Abstract
BACKGROUND
Recent studies in animals have shown a mechanistic link between intestinal microbial metabolism of the choline moiety in dietary phosphatidylcholine (lecithin) and coronary artery disease through the production of a proatherosclerotic metabolite, trimethylamine-N-oxide (TMAO). We investigated the relationship among intestinal microbiota-dependent metabolism of dietary phosphatidylcholine, TMAO levels, and adverse cardiovascular events in humans.
METHODS
We quantified plasma and urinary levels of TMAO and plasma choline and betaine levels by means of liquid chromatography and online tandem mass spectrometry after a phosphatidylcholine challenge (ingestion of two hard-boiled eggs and deuterium [d9]-labeled phosphatidylcholine) in healthy participants before and after the suppression of intestinal microbiota with oral broad-spectrum antibiotics. We further examined the relationship between fasting plasma levels of TMAO and incident major adverse cardiovascular events (death, myocardial infarction, or stroke) during 3 years of follow-up in 4007 patients undergoing elective coronary angiography.
RESULTS
Time-dependent increases in levels of both TMAO and its d9 isotopologue, as well as other choline metabolites, were detected after the phosphatidylcholine challenge. Plasma levels of TMAO were markedly suppressed after the administration of antibiotics and then reappeared after withdrawal of antibiotics. Increased plasma levels of TMAO were associated with an increased risk of a major adverse cardiovascular event (hazard ratio for highest vs. lowest TMAO quartile, 2.54; 95% confidence interval, 1.96 to 3.28; P<0.001). An elevated TMAO level predicted an increased risk of major adverse cardiovascular events after adjustment for traditional risk factors (P<0.001), as well as in lower-risk subgroups.
CONCLUSIONS
The production of TMAO from dietary phosphatidylcholine is dependent on metabolism by the intestinal microbiota. Increased TMAO levels are associated with an increased risk of incident major adverse cardiovascular events. (Funded by the National Institutes of Health and others.)
The phospholipid phosphatidylcholine (lecithin) is the major dietary source of choline, a semiessential nutrient that is part of the B-complex vitamin family.1,2Choline has various metabolic roles, ranging from its essential involvement in lipid metabolism and cell-membrane structure to its role as a precursor for the synthesis of the neurotransmitter acetylcholine. Choline and some of its metabolites, such as betaine, can also serve as a source of methyl groups that are required for proper metabolism of certain amino acids, such as homocysteine and methionine.3
There is a growing awareness that intestinal microbial organisms, collectively termed micro-biota, participate in the global metabolism of their host.4–6 We recently described the potential role of a complex phosphatidylcholine–choline metabolic pathway involving gut microbiota in contributing to the pathogenesis of atherosclerotic coronary artery disease in animal models.7 We also reported an association between a history of cardiovascular disease and elevated fasting plasma levels of trimethylamine-N-oxide (TMAO), an intestinal microbiota-dependent metabolite of the choline head group of phosphatidylcholine that is excreted in the urine.7–12
Here, we examine the relationship between oral intake of phosphatidylcholine and the involvement of the intestinal microbiota in the formation of TMAO in humans. We also examine the relationship between fasting plasma levels of TMAO and the long-term risk of incident major adverse cardiovascular events.
METHODS
STUDY DESIGNS
We designed and performed two prospective clinical studies, which were funded by the National Institutes of Health and approved by the institutional review board at the Cleveland Clinic. All participants provided written informed consent. In the first study (phosphatidylcholine challenge), we enrolled 40 healthy adults who had no chronic illnesses (including a known history of heart, renal, pulmonary, or hematologic disease), had no active infections, and had not taken antibiotics or probiotics within the past month. Participants underwent a dietary phosphatidylcholine challenge during visit 1 (as described below). Six of these study participants were then given metronidazole (500 mg twice daily) plus ciprofloxacin (500 mg once daily) for 1 week. After receipt of the antibiotics, a repeat phosphatidylcholine challenge was performed at visit 2. A third and final phosphatidylcholine challenge was performed 1 month or more after the withdrawal of antibiotics (and subsequent reacquisition of gut flora), at visit 3. After each challenge, choline metabolites were measured in plasma and urine, as described below.
In the second study (clinical outcomes), we enrolled 4007 adults who were undergoing elective diagnostic cardiac catheterization; the participants had no evidence of an acute coronary syndrome (cardiac troponin T level, <0.1 µg per liter, if available). A history of cardiovascular disease was defined as a documented history of coronary artery disease, peripheral artery disease, coronary or peripheral revascularization, stenosis of 50% or more in one or more vessels observed during coronary angiography, or a remote history of either myocardial infarction or stroke. Fasting blood samples were obtained from all participants at the time of cardiac catheterization.
LABORATORY TESTING
Routine laboratory tests were performed, and samples were measured on the Abbott Architect platform (Abbott Laboratories), except for testing of myeloperoxidase, which was measured with the use of the CardioMPO test (Cleveland Heart Laboratories). Creatinine clearance was estimated with the use of the Cockcroft–Gault equation. TMAO was measured in plasma, as described below. Major adverse cardiovascular events (defined as death from any cause, nonfatal myocardial infarction, and nonfatal stroke) were ascertained and adjudicated for all participants during 3 years of follow-up.
