Abstract
The tumor microenvironment is a complex milieu of tumor and host cells. Host cells can include tumor-reactive T cells capable of killing tumor cells. However, more frequently the tumor and host components interact to generate a highly immune suppressive environment that frustrates T cell cytotoxicity and promotes tumor progression through a variety of immune and non-immune mechanisms. Myeloid-derived suppressor cells (MDSC) are a major host component contributing to the immune suppressive environment. In addition to their inherent immune suppressive function, MDSC amplify the immune suppressive activity of macrophages and dendritic cells via cross-talk. This article will review the cell–cell interactions used by MDSC to inhibit anti-tumor immunity and promote progression, and the role of inflammation in promoting cross-talk between MDSC and other cells in the tumor microenvironment.
Keywords: Tumor immunity, Immune escape, Tumor microenvironment, Tumor-associated macrophages
The past decade has seen a large expansion in the number of experimental cancer immunotherapies being tested in clinical trials. Despite isolated responses in some patients, most of these trials have shown minimal impact on the clinical status of the majority of patients. The exceptions have been trials focused on reducing immune suppressive mechanisms that are present in most cancer patients, and include treatment with the mAbs ipilimumab and MDX-1106 which inhibit Cytotoxic T Lymphocyte Activation- 4 (CTLA-4) [1,2] and Programmed Death-1 (PD-1) [3] inhibitory molecules on T cells, respectively. In conjunction with extensive animal data showing that immune suppression is wide-spread in cancer patients, these clinical trials indicate that successful cancer immunotherapy will necessitate decreasing/eliminating immune suppressive mechanisms. In addition to suppression via molecules such as CTLA-4 and PD-1, myeloid lineage cells constitute a network of immune suppressive cells that are present in most cancer patients and which profoundly inhibit the generation of anti-tumor immunity. This network includes myeloid-derived suppressor cells (MDSC), tumor-associated macrophages (TAMS), and dendritic cells (DC). Each of these cell populations has inherent immune suppressive activity which is enhanced through their interactions with each other. These interactions can be either one-way interactions or mutually beneficial interactions (cross-talk) that are often exacerbated by inflammation in the tumor microenvironment. After a brief description of each of these cell populations, this article will focus on how interactions and cross-talk between the various myeloid cell populations enhance immune suppression and promote tumor growth.
1. Myeloid-derived suppressor cells (MDSC)
MDSC are immune suppressive immature myeloid cells that are elevated in virtually all patients and experimental mice with malignancies. MDSC include two major subpopulations of cells: monocytic and granulocytic (polymorphonuclear) MDSC, as defined by their expression of plasma membrane markers and their content of immune suppressive molecules. They enhance tumor growth through both non-immune and immune suppressive mechanisms. Their principle non-immune mechanism is the promotion of angiogenesis [4]. They inhibit both adaptive and innate anti-tumor immunity through a variety of diverse mechanisms. (i) MDSC inhibit T cell activation and function by producing reactive oxygen and nitrogen species (ROS and RNS) which down-regulate or dissociate the CD3-associated ζ chain from the T cell receptor (TcR) [5,6], by disrupting signaling through the IL-2 receptor [7], and by preventing antigen/MHC peptide recognition by nitrating the TcR [8] and MHC class I [9] molecules. (ii) MDSC deplete their environment of arginine [10] and l-cysteine [11], amino acids required for T cell activation and proliferation. (iii) MDSC disrupt T cell migration to lymph nodes by releasing ADAM 17 which downregulates the homing receptor CD62L (l-selectin) on T cells [12] and they inhibit intratumoral migration of effector CD8+ T cells by peroxynitrite modification of the chemoattractant CCL2 [13]. (iv) MDSC promote the expansion of natural T regulatory cells and drive the development of induced T regulatory cells through their production of IL-10, TGFβ, IFNγ and through CD40–CD40L interactions [14–16].
In addition to these mechanisms which directly affect CD4+ and CD8+ T cells, MDSC also use diverse mechanisms to suppress innate immunity. They inhibit natural killer cell cytotoxicity and inhibit NK cell production of IFNγ through a cell-contact-dependent mechanism [17–20] that involves membrane-bound TGFβ [17] or recognition of the NK cell receptors NKp30 [21] or NKG2D [17]. Innate anti-tumor immunity is also impacted by the effects of NKT cells on MDSC. There are two types of NKT cells: NKT I or inducible NKT (iNKT) cells facilitate tumor rejection [22] while NKT II cells promote tumor growth [23]. IL-13 production by NKT II cells increases the accumulation of MDSC [23,24].
