis laboratory at the University of Nottingham. Cells were incubated for 18 h at 37°C, 5% CO2. Intracellular staining for IFN-γ conjugated to FITC (5 μl) and surface staining with anti-CD69 conjugated to PE (5 μl) and CD154 conjugated to PC5 (2 μl) was performed as described above. Cells were centrifuged and fixed with 0.5% formaldehyde. Data acquisition was performed on a Beckman Coulter flow cytometer and data was analyzed by using the WinMDI software.
Proliferation Assay
Ninety-six-well plates were coated with 1 μg/ml of anti-CD3 (Beckman Coulter, Roissy, France) for 90 min at 37°C and 5% CO2. The plates were washed 3 times with 200 μl ice-cold phosphate-buffered saline (Sigma Aldrich). Increasing concentrations of NAP peptide were added to the wells (0, 0.25, 0.5 and 1 μg/ml).
Each condition was done in triplicate; 5 × 105 PBMCs were plated out in a volume of 200 μl and incubated for 24 h at 37°C and 5% CO2. The cells were pulsed with 50 μl of (methyl-3H) thymidine (Amersham, Little Chalfont, UK) and further incubated for 16 h at 37°C and 5% CO2. Cells were harvested onto a Packard uniFilter plate using a MicroMate 96 cell harvester. The plate was left to dry for 4 h. Fifty microliters of scintillation fluid (Microscint TM Packard, Groningen, The Netherlands) were added to each well. Incorporation of methyl-3H thymidine was measured in a β-counter (microplate scintillation counter; Packard, Erskine, UK) and expressed as average counts per minute.
Quantitative Real-Time Polymerase Chain Reaction
Peripheral blood was obtained from healthy volunteers as well as age- and gender-matched patients with MS. Blood samples were left at room temperature for 3 h in Paxgene blood RNA tubes (PreAnalytix, Crawley, UK) before processing for total RNA extraction following the manufacturer's instructions. Extracted RNA from each sample was quantified (40–175 ng/μl) and quality evaluated using a spectrophotometer (ND-1000 v3.3.0; Nanodrop, Wilmington, Del., USA). RNA was converted to cDNA.
The cDNA was diluted and 5 μl was used for real-time polymerase chain reaction (PCR). FullVerocity SYBR Green QPCR Master Mix (Stratagene, La Jolla, Calif., USA) was used to measure ADNP expression by real-time PCR. Primer pairs were synthesized by MWG Operon (Eurofin, Ebersberg, Germany). Quantitative PCR was performed with the ADNP primer set 5′-AGGCCAGGGTTACAGTGTTG-3′ (forward) and 5′-CTCTGGATG-CCTGTGACTGA-3′ (reverse).
ADNP mRNA results were based on normalised ratio to the internal standard beta-2 microglobulin (B2MG) (5′-GTGGCCTTAGCTGTG-3, forward and 5′-TGG ATG AAA CCC AGA CAC ATA G-3′, reverse). PCR was performed in triplicate conditions; 10 min at 95°C and 40 cycles of 15 s at 95°C and 1 min at 60°C. The dissociation curve method was utilised to assess the specificity of the amplified product. Quantitative PCR was performed on a MX4000 Multiplex qPCR system (Stratagene).
Statistical Analysis
A repeated measure ANOVA test and paired t test were used to analyze the expression of CD69, CD154 and IFN-γ between groups. A Mann-Whitney test was employed to observe statistical differences between the mRNA expression of ADNP in peripheral blood from MS patients and healthy controls.
Results
Expression of ADNP on Normal PBMC
Expression of ADNP was determined by flow cytometry in PBMCs from 3 healthy control donors and 3 relapsing remitting MS patients in remission. ADNP expression was predominantly localized to the T cells and the monocyte/macrophage lineage. Naturally occurring CD4+CD25+ regulatory T cells did not express ADNP. There were no differences in ADNP between the normal and MS PBMC subpopulations above; however, there was statistically significant difference in the expression of ADNP in NK cells, which was higher in controls compared to MS patients (p = 0.001). Table 1 shows the expression of ADNP on various cells of the immune system.