PHOSPHATIDYLCHOLINE CHALLENGE
A simple dietary phosphatidylcholine–choline challenge was administered to all participants in the first study. For each participant, baseline blood and spot urine samples were obtained after an overnight fast (≥12 hours). At baseline, participants were given two large hard-boiled eggs including yolk (containing approximately 250 mg of total choline each) to be eaten within a 10-minute period together with 250 mg of deuterium-labeled phosphatidylcholine (d9-phosphatidylcholine), as a tracer, contained in a gelatin capsule, which was administered under an investigational new drug exemption (IND number, 107940). Serial venous blood sampling was performed at 1, 2, 3, 4, 6, and 8 hours after baseline, along with a 24-hour urine collection.
The high-purity d9-phosphatidylcholine (>98% isotope enrichment) that was provided was synthesized from 1-palmitoyl-2-palmitoyl-sn-glycero-3-phosphoethanolamine after exhaustive methylation with d3-methyliodide (Cambridge Isotopes Laboratories). The d9-phosphatidylcholine was isolated by means of sequential preparative thin-layer chromatography and high-performance liquid chromatography and was crystallized and dried under vacuum. Its purity (>99%) was confirmed by both multinuclear nuclear magnetic resonance spectroscopy and mass spectrometry.
MEASUREMENTS OF CHOLINE METABOLITES
Plasma aliquots were isolated from whole blood collected in EDTA tubes, maintained at 0 to 4°C until processing within 4 hours and stored at −80°C. An aliquot from each 24-hour urine collection was spun to precipitate any potential cellular debris, and supernatants were stored at −80°C until analysis. TMAO, trimethylamine, choline, betaine, and their d9-isotopologues were quantified with the use of a stable-isotope-dilution assay and high-performance liquid chromatography with on line electrospray ionization tandem mass spectrometry on an AB SCIEX QTRAP 5500 mass spectrometer; d4(1,1,2,2)-choline, d3(methyl)-trimethylamine-N-oxide, and d3(methyl)-trimethylamine were used as internal standards.7 For measurement of trimethylamine in plasma, a sample aliquot was acidified to a final concentration of 60 mM of hydrochloric acid before storage at −80°C. Levels of TMAO in urine were adjusted for urinary dilution by analysis of the urine creatinine level.
STATISTICAL ANALYSIS
In the clinical-outcomes study, we used Student’s t-test and the Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables to analyze the differences between participants who had major adverse cardiovascular events during follow-up and those who did not. For most analyses of outcomes, we divided plasma TMAO levels into quartiles. Where indicated, TMAO was also analyzed as a continuous variable, with the hazard ratio determined per standard-deviation change in the TMAO level. We used Kaplan–Meier analysis with Cox proportional-hazards regression for the time-to-event analysis to determine hazard ratios and 95% confidence intervals for major adverse cardiovascular events.
We performed Cox proportional-hazards regression analysis with adjustment for traditional cardiovascular risk factors (age, sex, systolic blood pressure, presence or absence of diabetes mellitus, low-density and high-density lipoprotein cholesterol levels, triglyceride levels, and smoking status) and for log-transformed high-sensitivity C-reactive protein levels (both alone and with myeloperoxidase), the log-transformed estimated glomerular filtration rate, total leukocyte count, body-mass index, medication history, and angiographic extent of coronary artery disease. For subgroup analyses, Cox proportional-hazards regression analysis was performed with adjustment for traditional cardiovascular risk factors and log-transformed high-sensitivity C-reactive protein levels. Improvement in model performance that was introduced by the inclusion of TMAO levels was evaluated with the use of net reclassification improvement. We calculated the C statistic using the area under the receiver-operating-characteristic (ROC) curve. Three-year predicted probabilities of a major adverse cardiovascular event were estimated from the Cox model. All analyses were performed with the use of R software, versions 2.8.0 and 2.15.1. A two-sided P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