2. Tumor-associated macrophages
In healthy individuals macrophages are key cells that promote host survival by regulating adaptive immunity, promoting wound healing, and eliminating infectious agents (reviewed in [25]). Similar to MDSC, macrophages are a diverse population of myeloid cells and facilitate tumor progression via both immunological and non-immunological mechanisms. They form a continuous spectrum of cells that range in phenotype from M1-like or classically activated macrophages to M2-like or alternatively-activated macrophages [26]. M1-like macrophages are typically activated by IFNγ and lipopolysaccharide, and are characterized by their high expression of IL-12 and low expression of IL-10. This cytokine profile promotes the development of a type 1 T cell response which facilitates anti-tumor immunity. In addition, M1-like macrophages can be tumoricidal. In contrast, M2-like macrophages are activated by IL-4, IL-13, IL-10, and glucocorticoid hormones, produce high levels of IL-10 and low levels of IL-12, and promote tumor progression. Recent studies have identified up to seven phenotypically distinct macrophage subpopulations within tumors, demonstrating the complexity of this cell population [27]. Macrophage phenotype is driven by their local tissue microenvironment and the tumor microenvironment strongly polarizes macrophages towards an M2-like phenotype, giving rise to so-called “tumor associated macrophages” (TAMs). In addition to their immune suppressive activity, TAMs also promote tumor progression through multiple non-immune mechanisms including facilitating angiogenesis [28], promoting tumor cell invasion and metastasis [29], and protecting tumor cells from chemotherapy-induced apoptosis [30].
3. Dendritic cells
The major function of dendritic cells (DC) is to process and present antigen for the activation of CD4+ and CD8+ T cells. Endocytosis of antigen by immature DC drives DC maturation and the subsequent presentation of antigen to T cells. However, the tumor microenvironment systemically perturbs this process by increasing the accumulation of immature DC and decreasing DC maturation [31]. As a result, DC fail to activate tumor-reactive T cells and/or become tolerogenic. Defective dendritic cell function has been found in many patients with a variety of cancers, as well as in most mice with transplanted or spontaneous tumors [31–37]. Multiple conditions and factors within the tumor microenvironment, including hypoxia, lactic acid build-up, and adenosine accumulation, cause DC abnormalities (reviewed in [38]). As discussed below, many of these same conditions also foster cross-talk between the myeloid cell populations.
4. Bidirectional cross-talk between MDSC and macrophages exacerbates immune suppression
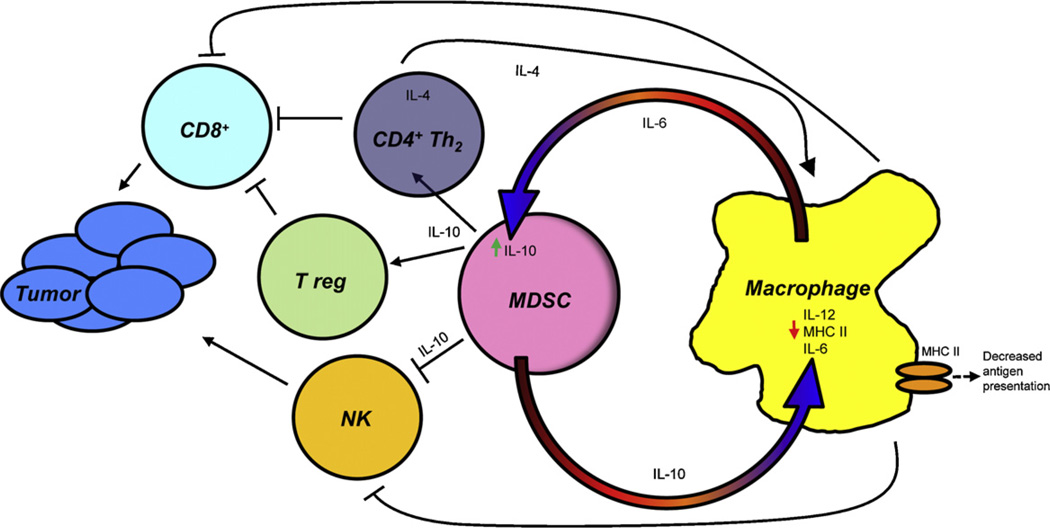
In individuals with tumors, the accumulation and suppressive activity of MDSC and TAMs is initiated by factors produced by tumor cells. Many of these factors act directly on MDSC and TAMs. However, interactions between MDSC and macrophages further exacerbate suppression by these cells by altering cytokine production and expression of cell surface molecules important for cellular function (Fig. 1).
Fig. 1.
Cross-talk between MDSC and macrophages polarizes immunity towards a type 2 response that promotes tumor progression. MDSC and macrophages interact with each other through a variety of soluble mediates and cell contact-dependent mechanisms that enhance the suppressive activity of each cell type. Cross-talk results in increased production of IL-10, decreased production of IL-12 and IL-6, and down-regulation of macrophage MHC II. The down-stream effects are the activation of CD4+ Th2 and T regulatory cells and decreased antigen presentation which impair cytotoxic CD8+ T cell activity and impairment of NK cell cytotoxicity. Green arrows indicate molecules that are increased; small red arrows indicate molecules that are decreased; large orange/red semi-circular arrows indicate donor to recipient cell effects.