Table 1.
The expression of ADNP on various cells of the immune system in MS patients and healthy controls
| Cell type | Marker | Controls mean % ADNP+ cells | Patients mean % ADNP+ cells |
|---|---|---|---|
| T cells | CD3 | 72 (6.49) | 62 (13) |
| T cells | CD4 | 43.3 (8.51) | 46 (9.3) |
| T cells | CD25 | 5.71 (1) | 8.6 (1.8) |
| NK cells | CD3−, CD56+ | 4.58 (0.66) | 0.12 (0.03)* |
| B cells | CD19 | 10.9 (2.39) | 16 (3.5) |
| Monocytes | CD14 | 79.2 (2.05) | 68 (19) |
Figures in parentheses are ± SD.
* Statistically significant (p = 0.001); other differences were not statistically significant.
Effects of NAP on T Cell Activation
As expected, upregulation of the activation marker CD69 was observed on PBMCs from healthy volunteers stimulated with 1 μg anti-CD3 and anti-CD28. Upregulation of CD69 was reduced in the presence of increasing concentrations of NAP (table 2). The difference was significant at 1 μg/ml NAP (p = 0.02) and 0.5 μg/ml NAP (p = 0.02), but not at 0.25 μg/ml, although a trend was seen (p = 0.07). Similarly, NAP reduced the expression of CD154 on PDB/ionomycin stimulated PBMCs from healthy controls, at all concentrations: 1 μg/ml NAP (p = 0.01), 0.5 μg/ml NAP (p = 0.003) and 0.25 μg/ml NAP (p = 0.04; table 3). Baseline expression of CD69 and CD154 in the absence of stimuli were significantly increased in MS compared to controls. Although the PBMCs of MS patients showed very high expression of CD69 which remained high despite NAP (data not shown), a significant downregulation with NAP was seen in PBMCs isolated from MS patients with the activation marker CD154. There was a statistically significant difference at 1 μg/ml NAP (p = 0.01) and 0.5 μg/ml NAP (p = 0.008) but not at 0.25 μg/ml (p = 0.25; table 3).
Table 2.
The expression of CD69 in healthy controls after treatment with NAP
| Stimuli | Concentration of NAP, μg/ml | Mean % CD69+ cells |
|---|---|---|
| 0 | 0 | 62 (8.3) |
| 1 μg/ml anti-CD3/CD28 | 0 | 79 (10)* |
| 1 μg/ml anti-CD3/CD28 | 1 | 70 (12)* |
| 1 μg/ml anti-CD3/CD28 | 0.5 | 71 (9.5)* |
| 1 μg/ml anti-CD3/CD28 | 0.25 | 71 (3.1) |
Figures in parentheses are ± SD.
* p < 0.05.
Table 3.
The expression of CD154 in MS patients and healthy controls after treatment with NAP
| Stimuli | Concentration of NAP, μg/ml | Controls mean % CD154 | Patients mean % CD154 |
| 0 | 0 | 4.6 (1.8) | 31 (18)* |
| PDB 20 ng/ml, ionomycin 5 μg/ml | 0 | 18 (3.6) | 79 (6.8) |
| PDB 20 ng/ml, ionomycin 5 μg/ml | 1 | 12 (5.7) | 71 (5.9) |
| PDB 20 ng/ml, ionomycin 5 μg/ml | 0.5 | 9.8 (3.9) | 60 (5.2) |
| PDB 20 ng/ml, ionomycin 5 μg/ml | 0.25 | 7.9 (2.3) | 69 (16) |
Figures in parentheses are ± SD.
* p < 0.05.
