Abstract
Myotonic dystrophy type 1 (DM1) is the most common muscular dystrophy in adults and as yet no cure for DM1. Here, we report the potential of manumycin A for a novel DM1 therapeutic reagent. DM1 is caused by expansion of CTG repeat. Mutant transcripts containing expanded CUG repeats lead to aberrant regulation of alternative splicing. Myotonia (delayed muscle relaxation) is the most commonly observed symptom in DM1 patients and is caused by aberrant splicing of the skeletal muscle chloride channel (CLCN1) gene. Identification of small-molecule compounds that correct aberrant splicing in DM1 is attracting much attention as a way of improving understanding of the mechanism of DM1 pathology and improving treatment of DM1 patients. In this study, we generated a reporter screening system and searched for small-molecule compounds. We found that manumycin A corrects aberrant splicing of Clcn1 in cell and mouse models of DM1.
Myotonic dystrophy (DM), a genetic disorder, is the most common type of muscular dystrophy in adults1. The types of DM disease2, DM types 1 (DM1) and 2 (DM2), differ genetically but are similar clinically. DM1 is caused by expansion of a CTG repeat in the 3′-untranslated region (UTR) of the DM protein kinase (DMPK) gene, whereas DM2 is caused by expansion of a CCTG repeat in intron 1 of the zinc finger 9 (ZNF9) gene3,4,5. DM1 presents with a variety of symptoms, such as myotonia, progressive muscle wasting, cataracts, insulin resistance, and intellectual deficits1,6. Identification of the DM2 mutation5 and studies using DM1 mice that express the expanded CUG repeat7 suggest that RNA gain-of-function causes the DM1 phenotype.
How does nucleotide expansion within a non-coding region cause DM1? An investigation of the molecular mechanism of DM1 proposed that expanded repeat RNA transcripts form hairpin structures that retain nuclear foci in DM1 cells8, and affect the function of RNA-binding proteins9,10,11,12. ‘Muscleblind-like' (MBNL) and ‘CUGBP and ETR-3 like factor' (CELF) proteins are well-studied RNA-binding proteins. Sequestration of MBNL1 by toxic expanded RNA transcripts11,13 and up-regulation of CUGBP-114 result in aberrant regulation of alternative splicing events in DM12. Our previous studies indicated aberrant regulation of MYOM1 and PDLIM3 in DM1 patients15,16; more than 25 genes have been found to be misregulated in patients with DM17. Missplicing of the following genes has been associated with DM1 symptoms: CLCN1, which results in myotonia7,18, BIN1, which is associated with T-tubule alterations and muscle weakness19, and INSR, which contributes to insulin resistance20. However, there is no evident relationship between additional misspliced genes and DM1 symptoms. Therefore, further studies are needed to elucidate this association.
Myotonia is one of the features observed most commonly in individuals with DM1. People with myotonia are unable to relax certain muscles after use. For example, a person may not be able to release their grip on a doorknob or handle. In DM1 patients, the inclusion of alternative exons 6B and/or 7A, and retention of intron 2 of CLCN1, are observed due to aberrant regulation of alternative splicing18. Abnormal splicing of CLCN1 results in a frameshift and produces premature termination codons in transcripts, leading to nonsense-mediated mRNA decay (NMD) or the production of a truncated protein with a dominant-negative effect21. A mouse model of DM1 expressing an expanded CUG repeat (HSALR) also shows increased inclusion of Clcn1 exon 7A and displays myotonia7. Our previous study using a Clcn1 minigene identified regulation of exon 7A by MBNL and CELF proteins22.
The identification of small-molecule compounds that correct missplicing events in DM1 would benefit both our understanding of novel aspects of DM1 pathogenesis and DM1 therapy. In DM1, there may be other key players in addition to MBNL and CELF. There are several approaches to DM1 therapy, such as overexpression of MBNL123, RNA interference targeting CUG repeat transcripts24, inhibition of MBNL1 sequestration through use of a CAG oligonucleotide that binds to the CUG repeats25 or a small molecule26, and degradation of expanded CUG repeat transcripts through the RNase H pathway, which occurs through induction of 2′ methoxyethyl (MOE) gapmers27. Although use of an antisense oligonucleotide showed remarkable effects, there was a difficulty with body-wide delivery while small-molecule compounds have the advantage of oral formulation.
In this study, we established a Clcn1-L minigene reporter assay and found that manumycin A corrects abnormal splicing of Clcn1. Furthermore, we confirmed that injection of manumycin A corrects missplicing of Clcn1 in a mouse model of DM1 via H-Ras pathway.