Tumoricidal M1-like macrophages have a phenotype of IL-12hiIL-10lo and are activated by LPS and IFNγ. However, co-culture of LPS and IFNγ-activated peritoneal macrophages with tumor-induced MDSC for 16 h down-regulates macrophage production of IL-12 by >80% [39]. Similar to MDSC suppression of T cell activation [7,40,41], MDSC-mediated down-regulation of IL-12 requires MDSC–macrophage cell contact. MDSC produce high levels of IL-10 and IL-10 is a key cytokine for regulating IL-12 transcription [42]. Co-cultures of tumor-induced MDSC from IL-10-deficient mice with wild type macrophages confirmed that MDSC production of IL-10 mediated IL-12 down-regulation. Interestingly, macrophages themselves exacerbate their polarization towards an M2 phenotype because they stimulate MDSC to synthesize more IL-10 [39].
Increased MDSC production of IL-10 and decreased macrophage production of IL-12 are also likely to impact CD4+ T cells and natural killer (NK) cells. IL-10 drives the differentiation of type 2 CD4+ T (Th2) cells, while IL-12 induces differentiation of type 1 CD4+ T (Th1) cells and NK cells. Increased production of IL-10 and the absence of IL-12 therefore favor the development of Th2 cells and a decrease in NK cells. Th2 cells counter-act the development of cytotoxic CD8+ T cells (CTL). They also produce IL-4 which contributes to the development of tumor-associated macrophages [43]. Because IL-10 is also a potent inducer of CD4+ T regulatory cells (Tregs) [44], MDSC-produced IL-10 is also likely to facilitate the development of Tregs.
MDSC and macrophage bidirectional cross-talk also alters macrophage expression of MHC class II molecules. Although not as efficient as DC, macrophages are effective antigen presenting cells and cross-prime and cross-present both MHC class I and MHC class II-restricted peptides. Co-culture studies with tumor-induced mouse MDSC and macrophages demonstrated that MDSC significantly reduced macrophage expression of MHC II molecules. MHC II down-regulation required MDSC–macrophage cell-to-cell contact and experiments with IL-10-deficient MDSC indicated that the reduction was mediated by IL-10 produced by MDSC (Clements and Ostrand-Rosenberg, unpublished results). IL-10 may downregulate MHC II by increasing transcription of March 1, a ligase that ubiquitinates the cytoplasmic tail of MHC II molecules in monocytes [45–47]. The net result is that macrophages are less effective antigen presenting cells, further diminishing T cell activation and enhancing immune suppression/tolerance.
Studies with human monocytes/macrophages and T cells from mice with Leishmania donovani have demonstrated that IL-10 and IL-12 are at least partially regulated through the phosphatidylinositol-3-kinase (PI3K) – mammaliam target of rapamycin (mTOR) pathway. In these reports, rapamycin, an inhibitor of the mTOR pathway, reduced IL-10 production in macrophages [48] and in Th2 CD4+ T cells [49], and increased IL-12 production in Th2 CD4+ T cells [49]. These findings raised the possibility that rapamycin may inhibit macrophage-MDSC cross-talk and reduce MDSC production of IL-10 and restore macrophage production of IL-12. MDSC–macrophage co-cultures treated with rapamycin produce less IL-10 and more IL-12. However, rapamycin is only effective if both MDSC and macrophages are present, indicating that the drug is not acting directly on MDSC to inhibit IL-10 production or directly on macrophages to promote IL-12 production (Clements and Ostrand-Rosenberg, unpublished results). These findings suggest that rapamycin or other mTOR inhibitors, may be useful therapeutic agents to diminish MDSC–macrophage crosstalk.
The bidirectional nature of MDSC–macrophage interactions significantly amplifies the levels of IL-10 and decreases the levels of IL-12. Therefore, in the tumor microenvironment where both MDSC and macrophage co-exist, IL-10 and IL-12 levels are most likely dramatically increased or decreased, respectively, relative to the cytokine level of either cell population by itself. As a result, MDSC–macrophage bidirectional cross-talk has the potential to further enhance immune suppression.