PHOSPHATIDYLCHOLINE CHALLENGE
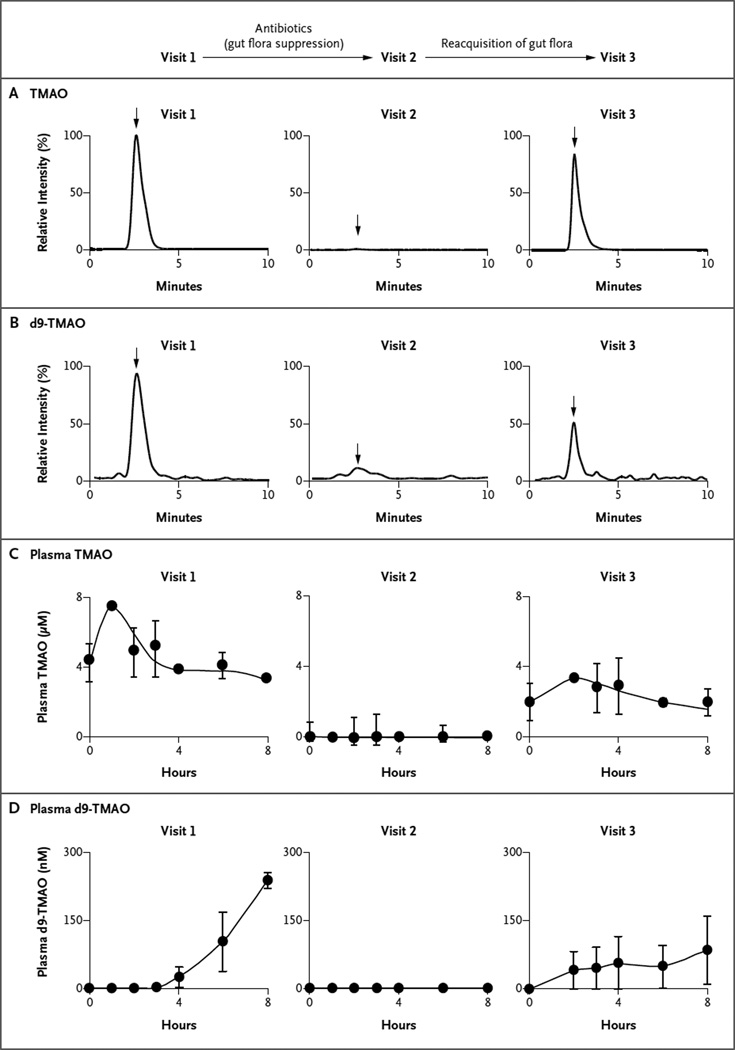
For the 40 participants in the phosphatidylcholine challenge, plasma levels of TMAO are shown in Figure 1, and plasma levels of choline and betaine in Figure S1 in the Supplementary Appendix (available with the full text of this article at NEJM.org). Endogenous (unlabeled) TMAO (Fig. 1C) and choline and betaine (Fig. S1 in the Supplementary Appendix) were present in fasting plasma samples at baseline. Both TMAO and d9-TMAO were readily detected in plasma after the dietary phosphatidylcholine challenge at visit 1 (Fig. 1A and 1B). Time-dependent increases in the levels of both TMAO (Fig. 1C) and d9-TMAO (Fig. 1D) were also observed postprandially. Examination of 24-hour urine specimens after the phosphatidylcholine challenge also showed the presence of both TMAO and d9-TMAO (Fig. S2 in the Supplementary Appendix). A strong correlation was observed between plasma levels of TMAO and the absolute urine TMAO level (Spearman’s r = 0.58, P<0.001) and the urinary ratio of TMAO to creatinine (Spearman’s r = 0.91, P<0.001). Time-dependent increases in the plasma levels of both the natural isotopes and d9-tracer forms of choline and betaine also increased after the phosphatidylcholine challenge (Fig. S1A and S1B in the Supplementary Appendix).
Figure 1. Effects of a Phosphatidylcholine Challenge and Administration of Antibiotics on Mean Levels of Trimethylamine-N-Oxide (TMAO) and Its d9 Isotopologue (d9-TMAO).
All 40 study participants underwent the first dietary phosphatidylcholine challenge (visit 1), which consisted of the ingestion of deuterium-labeled phosphatidylcholine (d9-phosphatidylcholine) and two hard-boiled eggs. Six participants then received broad-spectrum antibiotics for 1 week, followed by a second phosphatidylcholine challenge (visit 2). These same participants returned again at least 1 month after discontinuing the antibiotics for a third challenge (visit 3). Shown are the results of assays for TMAO (Panel A) and d9-TMAO (Panel B) after the phosphatidylcholine challenge, before and after the administration of antibiotics, with the intensity of stable-isotope-dilution assays measured by means of high-performance liquid chromatography with online electro-spray ionization tandem mass spectrometry. The arrows indicate retention time where authentic isotope-labeled TMAO standards elute. Also shown are the plasma levels of TMAO (Panel C) and d9-TMAO (Panel D) at each visit. The plasma levels of TMAO were markedly suppressed after the administration of antibiotics and subsequently reappeared after the cessation of antibiotics, indicating that the production of TMAO from dietary phosphatidylcholine is dependent on metabolism by the intestinal microbiota.
The suppression of intestinal microbiota by the administration of oral broad-spectrum antibiotics for 1 week in six of the participants resulted in near-complete suppression of detectable TMAO and d9-TMAO after the phosphatidylcholine challenge during visit 2 in both plasma (Fig. 1) and urine (Fig. S2 in the Supplementary Appendix). In parallel analyses, postprandial elevations in plasma trimethlyamine and d9-trimethylamine levels were observed after the phosphatidylcholine challenge, but the levels were undetectable after the administration of antibiotics (data not shown). In contrast, the time courses for postprandial changes in free choline and betaine (natural isotopes and d9-isotopologues) were not altered by suppression of intestinal micro-biota (Fig. S1 in the Supplementary Appendix).