Results
Generation of the Clcn1-L reporter assay system
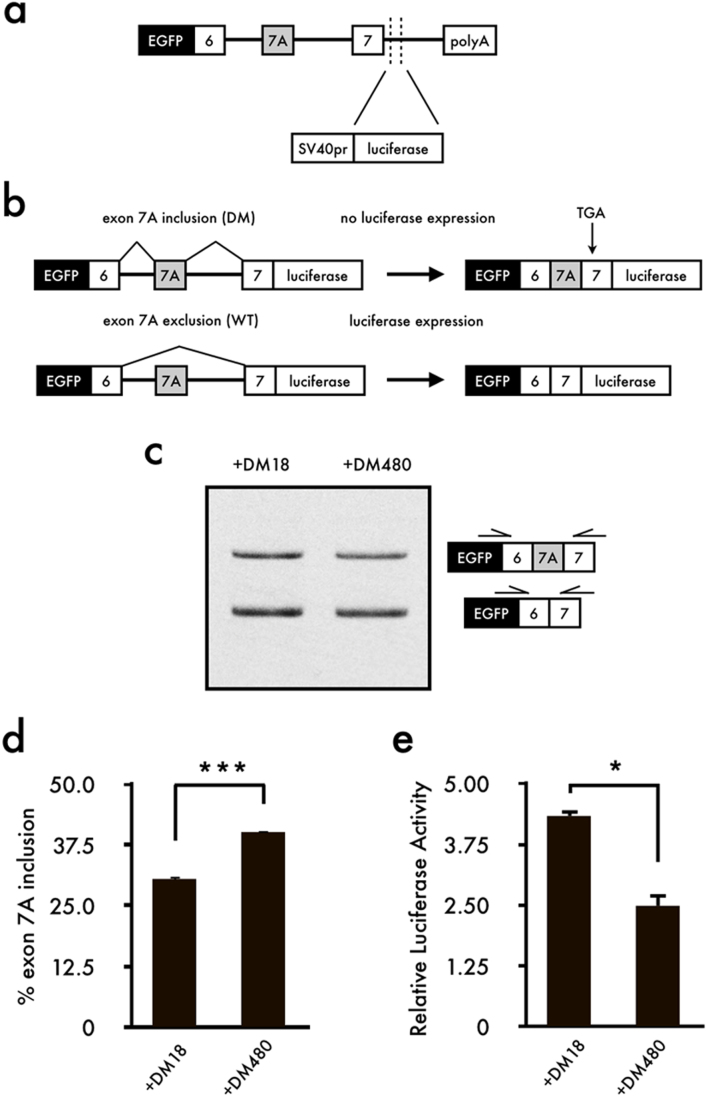
To identify small chemical compounds effective against aberrant splicing of CLCN1 in DM1, we generated a minigene reporter vector containing the mouse Clcn1 gene from exons 6 to 7 and the firefly luciferase gene (Fig. 1a). As shown in Fig. 1b, luciferase expression was obtained upon exclusion of exon 7A; however, inclusion of exon 7A produced a termination codon, resulting in a lack of luciferase expression. Next, we confirmed the co-transfection of Clcn1-L and DMPK constructs harboring either CTG18 (DM18) or interrupted CTG480 (DM480) repeats. RT-PCR analysis revealed that inclusion of exon 7A was significantly increased upon co-expression of Clcn1-L and DM480 compared to co-expression with DM18 (Fig. 1c,d). According to the luciferase analysis, co-expression of Clcn1-L and DM18 displayed a higher activity than did co-expression with DM480 (Fig. 1e). Note that we did not delete the initiation codon of luciferase gene. It is possible that an extra ATG codon in the Clcn1-L might induce translation of luciferase irrespective of exon 7A exclusion. However, in our experiments, the luciferase activity decreased when DM480 was transfected (Fig. 1e). Therefore, we concluded that this reporter system was properly working.
Figure 1. Construction of the Clcn1-L reporter minigene and effect of triplet repeat expansion on the reporter vector Clcn1-L.
(a) Schematic structure of the Clcn1-L minigene reporter. A genomic segment of mouse Clcn1 containing exons 6 to 7 (including the intron) was sub-cloned downstream of EGFP in the pEGFP-C1 plasmid. Firefly luciferase was inserted in-frame with a correct Clcn1 splicing pattern. (b) Schematic of how Clcn1-L functions. Exon 7A exclusion results in luciferase expression to detect correct Clcn1 splicing. (c) Inclusion of Clcn1 exon 7A increased upon expression of the expanded CUG repeat. (d) Bar charts show the quantified percentages of exon 7A inclusion (mean + SEM, n = 4). (e) Luciferase analysis showed that relative luciferase activity decreased upon expression of the expanded CUG repeat (mean + SEM, n = 3). The gel image was cropped around the region of interest and the samples (n = 4) were resolved in the same gel. Statistical significances were determined using t-tests (*p < 0.05, ***p < 0.001).