5. Inflammation exacerbates bidirectional cross-talk between MDSC and macrophages
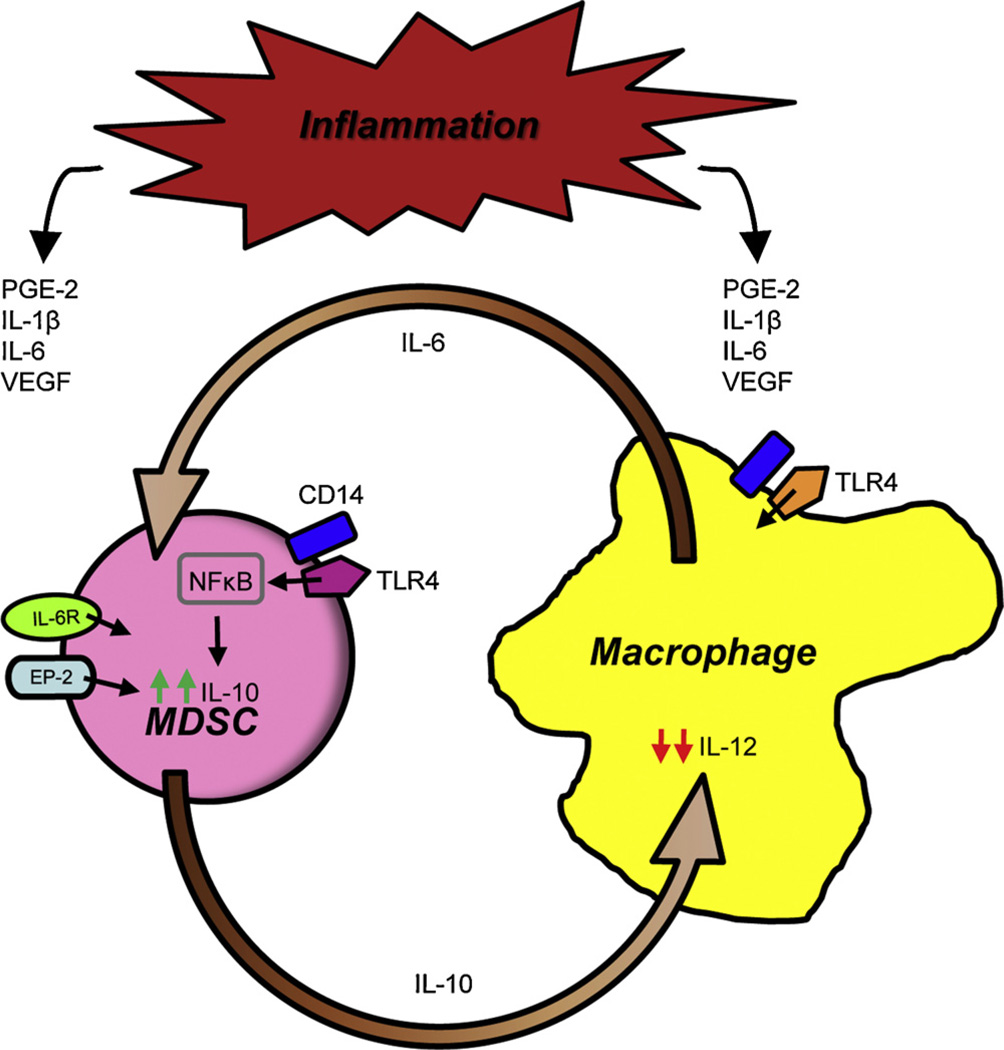
The accumulation of MDSC as well as the immune suppressive mechanisms used by MDSC are exacerbated by chronic inflammation [50–53], and inflammation also increases cross-talk between MDSC and macrophages (Fig. 2). The effect of inflammation on MDSC–macrophage cross-talk was demonstrated using two approaches to increase the inflammatory milieu. In one approach, tumor cells were transfected with the gene encoding IL-1β so the tumor microenvironment contained heightened levels of IL-1β which is upstream of many additional pro-inflammatory mediators. In a second approach MDSC were generated in IL-1 receptor antagonist-deficient (IL-1Ra−/−) mice. In the absence of IL-1Ra, mice cannot attenuate IL-1β signaling and therefore have heightened inflammation. MDSC induced under conditions of high IL-1β (“inflammatory” MDSC) synthesize more IL-10 than MDSC induced in less inflammatory settings (“conventional” MDSC), and the presence of macrophages further increases the production of IL-10 by inflammatory MDSC [54]. This increase in IL-10 is due to macrophage production of IL-6 since co-cultures of MDSC and IL-6-deficient macrophages contain less IL-10 than co-cultures of MDSC and wild type macrophages (Beury and Ostrand-Rosenberg, unpublished results). Since IL-1β is a key regulator of IL-6 [55], IL-1β most likely increases MDSC production of IL-10 by increasing macrophage and MDSC synthesis of IL-6 which in turn increases MDSC production of IL-10.
Fig. 2.
Inflammation enhances MDSC–macrophage cross-talk. Tumor and stromal cells within the tumor microenvironment secrete a variety of inflammatory mediators. For example, tumor cells produce PGE2 which activates MDSC through the EP receptors and COX2 which promotes the production of PGE2 from arachidonic acid. Tumor cells and MDSC produce VEGF, IL-6 and S100A8/A9 proteins which bind to their respective receptors on MDSC, as well as IL-1β which acts via IL-6. These mediators accentuate the cross-talk between MDSC and macrophages and further increase MDSC production of IL-10 and decrease macrophage production of IL-12. Inflammation-increased MDSC production of IL-10 is TLR4-dependent and involves up-regulation of CD14.