After the withdrawal of antibiotics and subsequent reappearance of intestinal microbiota over a period of 1 month or longer, the phosphatidylcholine challenge during visit 3 again resulted in readily detectable and time-dependent changes in TMAO and d9-TMAO in plasma (Fig. 1) and urine (Fig. S2 in the Supplementary Appendix). The extent to which TMAO levels in plasma at visit 3 returned to preantibiotic levels was variable, a finding that is consistent with reports describing variable recovery of intestinal microbiota after antibiotic cessation.13,14
CLINICAL OUTCOMES
TMAO Levels and Cardiovascular Events
The baseline characteristics of the 4007 participants in the clinical-outcomes study are shown in Table 1, according to whether they had a major adverse cardiovascular event during the 3-year follow-up. The mean age of the participants was 63 years, and two thirds were men; the prevalence of cardiovascular risk factors was high, and many of the participants had at least single-vessel coronary disease. Participants who had incident major adverse cardiovascular events during 3 years of follow-up had higher risk profiles at baseline than those without events, including an older age, higher fasting glucose levels, and higher rates of diabetes, hypertension, and previous myocardial infarction.
Table 1.
Baseline Characteristics of the Participants in the Clinical-Outcomes Study, According to Status with Respect to Major Adverse Cardiovascular Events at 3 Years.*
| Characteristic | All Participants (N = 4007) |
Participants without Events (N = 3494) |
Participants with Events (N = 513) |
P Value† |
|---|---|---|---|---|
| Age — yr | 63±11 | 62±11 | 68±10 | <0.001 |
| Male sex — % | 64 | 65 | 62 | 0.16 |
| Median body-mass index (interquartile range)‡ | 28.7 (25.6–32.5) | 28.7 (25.7–32.5) | 28.1 (24.8–32.4) | 0.03 |
| Diabetes mellitus — % | 32 | 30 | 43 | <0.001 |
| Hypertension — % | 72 | 71 | 79 | <0.001 |
| History of myocardial infarction — % | 42 | 40 | 53 | <0.001 |
| No. of coronary vessels with ≥50% stenosis — % | ||||
| 0 | 26 | 28 | 15 | <0.001 |
| 1 | 20 | 21 | 15 | 0.004 |
| 2 | 20 | 20 | 21 | 0.51 |
| 3 | 34 | 32 | 48 | <0.001 |
| Current or former smoker — % | 65 | 65 | 69 | 0.05 |
| Median cholesterol (interquartile range) — mg/dl | ||||
| Low-density lipoprotein | 96 (78–117) | 96 (78–117) | 96 (75–116) | 0.34 |
| High-density lipoprotein | 34 (28–41) | 34 (28–41) | 33 (28–40) | 0.03 |
| Median triglycerides (interquartile range) — mg/dl | 118 (85–170) | 118 (85–169) | 124 (86–173) | 0.52 |
| Median apolipoprotein (interquartile range) — mg/dl | ||||
| B | 82 (69–96) | 82 (69–96) | 82 (68–96) | 0.86 |
| A1 | 116 (103–133) | 117 (103–133) | 114 (100–129) | 0.002 |
| Median fasting glucose (interquartile range) — mg/dl | 102 (93–119) | 102 (92–117) | 106 (94–135) | <0.001 |
| Median high-sensitivity C-reactive protein (interquartile range) — ng/liter |
2.4 (1–5.9) | 2.3 (1–5.5) | 3.9 (1.8–9.8) | <0.001 |
| Median myeloperoxidase (interquartile range) — pM | 115.2 (76.4–245.7) | 113.2 (75.4–238.3) | 136.3 (84.7–329.3) | <0.001 |
| Median estimated glomerular filtration rate (interquartile range) — ml/min/1.73 m2 |
82 (69–95) | 83 (71–96) | 75 (56–89) | <0.001 |
| Median white-cell count (interquartile range) — per mm3 | 6100 (5100–7500) | 6100 (5000–7500) | 6400 (5300–8100) | 0.001 |
| Medications — % | ||||
| Aspirin | 74 | 74 | 70 | 0.04 |
| ACE inhibitor or ARB | 50 | 49 | 58 | <0.001 |
| Statin | 60 | 61 | 56 | 0.06 |
| Beta-blocker | 63 | 63 | 65 | 0.41 |
| Median TMAO (interquartile range) — µM | 3.7 (2.4–6.2) | 3.5 (2.4–5.9) | 5.0 (3.0–8.8) | <0.001 |
Plus–minus values are means ±SD. To convert the values for cholesterol to millimoles per liter, multiply by 0.02586. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. To convert the values for glucose to millimoles per liter, multiply by 0.05551. ACE denotes angiotensin-converting enzyme, ARB angiotensin-receptor blocker, and TMAO trimethylamine-N-oxide.
The P value is for the comparison between patients who had a major adverse cardiovascular event and those who did not.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Participants who had major adverse cardiovascular events also had higher baseline levels of TMAO, as compared with those who did not have cardiovascular events (median, 5.0 µM [interquartile range, 3.0 to 8.8] vs. 3.5 µM [interquartile range, 2.4 to 5.9]; P<0.001) (Table 1). As compared with participants in the lowest quartile of TMAO levels, those in the highest quartile had a significantly increased risk of an event (hazard ratio, 2.54; 95% confidence interval [CI], 1.96 to 3.28; P<0.001) (Table 2).
Table 2.