Identification of small-molecule compounds that correct aberrant splicing of Clcn1 under the expression of expanded CUG repeats in vitro
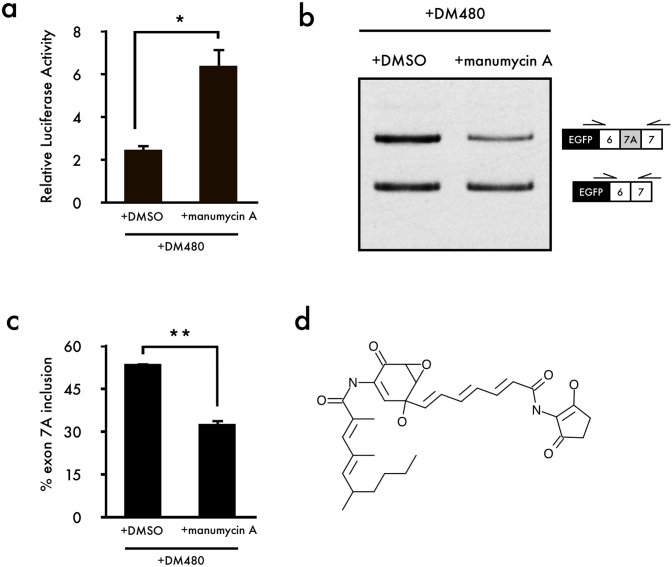
The ICCB Known Bioactives Library is a collection of compounds with defined biological activities. The library was screened to identify compounds that corrected aberrant splicing of Clcn1 using our luciferase reporter assay. Because expanded CUG repeat expression causes aberrant regulation of alternative splicing in DM1, we transfected C2C12 cells with both the Clcn1-L luciferase reporter vector and DM480, which expresses the expanded CUG repeat. Although most compounds showed little effect compared to that of DMSO treatment (control, Table S1), some of the compounds showed high luciferase activity (Fig. S1), and Ro 31-8220, AGC, and manumycin A caused little toxicity to cells (Fig. S2). Since Ro 31-8220 was well studied with DM128 and AGC caused large SEM, manumycin A was chosen in this study for further analysis. Manumycin A (20 μM) showed high luciferase activity in the presence of the expanded CUG repeat (Fig. 2a and Fig. S1).
Figure 2. Identification of small-molecule compounds that correct aberrant splicing of Clcn1.
(a) A luciferase reporter assay showed that manumycin A corrected aberrant splicing in the presence of the expanded CUG repeat (mean + SEM, n = 3). (b) Cellular splicing analysis showed that manumycin A corrects aberrant splicing of Clcn1. (c) Quantification of the results shown in (b) (mean + SEM, n = 3). (d) Structure of manumycin A. The gel image was cropped around the region of interest and the samples (n = 3) were resolved in the same gel. Statistical significance was determined using t-tests (*p < 0.05, **p < 0.01).
Next, we performed RT-PCR analysis to investigate whether manumycin A corrects aberrant Clcn1 splicing caused by expression of the expanded CUG repeats. Results from RT-PCR showed that the addition of manumycin A effectively corrects aberrant splicing of Clcn1 in the presence of the expanded CUG repeat (Fig. 2b,c). Percentages of Clcn1 exon 7A inclusion showed that manumycin A treatment rescued abnormal exon 7A inclusion levels caused by expression of the expanded CUG repeat to levels similar to those for the normal CUG repeat (Fig. 2c). Additionally, we tested the dosage of manumycin A (~10-40 μM), and found that the effects of manumycin A were concentration-dependent (Fig. S3). High-dosage manumycin A rescued aberrant splicing in the presence of the expanded CUG repeat and showed skipping of exon 7A (Fig. S3) compared to the control (Fig. 2c).
Manumycin A corrects aberrant splicing of Clcn1 in a mouse model of DM1
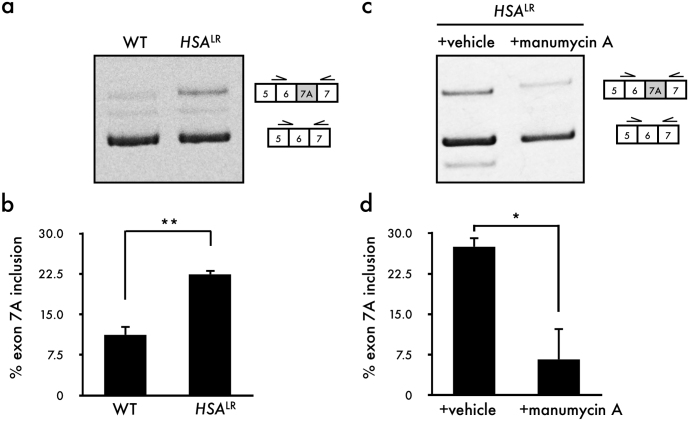
Next, we examined the ability of manumycin A to rescue aberrant splicing of Clcn1 in a mouse model (HSALR) of DM1, in which 250 CUG repeats are expressed under the control of the actin promoter. RT-PCR analysis showed elevated inclusion of Clcn1 exon 7A in the HSALR mice compared with the wild-type mice (Fig. 3a,b). Injection of manumycin A induced a remarkable reduction in Clcn1 exon 7A inclusion (Fig. 3c,d). However, manumycin A did not rescue aberrant splicing of Serca1 and m-Titin (Fig. S4).