In addition to IL-1β, pro-inflammatory bioactive lipids also increase MDSC–macrophage cross-talk to promote immune suppression. Prostaglandin E2 (PGE2), a product of arachidonic acid metabolism, binds to all four prostanoid receptors (EP-1, -2, -3, and -4) and Butaprost, a PGE2 analogue that only binds to EP2, both drive the differentiation of MDSC from c-kit+ hematopoietic progenitor cells [56]. PGE2 and Butaprost also increase MDSC production of IL-10 in the presence of macrophages. In contrast to the effects of IL-1β, this cross-talk-mediated increase in IL-10 does not require MDSC–macrophage cell-to-cell contact, indicating that strictly soluble factors are responsible (Clements and Ostrand-Rosenberg, unpublished data).
At a mechanistic level, the increase in IL-10 is mediated by signaling through MDSC-expressed TLR4 because MDSC from TLR4-deficient mice do not have higher levels of IL-10. Interestingly, macrophage-induced up-regulation of IL-10 by MDSC also requires signaling through TLR4 on macrophages since TLR4-deficient macrophages are unable to increase MDSC production of IL-10.
The MDSC–macrophage cross-talk experiments described above were performed in the presence of lipopolysaccharide (LPS), a known activator of macrophages. Signaling through TLR4 typically involves the binding of LPS to the LPS binding protein, which subsequently transfers LPS to the membrane-bound receptor CD14. CD14 then complexes with TLR4 to initiate TLR4 signaling and down-stream activation of NFκB [57]. CD14 levels are increased on inflammatory MDSC during cross-talk with macrophages and this increase is TLR4-dependent because TLR4-deficient inflammatory MDSC do not display elevated levels of CD14 [54]. Therefore, inflammation most likely increases CD14 levels which may make MDSC more responsive to LPS and other TLR4 activating ligands, thereby driving MDSC production of IL-10 and the resulting in immune suppression.
As a result of their increased production of IL-10, inflammatory MDSC are significantly more efficient at down-regulating macrophage production of IL-12. These findings demonstrate a direct role of inflammation in promoting M2 polarization of macrophages and thereby promoting immune evasion.
6. Inflammation increases MDSC–NK cell cross-talk
In addition to their cross-talk with other myeloid cells, MDSC also impact NK cells and reduce their suppressive activity [18], and inflammation increases these effects in a unidirectional fashion [20]. NK cell differentiation is characterized by the expression of CD27 on immature NK cells and increasing expression of CD11b and KLRG-1 as NK cells mature [58]. Inflammation, via IL-1β, decreases the levels of CD27 on immature CD27+ NK cells in the bone marrow, and eliminates CD11b+KLRG-1+ NK cells in the spleen. Inflammation also decreases NK cell expression of the NK cell activating receptor NKG2D, presumably making it more difficult to activate NK cells, and reduces NK cytotoxic activity. These effects are mediated by a Ly6Clow subpopulation of granulocytic MDSC which are preferentially expanded by IL-1β [20].
In contrast to the Ly6Clow MDSC population of the previous paragraph, monocytic MDSC have been reported to express the NK activating ligand Rae1 and activate NK cells through the NK receptor NKG2D. NK cells activated by MDSC subsequently kill MDSC [59]. This report and that of [20] each used only one tumor. Therefore, not enough studies have been completed using different tumor systems to determine if NK cell suppression and activation are characteristic of granulocytic Ly6Clow and monocytic MDSC, respectively, or if these findings are limited to the MDSC induced by the particular tumors used in these reports.
7. MDSC–macrophage cross-talk reduces inflammation
Within the tumor microenvironment tumor cells and stromal cells, including MDSC and macrophages, generate a proinflammatory environment. Different tumor cells produce a variety of pro-inflammatory mediators including prostaglandins, cyclooxygenases, IL-6, TNFα, as well as many other mediators [60]. Although inflammation drives MDSC accumulation and suppressive potency [53], and MDSC themselves produce inflammatory mediators [61,62], MDSC also reduce inflammation through their production of the anti-inflammatory cytokine IL-10. MDSC further decrease inflammation by down-regulating macrophage production of the pro-inflammatory cytokine IL-6. This down-regulation requires MDSC–macrophage cell-to-cell contact, and is enhanced by rapamycin treatment (Beury, Clements, and Ostrand-Rosenberg, unpublished results), consistent with the concept that IL-6 is at least partially regulated by mTOR [63].