Risk of a Major Adverse Cardiovascular Event at 3 Years, According to Quartile of TMAO Level.*
| Risk of Event | TMAO Level | ||||||
|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||
| reference | hazard ratio (95% CI) |
P value | hazard ratio (95% CI) |
P value | hazard ratio (95% CI) |
P value | |
| Unadjusted hazard ratio | 1.00 | 1.24 (0.93–1.66) | 0.15 | 1.53 (1.16–2.02) | 0.003 | 2.54 (1.96–3.28) | <0.001 |
| Adjusted hazard ratio | |||||||
| Model 1† | 1.00 | 1.14 (0.86–1.53) | 0.37 | 1.29 (0.98–1.71) | 0.07 | 1.88 (1.44–2.44) | <0.001 |
| Model 2‡ | 1.00 | 1.08 (0.79–1.48) | 0.61 | 1.15 (0.85–1.56) | 0.36 | 1.49 (1.10–2.03) | 0.01 |
| Model 3§ | 1.00 | 1.06 (0.77–1.45) | 0.72 | 1.11 (0.82–1.51) | 0.50 | 1.43 (1.05–1.94) | 0.02 |
A major adverse cardiovascular event was defined as death, myocardial infarction, or stroke. The quartiles of TMAO levels are as follows: quartile 1, less than 2.43 µM; quartile 2 2.43 to 3.66 µM; quartile 3, 3.67 to 6.18 µM; and quartile 4, more than 6.18 µM. Hazard ratios and P values are for the comparison with quartile 1.
In model 1, hazard ratios were adjusted for traditional risk factors (age, sex, smoking status, systolic blood pressure, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, and status with respect to diabetes mellitus), plus log-transformed high-sensitivity C-reactive protein level.
In model 2, hazard ratios were adjusted for all factors in model 1, plus myeloperoxidase level, log-transformed estimated glomerular filtration rate, total white-cell count, body-mass index, and status with respect to receipt of certain medications (aspirin, statin, ACE inhibitor, ARB, or beta-blocker).
In model 3, hazard ratios were adjusted for all factors in model 2, plus the extent of disease, as seen on angiography.
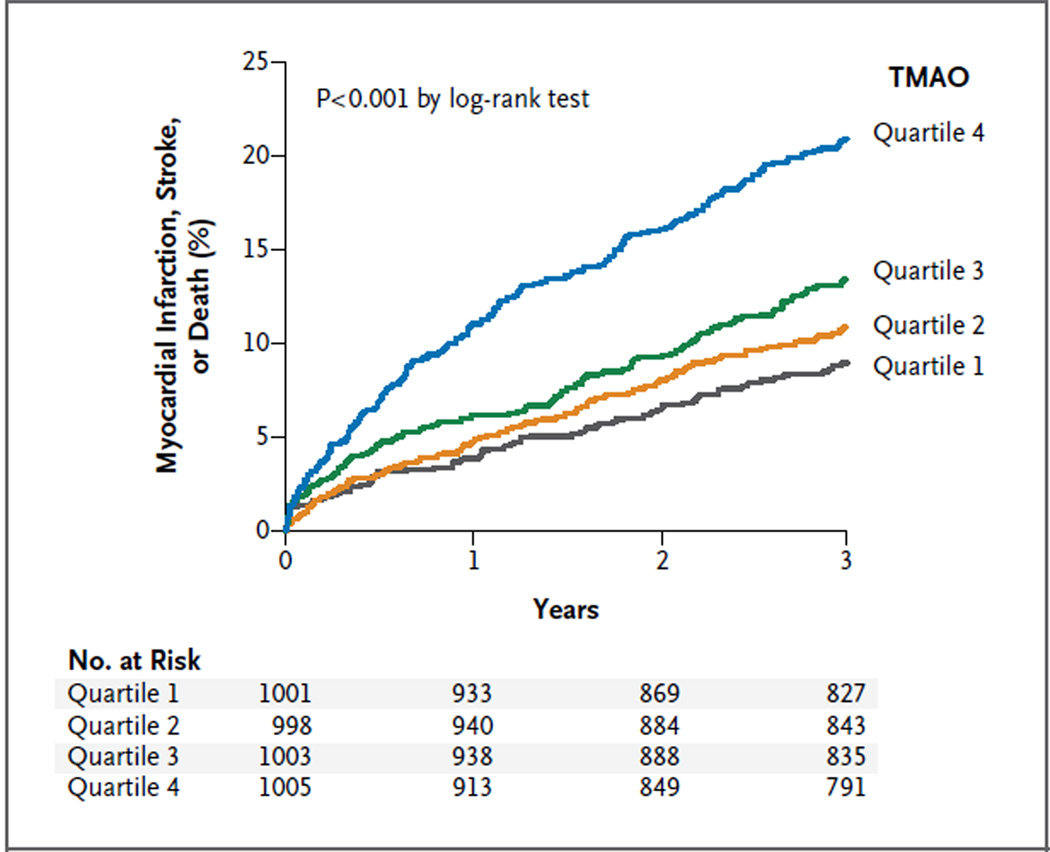
After adjustment for traditional risk factors and other baseline covariates, elevated plasma levels of TMAO remained a significant predictor of the risk of major adverse cardiovascular events (Table 2). We observed a graded increase in risk with increasing levels of TMAO, as illustrated in the Kaplan–Meier analysis shown in Figure 2. A similar graded increase in risk was observed when levels of TMAO were analyzed as a continuous variable in increments of 1 SD (unadjusted hazard ratio, 1.40 [95% CI, 1.29 to 1.51; P<0.001]; adjusted hazard ratio, 1.30 [95% CI, 1.20 to 1.41; P<0.001]).