Figure 3. Manumycin A corrects aberrant splicing of Clcn1 in HSALR DM1 model mice.
(a) RT-PCR analysis showed increased inclusion of Clcn1 exon 7A in HSALR mouse. (b) Quantification of the results shown in (a) (mean + SEM, n = 3). (c) RT-PCR analysis showed reduced inclusion of Clcn1 exon 7A 5 days after injection of manumycin A into TA muscles. (d) Quantification of the results shown in (a) (Mean + SEM, n = 3). The gel image was cropped around the region of interest and the samples (n = 3) were resolved in the same gel. Statistical significance was determined using t-tests (*p < 0.05, **p < 0.01).
H-Ras regulates alternative splicing of Clcn1 exon 7A
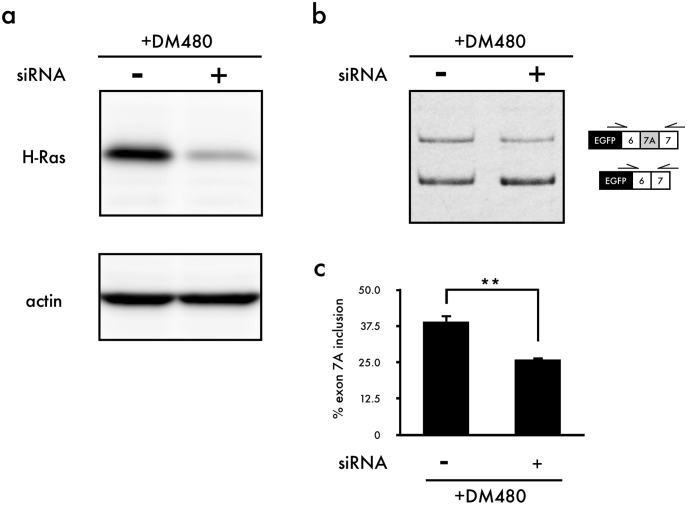
Manumycin A is an antibiotic generated by Streptomyces parvulus29. It acts as a selective and vigorous inhibitor of Ras farnesyltransferase30. After translation, Ras protein requires several modifications: isoprenylation, proteolysis, methylation and palmitoylation31,32,33. Isoprenylation by the enzyme farnesyltransferase (FTase) or geranylgeranyltransferase I (GGTase I) is the first step in the post-translational modification of Ras. Farnesylation or geranylgeranylation is necessary for Ras to attach to the inner side of the plasma membrane. Without attachment to the cell membrane, Ras is unable to be activated34. H-Ras, K-Ras and N-Ras are the members of the Ras family. It is important that H-Ras is only farnesylated, whereas K-Ras and N-Ras can be farnesylated and geranylgeranylated. Thus, inhibitors of farnesyltransferase are effective in reducing the activity of H-Ras but not that of K-Ras and N-Ras35. Correction of Clcn1 splicing by manumycin A may therefore be due to the inhibition of H-Ras activity. To test this possibility, we knocked down endogenous H-Ras expression using small interfering RNA (siRNA) and examined the effect of H-Ras inhibition on Clcn1 splicing. We confirmed the efficacy of the siRNA in modulating the expression of the H-Ras by Western blot analysis (Fig. 4a). RT-PCR analysis showed reduced inclusion of Clcn1 exon 7A in the presence of expanded CUG repeats (Fig. 4b,c). We also examined the effects of K-Ras and N-Ras knockdown and found that N-Ras knockdown reduced the inclusion of Clcn1 exon 7A, whereas K-Ras knockdown did not alter Clcn1 splicing (Fig. S5). Additionally, we tested whether expanded CUG repeats altered the expression levels of H-Ras. Although there was no significant difference between DM18- and DM480-transfected C2C12 cells, an upward trend was observed when cells were transfected with DM480 (Fig. S6).
Figure 4. The knockdown of H-Ras corrects aberrant splicing of Clcn1 in the presence of the expanded CUG repeat.
(a) Representative result of Western blot analysis of H-Ras in C2C12 cells. (b) Results of cellular splicing assays using Clcn1-L minigene, DM480 and siRNA. (c) Quantification of the results shown in (b) (mean + SEM, n = 3). The gel and blot image were cropped around the region of interest and the samples (n = 3) were resolved in the same gel or blot. Statistical significance was determined using t-tests (**p < 0.01).