The ability of MDSC–macrophage cross-talk to both promote and reduce inflammation may at first appear to be contradictory. However, these dual functions may exist as mechanisms to homeostatically balance the tumor microenvironment. Chronic inflammation is needed to promote the development of MDSC and TAMs; however, acute inflammation activates adaptive T cell immunity with the potential to mediate tumor rejection. By modulating the inflammatory tumor microenvironment, MDSC–macrophage cross-talk reduces the opportunity to activate tumor-reactive T cells and thereby provides an environment for immune escape and continued tumor progression.
8. MDSC–DC cross-talk contributes to DC dysfunction
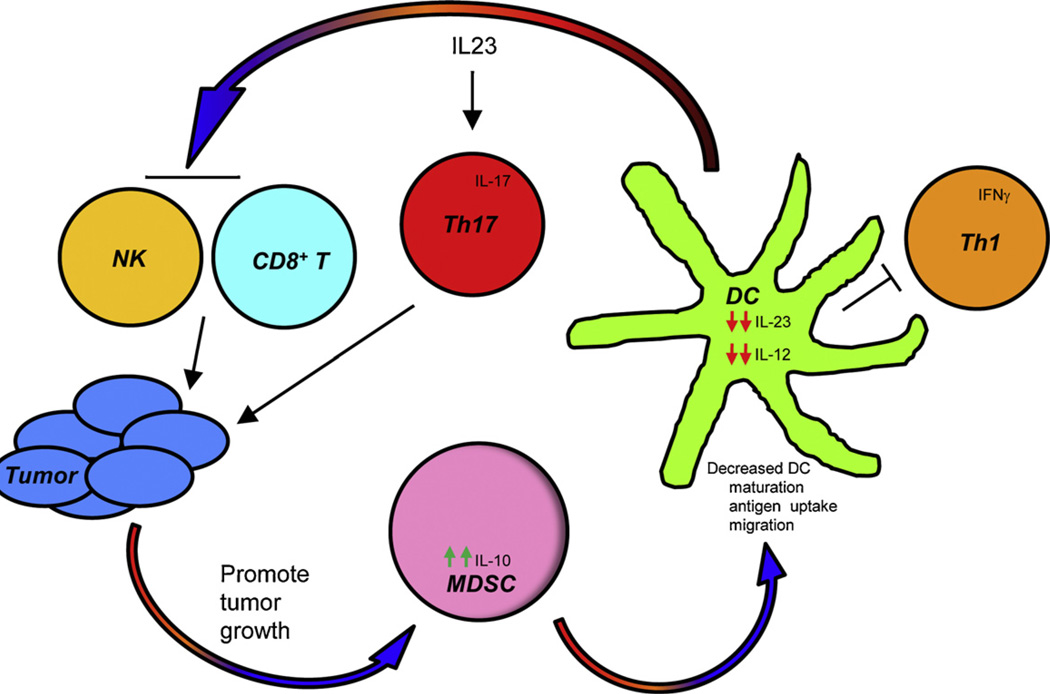
In contrast to MDSC–macrophage interactions, there is less information on cross-talk between MDSC and DC. As discussed above, in many cancer patients the numbers of mature DC are reduced and DC function is deficient. Although multiple factors are likely to contribute to DC dysfunction, evidence is accumulating that MDSC–DC cross-talk may at least be partially responsible. In vitro studies in which mouse MDSC were differentiated from ckit+ bone marrow progenitor cells in the presence of IL-4, GM-CSF, and PGE2 demonstrated that the numbers of mature DC decreased proportionately to the increasing numbers of MDSC [56]. The differentiation of murine DC was similarly reduced when mixtures of murine myeloid cells were treated with LPS and IFNγ [64]. IL-10 produced by hepatocellular carcinoma-induced MDSC has also been shown to decrease DC production of IL-12 [65]. Since MDSC and DC share a common progenitor cell, the reduction in mature DC observed in cancer patients may be due to the skewing of the common MDSC/DC progenitor towards the preferential differentiation of MDSC at the expense of DC (Fig. 3).
Fig. 3.
Cross-talk between MDSC and dendritic cells impairs DC function and promotes tumor progression. Tumor-associated DC produce IL-23 which reduces tumor infiltration of CD8+ T cells and suppresses NK cell cytotoxicity, thereby promoting tumor growth. IL-23 also induces Th17 cells that secrete IL-17 which supports tumor progression. Increased tumor burden facilitates the accumulation of MDSC which in turn decrease DC maturation, antigen uptake, migration, IL-23, IL-12 and T cell IFNγ production, thereby limiting the activation of CD8-mediated anti-tumor immunity.