Figure 2. Kaplan–Meier Estimates of Major Adverse Cardiovascular Events, According to the Quartile of TMAO Level.
Data are shown for 4007 participants in the clinical-outcomes study. The P value is for all comparisons.
When the components of the major adverse cardiovascular events were analyzed separately, increased levels of TMAO remained significantly associated with an increased risk of death (hazard ratio, 3.37; 95% CI, 2.39 to 4.75; P<0.001) and nonfatal myocardial infarction or stroke (hazard ratio, 2.13; 95% CI, 1.48 to 3.05; P<0.001). The inclusion of TMAO as a covariate resulted in a significant improvement in risk estimation over traditional risk factors (net reclassification improvement, 8.6% [P<0.001]; integrated discrimination improvement, 9.2% [P<0.001]; C statistic, 68.3% vs. 66.4% [P = 0.01]). In a separate analysis, we excluded all participants who underwent revascularization within 30 days after enrollment in the study. In this subcohort of 3475 participants, TMAO remained significantly associated with the risk of major adverse cardiovascular events (unadjusted hazard ratio for highest quartile vs. lowest quartile, 2.47 [95% CI, 1.87 to 3.27]; P<0.001).
Cardiovascular Risk in Low-Risk Subgroups
The prognostic value of elevated plasma levels of TMAO for cardiovascular risk remained significant in various subgroups associated with a reduced overall risk of major adverse cardiovascular events (Fig. S3 in the Supplementary Appendix). These subgroups included younger participants (<65 years of age), women, and participants who did not have a known history of coronary artery disease or coronary disease risk equivalents, had low lipid and apolipoprotein levels, had normal blood pressure, did not smoke, and had low levels of other known risk markers, such as C-reactive protein, myeloperoxidase, and white-cell count.
DISCUSSION
Studies in germ-free mice and cross-sectional clinical studies in humans have suggested a role for the intestinal microbiota in the pathogenesis of atherosclerosis in patients with a diet rich in phosphatidylcholine (with major sources including eggs, liver, beef, and pork) through the formation of the metabolite trimethylamine and conversion to TMAO.7,15 In our study, we describe the generation of the proatherogenic metabolite TMAO from dietary phosphatidylcholine through the use of stable-isotope-tracer feeding studies. We further found a role for the intestinal microbiota in the production of TMAO through its suppression by means of oral broad-spectrum antibiotics and then reacquisition of trimethylamine and the production of TMAO from dietary phosphatidylcholine after the withdrawal of antibiotics and subsequent intestinal recolonization. Finally, we describe the potential clinical significance of this intestinal microbiota-dependent metabolite by showing that fasting plasma TMAO levels predict the risk of incident major adverse cardiovascular events independently of traditional cardiovascular risk factors and the presence or extent of coronary artery disease and within multiple low-risk subgroups, including both participants without angiographic evidence of substantial coronary artery disease (i.e., stenosis of <50% in major coronary vessels) and those with low-risk lipid and apolipoprotein levels. Our findings suggest that pathways that are dependent on the intestinal microbiota may contribute to the pathophysiology of atherosclerotic coronary artery disease and suggest potential therapeutic targets.
The intestinal microbiota have previously been implicated in complex metabolic diseases such as obesity.4–6,16–18 However, the involvement of microbiota in the inception of atherosclerosis in humans has only recently been suggested.7 The ability of oral broad-spectrum antibiotics to temporarily suppress the production of TMAO is a direct demonstration that intestinal microorganisms play an obligatory role in the production of TMAO from phosphatidylcholine in humans. Intestinal microbiota convert the choline moiety of dietary phosphatidylcholine into trimethylamine, which is subsequently converted into TMAO by hepatic flavin-containing monooxygenases (Fig. 3).20–23 The observed delay in the detection of plasma d9-TMAO levels after the ingestion of d9-phosphatidylcholine may reflect the time required for the conversion of trimethylamine into TMAO,24 since separate analyses monitoring trimethylamine and d9-trimethylamine production showed a time course consistent with a precursor-to-product relationship (data not shown). TMAO has been identified in fish as an important osmolite,25 and the ingestion of fish raises urinary TMAO levels. Nevertheless, the high correlation between urine and plasma levels of TMAO argues for effective urinary clearance of TMAO. Hence, an efficient excretion mechanism may provide protection by preventing the accumulation of TMAO and does not undermine the mechanistic link between TMAO and cardiovascular risk.