Discussion
Misregulation of alternative splicing is a characteristic feature of DM1. Although the number of missplicing events is over 25, few genes have been reported to play a role in disease manifestations. Abnormal regulation of alternative splicing in the CLCN1 gene is one of the events that can account for myotonia, which is often recognized in DM1. In this investigation, we generated a Clcn1-L reporter assay system and searched for small-molecule compounds that affect abnormal splicing of Clcn1 in the presence of expanded CUG repeats. We found that manumycin A corrects aberrant splicing of Clcn1, which was confirmed in vivo. We also revealed that H-Ras was involved in the regulation of alternative splicing of Clcn1 exon 7A.
Injection of DM1 model mice with manumucin A corrected aberrant splicing of Clcn1; however, splicing of Serca1 and m-Titin, which is also abnormally regulated in DM136,37, was not changed. It has been reported that Serca1 is regulated by MBNL137,38 and CUGBP139 and that m-Titin is also regulated by MBNL137. Furthermore, the expression of MBNL1 and CUGBP1 was not altered by treatment with manumycin A (Fig. S7). For these reasons, we conclude that manumycin A did not alter Clcn1 splicing through effects on MBNL1 and CUGBP1. How, then, does manumycin A correct Clcn1 missplicing? Manumycin A is an inhibitor of Ras farnesyltransferase, and it could inhibit Ras activity. In this study, we demonstrated that H-Ras knockdown reduced the inclusion of Clcn1 exon7A, indicating that H-Ras is involved in a regulation of Clcn1 splicing.
Ras proteins perform functional roles in a large number of biological processes, leading to changes in cell morphology, survival, apoptosis, and gene expression40. Because Ras is positioned as the central molecular switch of these biological outcomes, it must interact with a variety of downstream targets. Recent studies implicated the Ras signaling pathway in alternative splicing regulation41. Activation of the Ras-PI3-kinase-PKB/Akt pathway alters the phosphorylation level of the serine/arginine-rich (SR) proteins SF2/ASF and 9G842. SR proteins are the best-characterized splicing regulatory factors, and it is well known that the activity of SR proteins is dependent on their phosphorylation level. Furthermore, the Ras-Raf-MEK-ERK pathway, another Ras pathway, is known to modulate alternative splicing41,43.
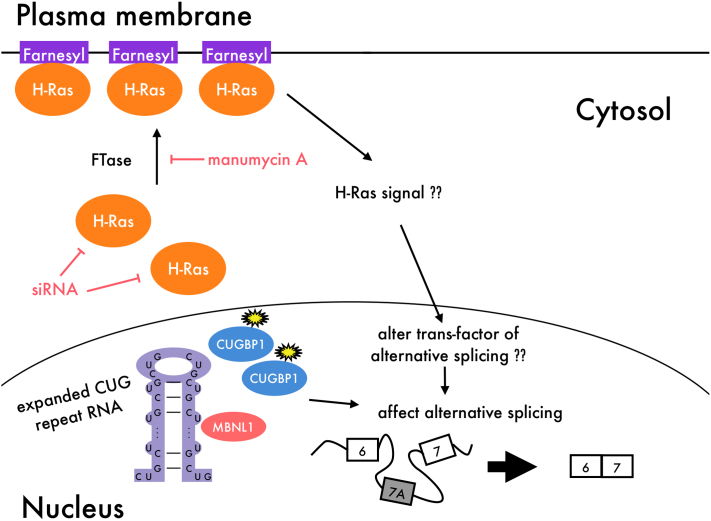
Considering these studies, it is possible that manumycin A inhibits H-Ras activity and alters the H-Ras signaling pathway, resulting in correction of Clcn1 splicing. Therefore, modulation of H-Ras signaling may influence a trans-acting factor other than MBNL1 and CUGBP1 and thereby contribute to Clcn1 splicing. The effect of manumycin A is illustrated in Figure 5, although the question remains as to what kind of trans-acting factor is influenced by manumycin A and H-Ras. Importantly, manumycin A treatment showed reduced total amount of Clcn1 mRNAs (with and without exon 7A) (Fig. 2b and Fig. 3c). Therefore, it is possible that manumycin A has another effect on either transcription or stability of Clcn1 pre-mRNA.
Figure 5. Model of how manumycin A affects Clcn1 splicing.
In DM1, it is known that expanded CUG repeat RNA transcripts trap MBNL1 and up-regulate CUGBP1, resulting in aberrant regulation of alternative splicing. However, manumycin A does not affect the expression of these proteins. Manumycin A might act as a Ras farnesyltransferase inhibitor, thereby altering H-Ras signaling. This could in turn result in alteration of a trans-acting factor involved in alternative splicing other than MBNL1 and CUGBP1.
A recent study revealed that oncogenic mutated H-Ras G12V inhibits muscle differentiation44. If H-Ras is involved in DM pathology, it may help us to better understand DM1. Interestingly, manumycin A only slightly changes the splicing of Clcn1 in the absence of DM480 expression (Fig. S8). Furthermore, transfection of C2C12 cells with the expanded CUG repeat tended to increase H-Ras expression (Fig. S6). Considering the transfection efficiency of C2C12 cells, it is likely that the expanded CUG repeat could alter H-Ras expression. However, involvement of Ras in DM1 has not been reported thus far, and further studies are needed in the future.