Recent in vitro studies assessing the effects of MDSC from cancer patients on DC differentiation further support a role for MDSC–DC cross-talk. Studies with MDSC from melanoma patients demonstrated that MDSC impaired DC maturation by reducing antigen uptake, preventing migration of immature and mature DC, blocking the ability of DC to induce IFNγ-producing T cells, and skewing DC cytokine production towards an anti-inflammatory phenotype [66]. DC production of the pro-inflammatory cytokine IL-23 and its downstream induction of Th17 cells may contribute to the effects of MDSC on DC. IL-23 closely resembles IL-12; however, it drives a divergent immunological pathway [67]. Whereas IL-12 induces IFNγ-producing Th1 cells which mediate cytotoxic responses and tumor rejection, IL-23 promotes tumor progression. IL-23 mediates these effects by driving the proliferation and inflammatory function of Th17 cells [68], which suppress immune surveillance and promote metastasis by impacting adaptive [69] and innate [70] immunity. To determine if MDSC impact IL-17 levels and DC production of IL-23, bone marrow-derived DC from healthy mice were co-cultured with MDSC and the DC subsequently sorted and incubated with transgenic CD4+ T cells and cognate peptide. IL-23 (p19/p40) levels were significantly reduced and IL-17 (IL-17A and IL-17AF) levels were modestly reduced in the presence of MDSC-conditioned DC relative to cultures with non-MDSC-conditioned DC (Sinha and Ostrand-Rosenberg, unpublished results). Because IL-23 and IL-17 promote tumor progression, these findings suggest that MDSC may reduce tumor progression by limiting IL-23 and IL-17 production. Therefore, the two studies identify apparently opposing roles for MDSC. These apparent differences could be due to the different species and subpopulations of MDSC used in the two studies since Poschke et al. used monocytic MDSC from melanoma patients and peripheral blood-derived human DC, while our study used murine granulocytic MDSC and bone-marrow-derived murine DC. In addition, Poschke et al. did not assess IL-23 or IL-17 levels. However, these findings may also represent the schizophrenic nature of MDSC as seen in their ability to limit tumor progression by activating NK cells [59]. Further studies are needed to clarify the role of MDSC in regulating DC function, and the complexity of the tumor microenvironment with respect to the milieu of cytokines and chemokines is likely to make this task difficult.
9. Conclusions
The tumor microenvironment includes diverse host cells that are chemoattracted and induced by tumor-produced factors to generate a highly immune suppressive environment. This review has described some of the host cell cross-talk between MDSC, macrophages, and DC that results in suppressing anti-tumor immunity. Because we are just beginning to understand the complexity of the tumor microenvironment, it is likely there are additional interactions that further promote tumor progression through currently unknown immunological and non-immunological mechanisms. Cross-talk between MDSC, macrophages, and DC promotes synergy amongst these cells and thereby amplifies the immune suppressive effects of the individual cell populations. In addition, induction of one population favors the development of the other populations, and chronic inflammation further increases suppressive potency. As a result, MDSC, macrophages, and DC in the tumor microenvironment are inextricably interconnected such that functions of one population are impacted by the quantity of the other populations. This co-dependency benefits the tumor, but also implies that therapies that down-regulate one population may also reduce the immune suppressive activity of other cell populations. This outcome is most likely applicable to MDSC and macrophages since these two myeloid populations directly impact each other in a reciprocal fashion via their production of IL-10 and IL-6, respectively. Reducing MDSC quantity or function is also likely to increase T cell activation by macrophages since macrophage levels of MHC II will be restored. Antigen presentation by DC is also likely to improve since reduced numbers of MDSC will eliminate the competition between MDSC and DC and promote the expansion and maturation of immunocompetent DC. Whether therapies that uniquely target DC will have down-stream effects on MDSC and macrophages remains unclear, since DC have not as yet been shown to impact MDSC or macrophage development or function. Regardless of whether DC affect MDSC and macrophages, future studies should be aimed at developing therapies that interfere with MDSC–macrophage–DC interactions such that the potent synergistic activity of these cells is neutralized and immune competence can be restored.
Acknowledgements
The authors thank Ms. Lakshmi Gorrepati for performing the initial IL-23 experiments and Jonathan Weiss for suggesting the rapamycin and mTOR experiments. Original studies were supported by NIH RO1CA115880, RO1CA84232 (SOR), and American Cancer Society IRG-97-153-07 (PS). DWB is supported by a pre-doctoral fellowship from the DOD Breast Cancer Program (W81XWH-11-1-0115).