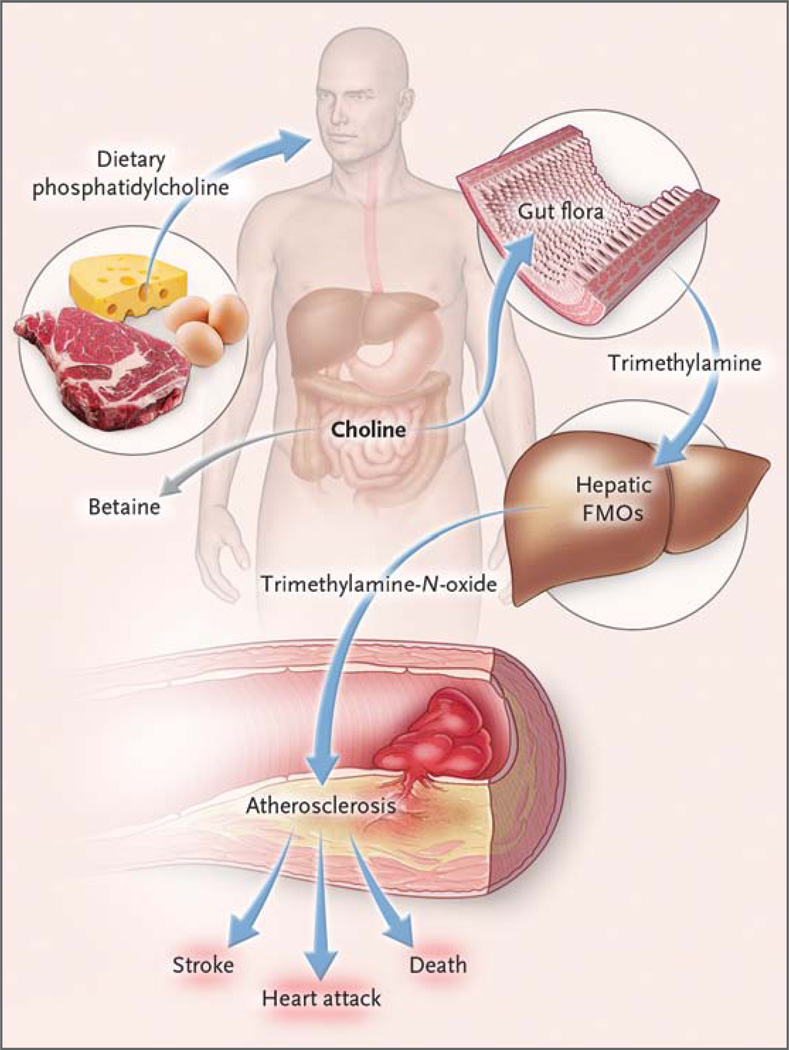
Figure 3. Pathways Linking Dietary Phosphatidylcholine, Intestinal Microbiota, and Incident Adverse Cardiovascular Events.
Ingested phosphatidylcholine (lecithin), the major dietary source of total choline, is acted on by intestinal lipases to form a variety of metabolic products, including the choline-containing nutrients glycerophosphocholine, phosphocholine, and choline. Choline-containing nutrients that reach the cecum and large bowel may serve as fuel for intestinal microbiota (gut flora), producing trimethylamine (TMA). TMA is rapidly further oxidized to trimethylamine-N-oxide (TMAO) by hepatic flavin-containing monooxygenases (FMOs). TMAO enhances the accumulation of cholesterol in macro-phages, the accumulation of foam cells in artery walls, and atherosclerosis,7 all factors that are associated with an increased risk of heart attack, stroke, and death. Choline can also be oxidized to betaine in both the liver and kid-neys.20 Dietary betaine can serve as a substrate for bacteria to form TMA21 and presumably TMAO.
Although an association between infectious organisms and atherosclerosis has previously been postulated, studies looking at the role of antimicrobial therapy in preventing disease progression have been disappointing.26,27 It is important to recognize that the choice of antimicrobial therapy in previous intervention trials was based largely on targeting postulated organisms rather than modulating the composition of intestinal microbiota or their metabolites. Furthermore, even if an antibiotic initially suppressed TMAO levels, the durability of that effect with long-term use remains unknown. In unpublished studies, we observed that long-term use of a single antibiotic (6 months of ciprofloxacin), which initially suppressed plasma TMAO levels in a rodent model, completely lost its suppressive effect, an observation that is consistent with the expansion of antibiotic-resistant intestinal microbiota. Thus, instead of suggesting that intestinal microbes should be eradicated with long-term use of antibiotics, our findings point to the possibility that plasma TMAO levels may identify a pathway within intestinal microbiota amenable to therapeutic modulation. For example, our data suggest that excessive consumption of dietary phosphatidylcholine and choline should be avoided; a vegetarian or high-fiber diet can reduce total choline intake.16 It should also be noted that choline is a semiessential nutrient and should not be completely eliminated from the diet, since this can result in a deficiency state. However, standard dietary recommendations, if adopted, will limit the intake of phosphatidylcholine- and cholinerich foods, since these foods are also typically high in fat and cholesterol content.1 An alternative potential therapeutic intervention is targeting the composition of the microbiota or biochemical pathways, with either a functional food such as a probiotic17 or a pharmacologic intervention. The latter hypothetically could take the form of treatment with an inhibitor to block specific microbial metabolic pathways or a short course of nonsystemic antibiotics to reduce the burden of TMAO-producing microbes, two therapies that have been used in the treatment of irritable bowel syndrome.28 Further studies are warranted to establish whether antimicrobial therapies can significantly reduce cardiovascular risk.