DM1 is the most common muscular dystrophy in adults, affecting approximately one in every 8,000 individuals. However, there is as yet no cure for DM. In this study, we showed that manumycin A corrects aberrant splicing of Clcn1 and revealed that H-Ras is involved in regulation of Clcn1 splicing. We hope that further studies on manumycin A and H-Ras will lead to a novel therapy for DM1.
Methods
Plasmid construction
Clcn1-L is a luciferase reporter vector containing a genomic segment of mouse Clcn1 (exon 6 to exon 7) and the firefly luciferase gene. Firefly luciferase (F-Luc) was amplified from the downstream of SV40 promoter of the pGL-3 promoter vector (Promega, Madison, WI, USA) by PCR using PfuUltra High-Fidelity DNA polymerase (STRATAGENE, La Jolla, CA, USA), generating a 1.7-kb product with the addition of a restriction site for SalI and BamHI. Therefore, this fragment did not contain SV40 promoter. The following primer pair was used: F-Luc forward, 5′-AAAGTCGACCCATGAAGACGCC-3′; and F-Luc reverse, 5′-CCGGATCCTTACACGGCGATCTT-3′. The fragment was inserted into the SalI-BamHI site of the Clcn1 minigene. The Clcn1 minigene contains PCR-amplified fragments of mouse Clcn1 (exon 6 to exon 7) and has been described previously22. The nucleotide sequences of the DNA inserts and reading frame were confirmed to be correct by sequencing. DM18 and DM480 contain a fragment of the 3′ region of DMPK with CTG18 or interrupted CTG480 repeats, respectively, and have been described previously23.
Cell culture and transfection
C2C12 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 20% (v/v) fetal bovine serum and then incubated at 37°C with 5% CO2. For the minigene and luciferase reporter assays, C2C12 cells were transfected with plasmids for expression of a minigene with toxic RNA transcripts using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). Transfection was conducted according to the manufacturer's protocol. Cells were cultured in 24- and 96-well plates for the minigene and luciferase reporter assays, respectively.
Luciferase reporter assay and drug screening
Culture, transfection, cell harvesting, and luciferase activity measurements were performed according to the standard methods of the Dual-Glo Luciferase Assay System (Promega). Clcn1-L and DM480 were co-expressed in cells. Plasmid pRL (Promega), which contains the sea pansy luciferase gene, was co-transfected as an internal control for normalization of transfection efficiency. In all experiments, luciferase activity was measured 48 h after transfection and was assayed using the Dual-Glo Luciferase Assay System (Promega). Firefly and sea pansy luciferase activities were measured using the Centro LB 960 (Berthold, Bad Wildbad, Germany), and the value of each sample was calculated as light units of firefly luciferase per light unit of sea pansy. The ICCB Known Bioactives Library (Enzo Life Sciences, Farmingdale, NY, USA) was referenced for drug screening. Chemical compounds were added 24 h after transfection at 0.1% (v/v), and luciferase activity was measured 24 h after the addition of chemical compounds.
Administration of manumycin A to DM mice
HSALR transgenic mice were used for animal experiments and have been described previously45. These mice express human skeletal actin mRNA, with approximately 250 CUG repeats in the 3′-UTR. Manumycin A was diluted to a final concentration of 75 ng/μl in saline containig 0.1% DMSO. Manumycin A (40 μl, 3.0 μg) or vehicle (0.1% DMSO in saline) was injected into the TA muscles of opposite limbs. Mice were killed 5 days after injection, and TA muscle was obtained for splicing analysis. All experiments were conducted according to the Regulations for Animal Experimentation at the University of Tokyo (Tokyo, Japan).
Identification of Clcn1 splice variants
Total RNA was isolated using a GenElute Mammalian Total RNA Miniprep kit (Sigma-Aldrich, St. Louis, MO, USA). cDNA synthesis was performed using a Prime-Script First Strand cDNA Synthesis Kit (TAKARA BIO, Otsu, Japan) with an oligo dT primer. The Clcn1-L minigene fragments were amplified by PCR (24–27 cycles) with the following primer pair: Clcn1-L forward, 5′-CATGGTCCTGCTGGAGTTCGTG-3′; and Clcn-L reverse, 5′-CTCCAAGTGGTGTTCCAAAACAGC-3′. To detect endogenous Clcn1 fragments, PCR amplification (32-34 cycles) was carried out with the following primer pair: Clcn1 forward, 5′-GCTGCTGTCCTCAGCAAGTT-3′; and Clcn1 reverse, 5′-CTGAATGTGGCTGCAAAGAA-3′. PCR products were resolved on 8% polyacrylamide gels and stained with ethidium bromide. Band intensities were quantified using an LAS-3000 instrument and Multigauge software (FUJIFILM, Tokyo, Japan). The ratio of exon 7A inclusion in Clcn1 was calculated as (7A inclusion)/(7A inclusion + 7A exclusion) × 100.