Effects of NAP on the Expression of IFN-γ
There was a statistically significant reduction in the IFN-γ expression of PBMCs treated with 0.25 μg/ml NAP. IFN-γ expression declined from 8.3 to 4.8% (p = 0.04). There were no statistically significant differences observed with the other peptide concentrations. Similar results were obtained with PBMCs from patients with MS: there was a statistically significant difference in IFN-γ expression with PBMCs treated with 0.25 μg/ml NAP. IFN-γ expression declined from 7 to 5% (p = 0.03). There was no statistically significant difference observed with the other peptide concentrations.
Proliferation Assay
The following proliferation results were seen with anti-CD3 treated/anti-CD28-treated PBMC with or without various concentrations of NAP (SD in parentheses): anti-CD3/anti-CD28 with no NAP, 14,000 cpm (±4,000); anti-CD3/anti-CD28 plus 0.25 μg NAP, 9,700 cpm (±5,400); anti-CD3/anti-CD28 plus 0.5 μg NAP, 11,000 cpm (±3,330); anti-CD3/anti-CD28 plus 1 μg NAP, 11,000 cpm (±3,800). The suppression caused by 1 and 0.5 μg of NAP was between 18 and 21%; 0.25 μg NAP caused 38% suppression. There was a statistically significant difference between no NAP versus 1 μg (p = 0.02), 0.5 μg (p = 0.02) and 0.25 μg (p = 0.03). With PBMC from MS patients, proliferation was reduced by 34, 6 and 9%, respectively, for the above doses in decreasing order, but this reduction did not reach statistical significance.
Expression of mRNA ADNP from MS Patients and Control Whole Blood
To confirm our gene expression studies indicating a reduced expression of ADNP mRNA in MS patients, we collected blood samples from 24 patients with MS, 17 females and 7 males, 1 with secondary progressive, 19 with relapsing remitting MS in remission and 4 relapsing remitting MS patients in relapse and measured ADNP mRNA by quantitative real-time PCR. None of the patients were on disease-modifying treatment. The patients were aged between 30 and 60 years (mean 40 years, SD ±10). The healthy volunteer group consisted of 14 females and 7 males aged between 20 and 60 (mean 40 years, SD ±10).
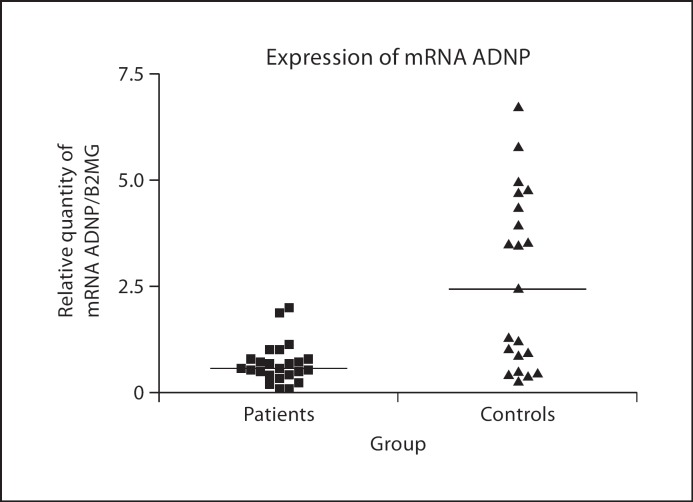
There was a statistically significant difference in the expression of ADNP mRNA, a lower expression being observed in the MS patients in comparison to the healthy controls (p < 0.001; fig. 1).
Fig. 1.
Expression of ADNP mRNA in peripheral blood of MS patients (n = 24) versus age- and gender-matched control subjects (n = 21). Results represent individual arbitrary units of ADNP mRNA normalised to β-2 microglobulin mRNA. The horizontal lines represent medians. The expression in controls was significantly higher than in MS patients (p < 0.001).
There were no statistically significant differences between MS clinical subgroups in terms of ADNP mRNA expression.