References
- 1.Trial watch: ipilimumab success in melanoma provides boost for cancer immunotherapy. Nat Rev Drug Discov. 2010;9:584. doi: 10.1038/nrd3245. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 5.Ezernitchi AV, Vaknin I, Cohen-Daniel L, Levy O, Manaster E, Halabi A, et al. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J Immunol. 2006;177:4763–4772. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- 6.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 8.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest. 2011;121:4015–4029. doi: 10.1172/JCI45862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate l-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–944. doi: 10.4049/jimmunol.0804253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 15.Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, et al. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70:99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 20.Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, et al. IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010;40:3347–3357. doi: 10.1002/eji.201041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stewart TJ, Smyth MJ, Fernando GJ, Frazer IH, Leggatt GR. Inhibition of early tumor growth requires J alpha 18-positive (natural killer T) cells. Cancer Res. 2003;63:3058–3060. [PubMed] [Google Scholar]
- 23.Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med. 2005;202:1627–1633. doi: 10.1084/jem.20051381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 28.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 29.Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, et al. A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS One. 2009;4:e6562. doi: 10.1371/journal.pone.0006562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, Cai Z, Wang S, Zhang X, Qian J, Hong S, et al. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood. 2009;114:3625–3628. doi: 10.1182/blood-2009-05-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 32.Bellone G, Carbone A, Smirne C, Scirelli T, Buffolino A, Novarino A, et al. Cooperative induction of a tolerogenic dendritic cell phenotype by cytokines secreted by pancreatic carcinoma cells. J Immunol. 2006;177:3448–3460. doi: 10.4049/jimmunol.177.5.3448. [DOI] [PubMed] [Google Scholar]
- 33.Lee BN, Follen M, Rodriquez G, Shen DY, Malpica A, Shearer WT, et al. Deficiencies in myeloid antigen-presenting cells in women with cervical squamous intraepithelial lesions. Cancer. 2006;107:999–1007. doi: 10.1002/cncr.22092. [DOI] [PubMed] [Google Scholar]
- 34.Ormandy LA, Farber A, Cantz T, Petrykowska S, Wedemeyer H, Horning M, et al. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J Gastroenterol. 2006;12:3275–3282. doi: 10.3748/wjg.v12.i20.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perrot I, Blanchard D, Freymond N, Isaac S, Guibert B, Pacheco Y, et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763–2769. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 36.Pinzon-Charry A, Ho CS, Maxwell T, McGuckin MA, Schmidt C, Furnival C, et al. Numerical and functional defects of blood dendritic cells in early- and late-stage breast cancer. Br J Cancer. 2007;97:1251–1259. doi: 10.1038/sj.bjc.6604018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinzon-Charry A, Maxwell T, Lopez JA. Dendritic cell dysfunction in cancer: a mechanism for immunosuppression. Immunol Cell Biol. 2005;83:451–461. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 38.Gabrilovich D, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumors. Nat Rev Immunol. doi: 10.1038/nri3175. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 40.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 41.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 42.Cao S, Liu J, Chesi M, Bergsagel PL, Ho IC, Donnelly RP, et al. Differential regulation of IL-12 and IL-10 gene expression in macrophages by the basic leucine zipper transcription factor c-Maf fibrosarcoma. J Immunol. 2002;169:5715–5725. doi: 10.4049/jimmunol.169.10.5715. [DOI] [PubMed] [Google Scholar]
- 43.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thibodeau J, Bourgeois-Daigneault MC, Huppe G, Tremblay J, Aumont A, Houde M, et al. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur J Immunol. 2008;38:1225–1230. doi: 10.1002/eji.200737902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 47.van Niel G, Wubbolts R, Ten Broeke T, Buschow SI, Ossendorp FA, Melief CJ, et al. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25:885–894. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Baker AK, Wang R, Mackman N, Luyendyk JP. Rapamycin enhances LPS induction of tissue factor and tumor necrosis factor-alpha expression in macrophages by reducing IL-10 expression. Mol Immunol. 2009;46:2249–2255. doi: 10.1016/j.molimm.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, et al. Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood. 2008;112:635–643. doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 51.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, et al. CD11b+/Gr− 1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol. 2005;175:8200–8208. doi: 10.4049/jimmunol.175.12.8200. [DOI] [PubMed] [Google Scholar]
- 53.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85:996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem. 2008;283:25900–25912. doi: 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 57.Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165:3541–3544. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- 58.Huntington ND, Vosshenrich CA, Di Santo JP. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol. 2007;7:703–714. doi: 10.1038/nri2154. [DOI] [PubMed] [Google Scholar]
- 59.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived suppressor cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaeffer V, Arbabi S, Garcia IA, Knoll ML, Cuschieri J, Bulger EM, et al. Role of the mTOR pathway in LPS-activated monocytes: influence of hypertonic saline. J Surg Res. 2011;171:769–776. doi: 10.1016/j.jss.2010.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greifenberg V, Ribechini E, Rossner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol. 2009;39:2865–2876. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- 65.Hu CE, Gan J, Zhang RD, Cheng YR, Huang GJ. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol. 2011;46:156–164. doi: 10.3109/00365521.2010.516450. [DOI] [PubMed] [Google Scholar]
- 66.Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother. 2011 doi: 10.1007/s00262-011-1143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 68.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 70.Teng MW, Andrews DM, McLaughlin N, von Scheidt B, Ngiow SF, Moller A, et al. IL-23 suppresses innate immune response independently of IL-17A during carcinogenesis and metastasis. Proc Natl Acad Sci U S A. 2010;107:8328–8333. doi: 10.1073/pnas.1003251107. [DOI] [PMC free article] [PubMed] [Google Scholar]