RNA interference
An siRNA specific for H-Ras (H-Ras siRNA) and a negative control siRNA (MISSION siRNA Universal Negative Control) were purchased from Sigma-Aldrich. The siRNA target sequences were as follows: mouse H-Ras siRNA sense, 5′- GUUGCAUCACAGUAAAUUAdTdT-3′; and mouse H-Ras siRNA antisense, 5′- UAAUUUACUGUGAUGCAACdTdT-3′. The efficacy of the RNAi-mediated knockdown of endogenous H-Ras and actin expression was determined by Western blot analysis. Antibodies specific for H-Ras (C-20; Santa Cruz Biotechnology, Santa Cruz, CA, USA,) and actin (A2066; Sigma-Aldrich) were used.
Author Contributions
K.O., Y.O. and S.I. designed the experiments; K.O. performed all experiments; Y.O. assisted with the construction of the reporter vector; S.S. assisted with the interpretation of the results; M.P.T. prepared the mice; S.T. prepared the small chemical compound library; N.I. supported the project; S.I. supervised the work; and K.O. analyzed the data and wrote the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Prof. Charles A. Thornton (University of Rochester) for providing us HSALR mice. We also thank Dr. Yoshihiro Kino for providing us the Clcn1 minigenes, DM18 and DM480 constructs. This work was supported by an intramural research grants (23–5) for Neurological and Psychiatric Disorders of NCNP from the Ministry of Health, Labour and Welfare, Japan, and by the Uesugi Foundation.
References
- Harper P. S. Myotonic Dystophy, third edn. (2001). [Google Scholar]
- Ranum L. P. & Cooper T. A. RNA-mediated neuromuscular disorders. Annu Rev Neurosci 29, 259–277 (2006). [DOI] [PubMed] [Google Scholar]
- Aslanidis C. et al. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature 355, (1992). [DOI] [PubMed] [Google Scholar]
- Brook J. D. et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell 69, 385 (1992). [DOI] [PubMed] [Google Scholar]
- Liquori C. L. et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 293, 864–867 (2001). [DOI] [PubMed] [Google Scholar]
- Machuca-Tzili L., Brook D. & Hilton-Jones D. Clinical and molecular aspects of the myotonic dystrophies: a review. Muscle Nerve 32, 1–18 (2005). [DOI] [PubMed] [Google Scholar]
- Mankodi A. et al. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol Cell 10, 35–44 (2002). [DOI] [PubMed] [Google Scholar]
- Taneja K. L., McCurrach M., Schalling M., Housman D. & Singer R. H. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J Cell Biol 128, 995–1002 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardaei M. et al. Three proteins, MBNL, MBLL and MBXL, co-localize in vivo with nuclear foci of expanded-repeat transcripts in DM1 and DM2 cells. Hum Mol Genet 11, 805–814 (2002). [DOI] [PubMed] [Google Scholar]
- Kino Y. et al. Muscleblind protein, MBNL1/EXP, binds specifically to CHHG repeats. Hum Mol Genet 13, 495–507 (2004). [DOI] [PubMed] [Google Scholar]
- Miller J. W. et al. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J 19, 4439–4448 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timchenko L. T. et al. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res 24, 4407–4414 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I. et al. Muscleblind-like proteins: similarities and differences in normal and myotonic dystrophy muscle. Am J Pathol 174, 216–227 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu-Martinez N. M., Wang G. S. & Cooper T. A. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell 28, 68–78 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebis M. et al. Alternative splicing of myomesin 1 gene is aberrantly regulated in myotonic dystrophy type 1. Genes Cells 16, 961–972 (2011). [DOI] [PubMed] [Google Scholar]
- Ohsawa N., Koebis M., Suo S., Nishino I. & Ishiura S. Alternative splicing of PDLIM3/ALP, for α-actinin-associated LIM protein 3, is aberrant in persons with myotonic dystrophy. Biochem Biophys Res Commun 409, 64–69 (2011). [DOI] [PubMed] [Google Scholar]
- Gomes-Pereira M., Cooper T. A. & Gourdon G. Myotonic dystrophy mouse models: towards rational therapy development. Trends Mol Med 17, 506–517 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet-B N. et al. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol Cell 10, 45–53 (2002). [DOI] [PubMed] [Google Scholar]
- Fugier C. et al. Misregulated alternative splicing of BIN1 is associated with T tubule alterations and muscle weakness in myotonic dystrophy. Nat Med 17, 720–725 (2011). [DOI] [PubMed] [Google Scholar]
- Savkur R. S., Philips A. V. & Cooper T. A. Aberrant regulation of insulin receptor alternative splicing is associated with insulin resistance in myotonic dystrophy. Nat Genet 29, 40–47 (2001). [DOI] [PubMed] [Google Scholar]