Discussion
We found that monocytes, B cells, T cells and other immune cells but very few CD4+CD25+ cells (a fraction that mainly includes regulatory T cells in the absence of activation stimuli) expressed intracellular ADNP. Treatment with the synthetic peptide NAP suppressed the proliferation of anti-CD3-stimulated PBMCs and decreased the activation of T cells as demonstrated by the reduced upregulation of CD69 and CD154 in response to TCR-mediated activation. CD154 was used as an additional, potentially more responsive marker for T cell activation because anti CD69 expression was too high, >90% in MS patients and not significantly suppressible. There was also a slight decrease in the percentage of cells expressing IFN-γ when activated in the presence of NAP. We report, for the first time, a reduced expression of ADNP mRNA in the blood of MS patients compared to controls. These results suggest that ADNP, which has a neuroprotective role in the CNS, also has an immunoregulatory role, which is likely independent of classical natural regulatory T cells.
This observation is in accord with our microarray results (Showe, L and Constantinescu, CS, unpubl. observations). Our observed ADNP downregulation in MS patients and its protective capacity in experimental autoimmune encephalomyelitis [3] suggests reduced immunoregulatory capacity via ADNP in MS patients. This suggests a role for ADNP/NAP as a potential therapy for MS as the ADNP deficiency may contribute to the defective immunoregulatory mechanisms in people with MS, who also show reduced natural regulatory T cell function [11].
ADNP has the potential to be a secreted protein [5] implying that its immunoregulatory and neuroprotective peptide NAP may be in the systemic circulation [7], and capable of regulating cells of the immune system, in a cytokine-like fashion, though potentially independent of surface receptors [6]. Inhibition by ADNP/NAP of the activation of autoreactive T cells may therefore be beneficial in MS [12]. As previously shown [13,14], both activation markers were expressed at higher level in MS patients compared to controls, even though the patients were in remission. Although, in this small sample, NAP seemed to have a weaker effect on the proliferation of PBMCs from MS patients compared to those from healthy subjects, the effects on activation as assessed by CD154 expression and on IFN-γ production were similar. Also, NK cells from MS patients had significantly lower ADNP, and one can speculate that MS patients’ NK cells may be less effective in regulating T cell proliferation [15]. Nevertheless, even if immune cells of MS patients may be less sensitive to immunomodulatory effects of ADNP/NAP, they may be sensitive enough to exhibit reduced inflammatory molecules. Future studies will address potential differences in ADNP responsiveness between immune cells of MS patients and healthy subjects.
It has previously been reported that pro-inflammatory cytokines contribute to peripheral T cell activation [16]. Our data suggest that NAP caused a downregulation of IFN-γ. A decrease in the transcription and secretion of IFN-γ may partially increase clinical recovery from MS, as previous studies have shown that IFN-γ exacerbates MS disease activity [17]. Interestingly, in contrast to the dose-dependent inhibition of CD69 and CD154 expression, IFN-γ expression and possibly proliferation were more efficiently inhibited at the lower dose of NAP, suggesting different pharmacological properties for different immune actions.
In conclusion, we have shown that ADNP is expressed in cells of the immune system and that its neuroprotective peptide has immunoregulatory properties. This is an example of neuroimmunomodulation that is relevant to nervous system diseases with both inflammatory and neurodegenerative components, like MS [18]. Our finding of a reduced expression of ADNP in peripheral blood cells of MS patients, that have been found to have reduced regulatory T cell function, suggest the possibility of an additional deficit of immunoregulation in this inflammatory demyelinating disease.
Acknowledgements
This study was supported in part by the MS Society of Great Britain and Northern Ireland and by the Stanhope Trust. We thank Anushka Naiken for technical assistance for the proliferation assay.