In conclusion, we have shown that intestinal microbes participate in phosphatidylcholine metabolism to form circulating and urinary TMAO. We also established a correlation between high plasma levels of TMAO and an increased risk of incident major adverse cardiovascular events that is independent of traditional risk factors, even in low-risk cohorts.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health and its Office of Dietary Supplements (R01HL103866 and 1P20HL113452). The clinical study GeneBank was supported by grants from the National Institutes of Health (P01HL098055, P01HL076491, R01HL103931, and R01DK080732) and a Cleveland Clinic/Case Western Reserve University Clinical and Translational Science Award (UL1TR000439). Dr. Hazen was supported by a gift from the Leonard Krieger Fund. Mass spectrometry instrumentation used was housed within the Cleveland Clinic Mass Spectrometry Facility with partial support through a Center of Innovation by AB SCIEX.
We thank Linda Kerchenski and Cindy Stevenson for their assistance in recruitment of study participants and Amber Gist and Naomi Bongorno for their assistance in the preparation of earlier versions of the figures and the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden JM. USDA database for the choline content of common foods: release two. 2008 ( http://www.ars.usda.gov/SP2UserFiles/Place/12354500/Data/Choline/Choln02.pdf)
- 2.Zhang AQ, Mitchell SC, Smith RL. Dietary precursors of trimethylamine in man: a pilot study. Food Chem Toxicol. 1999;37:515–520. doi: 10.1016/s0278-6915(99)00028-9. [DOI] [PubMed] [Google Scholar]
- 3.Zeisel SH. Choline: critical role during fetal development and dietary requirements in adults. Annu Rev Nutr. 2006;26:229–250. doi: 10.1146/annurev.nutr.26.061505.111156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen L, Ley RE, Volchkov PY, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de la Huerga J, Popper H. Urinary excretion of choline metabolites following choline administration in normals and patients with hepatobiliary diseases. J Clin Invest. 1951;30:463–470. doi: 10.1172/JCI102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simenhoff ML, Saukkonen JJ, Burke JF, Wesson LG, Schaedler RW. Amine metabolism and the small bowel in uraemia. Lancet. 1976;2:818–821. doi: 10.1016/s0140-6736(76)91207-1. [DOI] [PubMed] [Google Scholar]
- 10.Ihle BU, Cox RW, Dunn SR, Simenhoff ML. Determination of body burden of uremic toxins. Clin Nephrol. 1984;22:82–89. [PubMed] [Google Scholar]
- 11.Bain MA, Fornasini G, Evans AM. Trimethylamine: metabolic, pharmacokinetic and safety aspects. Curr Drug Metab. 2005;6:227–240. doi: 10.2174/1389200054021807. [DOI] [PubMed] [Google Scholar]
- 12.Erdmann CC. On the alleged occurrence of trimethylamine in the urine. J Biol Chem. 1910;8:57–60. [Google Scholar]
- 13.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 14.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216–3223. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 15.al-Waiz M, Mikov M, Mitchell SC, Smith RL. The exogenous origin of trimethylamine in the mouse. Metabolism. 1992;41:135–136. doi: 10.1016/0026-0495(92)90140-6. [DOI] [PubMed] [Google Scholar]
- 16.Stella C, Beckwith-Hall B, Cloarec O, et al. Susceptibility of human metabolic phenotypes to dietary modulation. J Proteome Res. 2006;5:2780–2788. doi: 10.1021/pr060265y. [DOI] [PubMed] [Google Scholar]
- 17.Martin FP, Wang Y, Sprenger N, et al. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill SR, Pop M, Deboy RT, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loscalzo J. Lipid metabolism by gut microbes and atherosclerosis. Circ Res. 2011;109:127–129. doi: 10.1161/RES.0b013e3182290620. [DOI] [PubMed] [Google Scholar]
- 20.Bennett BJ, de Aguiar Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Möller B, Hippe H, Gottschalk G. Degradation of various amine compounds by mesophilic clostridia. Arch Microbiol. 1986;145:85–90. doi: 10.1007/BF00413032. [DOI] [PubMed] [Google Scholar]
- 22.Lang DH, Yeung CK, Peter RM, et al. Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochem Pharmacol. 1998;56:1005–1012. doi: 10.1016/s0006-2952(98)00218-4. [DOI] [PubMed] [Google Scholar]
- 23.Bennett BJ, Vallim TQ, Wang Z, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Waiz M, Mitchell SC, Idle JR, Smith RL. The relative importance of N-oxidation and N-demethylation in the metabolism of trimethylamine in man. Toxicology. 1987;43:117–121. doi: 10.1016/0300-483x(87)90001-1. [DOI] [PubMed] [Google Scholar]
- 25.Yancey PH, Rhea MD, Kemp KM, Bailey DM. Trimethylamine oxide, betaine and other osmolytes in deep-sea animals: depth trends and effects on enzymes under hydrostatic pressure. Cell Mol Biol (Noisy-le-grand) 2004;50:371–376. [PubMed] [Google Scholar]
- 26.Cannon CP, Braunwald E, McCabe CH, et al. Antibiotic treatment of Chlamydia pneumoniae after acute coronary syndrome. N Engl J Med. 2005;352:1646–1654. doi: 10.1056/NEJMoa043528. [DOI] [PubMed] [Google Scholar]
- 27.Grayston JT, Kronmal RA, Jackson LA, et al. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637–1645. doi: 10.1056/NEJMoa043526. [DOI] [PubMed] [Google Scholar]
- 28.Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.