- Berg J., Jiang H., Thornton C. A. & Cannon S. C. Truncated ClC-1 mRNA in myotonic dystrophy exerts a dominant-negative effect on the Cl current. Neurology 63, 2371–2375 (2004). [DOI] [PubMed] [Google Scholar]
- Kino Y. et al. MBNL and CELF proteins regulate alternative splicing of the skeletal muscle chloride channel CLCN1. Nucleic Acids Res 37, 6477–6490 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanadia R. N. et al. Reversal of RNA missplicing and myotonia after muscleblind overexpression in a mouse poly(CUG) model for myotonic dystrophy. Proc Natl Acad Sci U S A 103, 11748–11753 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois M. A. et al. Cytoplasmic and nuclear retained DMPK mRNAs are targets for RNA interference in myotonic dystrophy cells. J Biol Chem 280, 16949–16954 (2005). [DOI] [PubMed] [Google Scholar]
- Wheeler T. M. et al. Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science 325, 336–339 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warf M. B., Nakamori M., Matthys C. M., Thornton C. A. & Berglund J. A. Pentamidine reverses the splicing defects associated with myotonic dystrophy. Proc Natl Acad Sci U S A 106, 18551–18556 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. E., Bennett C. F. & Cooper T. A. RNase H-mediated degradation of toxic RNA in myotonic dystrophy type 1. Proc Natl Acad Sci U S A 109, 4221–4226 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. S. et al. PKC inhibition ameliorates the cardiac phenotype in a mouse model of myotonic dystrophy type 1. J Clin Invest 119, 3797–3806 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler I., Thiericke R. & Zeeck A. The manumycin-group metabolites. Nat Prod Rep 15, 221–240 (1998). [DOI] [PubMed] [Google Scholar]
- Hara M. et al. Identification of Ras farnesyltransferase inhibitors by microbial screening. Proc Natl Acad Sci U S A 90, 2281–2285 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb M. H. Protein prenylation, et cetera: signal transduction in two dimensions. Science 275, 1750–1751 (1997). [DOI] [PubMed] [Google Scholar]
- Glomset J. A. & Farnsworth C. C. Role of protein modification reactions in programming interactions between ras-related GTPases and cell membranes. Annu Rev Cell Biol 10, 181–205 (1994). [DOI] [PubMed] [Google Scholar]
- Mumby S. M. Reversible palmitoylation of signaling proteins. Curr Opin Cell Biol 9, 148–154 (1997). [DOI] [PubMed] [Google Scholar]
- Scheffzek K. et al. The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277, 333–338 (1997). [DOI] [PubMed] [Google Scholar]
- Fiordalisi J. J. et al. High affinity for farnesyltransferase and alternative prenylation contribute individually to K-Ras4B resistance to farnesyltransferase inhibitors. J Biol Chem 278, 41718–41727 (2003). [DOI] [PubMed] [Google Scholar]
- Kimura T. et al. Altered mRNA splicing of the skeletal muscle ryanodine receptor and sarcoplasmic/endoplasmic reticulum Ca2+-ATPase in myotonic dystrophy type 1. Hum Mol Genet 14, 2189–2200 (2005). [DOI] [PubMed] [Google Scholar]
- Lin X. et al. Failure of MBNL1-dependent post-natal splicing transitions in myotonic dystrophy. Hum Mol Genet 15, 2087–2097 (2006). [DOI] [PubMed] [Google Scholar]
- Hino S. et al. Molecular mechanisms responsible for aberrant splicing of SERCA1 in myotonic dystrophy type 1. Hum Mol Genet 16, 2834–2843 (2007). [DOI] [PubMed] [Google Scholar]
- Ward A. J., Rimer M., Killian J. M., Dowling J. J. & Cooper T. A. CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1. Hum Mol Genet 19, 3614–3622 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. F. & Marais R. Ras protein signalling. Semin Immunol 12, 63–73 (2000). [DOI] [PubMed] [Google Scholar]
- Blaustein M., Pelisch F. & Srebrow A. Signals, pathways and splicing regulation. Int J Biochem Cell Biol 39, 2031–2048 (2007). [DOI] [PubMed] [Google Scholar]
- Blaustein M. et al. Concerted regulation of nuclear and cytoplasmic activities of SR proteins by AKT. Nat Struct Mol Biol 12, 1037–1044 (2005). [DOI] [PubMed] [Google Scholar]
- Weg-Remers S., Ponta H., Herrlich P. & König H. Regulation of alternative pre-mRNA splicing by the ERK MAP-kinase pathway. EMBO J 20, 4194–4203 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. et al. Proto-oncogenic H-Ras, K-Ras, and N-Ras are involved in muscle differentiation via phosphatidylinositol 3-kinase. Cell Res 20, 919–934 (2010). [DOI] [PubMed] [Google Scholar]
- Mankodi A. et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science 289, 1769–1773 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information