References
- 1.Zusev M, Gozes I. Differential regulation of activity-dependent neuroprotective protein in rat astrocytes by VIP and PACAP. Regul Pept. 2004;123:33–41. doi: 10.1016/j.regpep.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 2.Li H, Mei Y, Wang Y, Xu L. Vasoactive intestinal polypeptide suppressed experimental autoimmune encephalomyelitis by inhibiting T helper 1 responses. J Clin Immunol. 2006;26:430–437. doi: 10.1007/s10875-006-9042-2. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, Delgado M. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gozes I. Activity-dependent neuroprotective protein: from gene to drug candidate. Pharmacol Ther. 2007;114:146–154. doi: 10.1016/j.pharmthera.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Zamostiano R, Pinhasov A, Gelber E, Steingart RA, Seroussi E, Giladi E, Bassan M, Wollman Y, Eyre HJ, Mulley JC, Brenneman DE, Gozes I. Cloning and characterization of the human activity-dependent neuroprotective protein. J Biol Chem. 2001;276:708–714. doi: 10.1074/jbc.M007416200. [DOI] [PubMed] [Google Scholar]
- 6.Gozes I, Divinski I. The femtomolar-acting NAP interacts with microtubules: novel aspects of astrocyte protection. J Alzheimers Dis. 2004;6:S37–S41. doi: 10.3233/jad-2004-6s605. [DOI] [PubMed] [Google Scholar]
- 7.Quintana FJ, Zaltzman R, Fernandez-Montesinos R, Herrera JL, Gozes I, Cohen IR, Pozo D. NAP, a peptide derived from the activity-dependent neuroprotective protein, modulates macrophage function. Ann NY Acad Sci. 2006;1070:500–506. doi: 10.1196/annals.1317.069. [DOI] [PubMed] [Google Scholar]
- 8.Gozes I, Zamostiano R, Pinhasov A, Bassan M, Giladi E, Steingart RA, Brenneman DE. A novel VIP responsive gene. Activity dependent neuroprotective protein. Ann NY Acad Sci. 2000;921:115–118. doi: 10.1111/j.1749-6632.2000.tb06957.x. [DOI] [PubMed] [Google Scholar]
- 9.Gozes I, Bassan M, Zamostiano R, Pinhasov A, Davidson A, Giladi E, Perl O, Glazner GW, Brenneman DE. A novel signaling molecule for neuropeptide action: activity-dependent neuroprotective protein. Ann NY Acad Sci. 1999;897:125–135. doi: 10.1111/j.1749-6632.1999.tb07884.x. [DOI] [PubMed] [Google Scholar]
- 10.Gozes I, Zaltzman R, Hauser J, Brenneman DE, Shohami E, Hill JM. The expression of activity-dependent neuroprotective protein (ADNP) is regulated by brain damage and treatment of mice with the ADNP derived peptide, NAP, reduces the severity of traumatic head injury. Curr Alzheimer Res. 2005;2:149–153. doi: 10.2174/1567205053585873. [DOI] [PubMed] [Google Scholar]
- 11.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CFD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kierdorf K, Wang Y, Neumann H. Immune-mediated CNS damage. Results Probl Cell Differ 2009. [DOI] [PubMed]
- 13.Kosmaczewska A, Bilinska M, Ciszak L, Noga L, Pawlak E, Szteblich A, Podemski R, Frydecka I. Different patterns of activation markers expression and CD4+ T-cell responses to ex vivo stimulation in patients with clinically quiescent multiple sclerosis (MS) J Neuroimmunol. 2007;189:137–146. doi: 10.1016/j.jneuroim.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Balashov KE, Smith DR, Khoury SJ, Hafler DA, Weiner HL. Increased interleukin 12 production in progressive multiple sclerosis: Induction by activated CD4+ T cells via CD40 ligand. Proc Natl Acad Sci USA. 1997;94:599–603. doi: 10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segal BM. The role of natural killer cells in curbing neuroinflammation. J Neuroimmunol. 2007;191:2–7. doi: 10.1016/j.jneuroim.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martino G, Grohovaz F, Brambilla E, Codazzi F, Consiglio A, Clementi E, Filippi M, Comi G, Grimaldi LM. Proinflammatory cytokines regulate antigen-independent T-cell activation by two separate calcium-signaling pathways in multiple sclerosis patients. Ann Neurol. 1998;43:340–349. doi: 10.1002/ana.410430312. [DOI] [PubMed] [Google Scholar]
- 17.Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- 18.Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]