Abstract
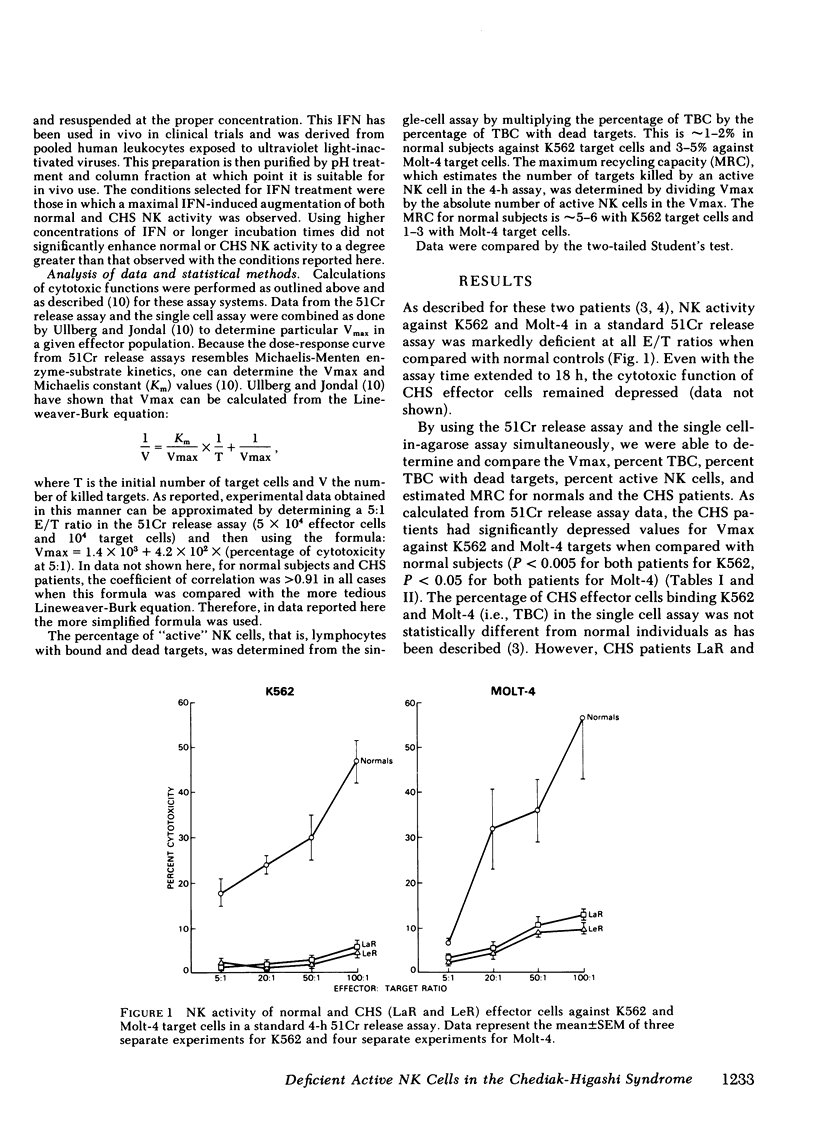
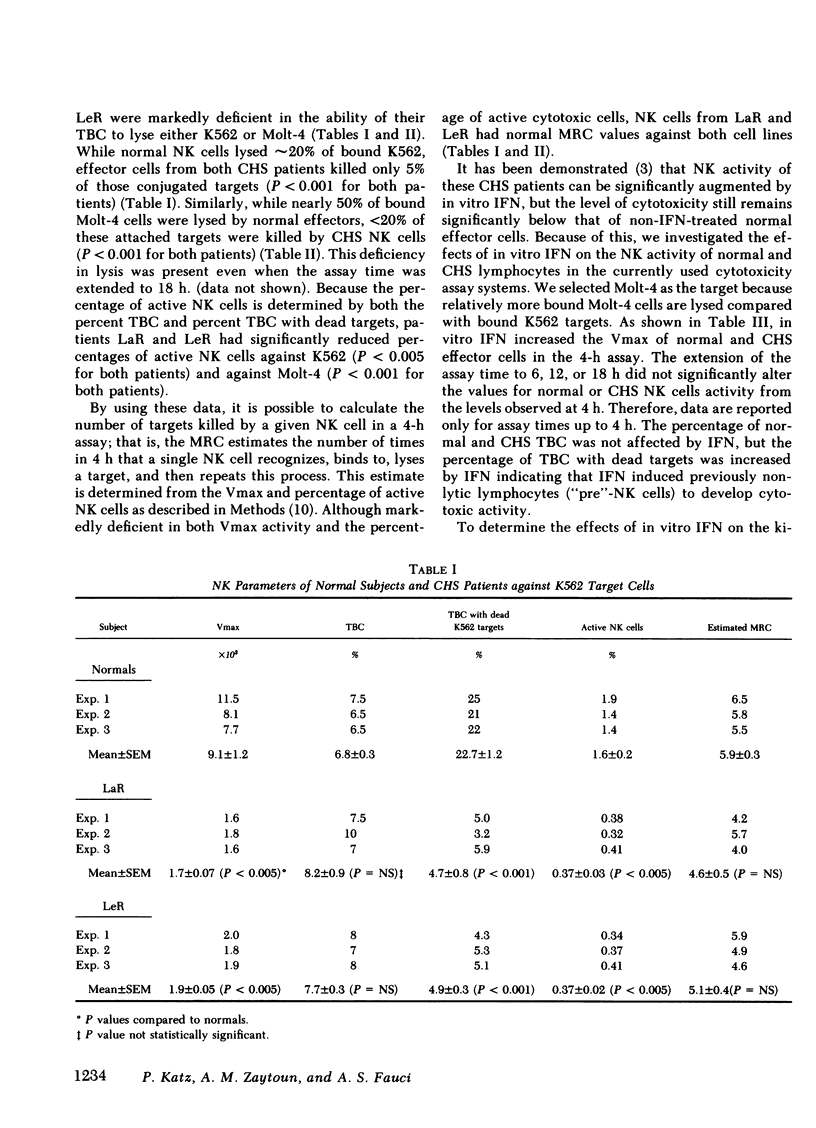
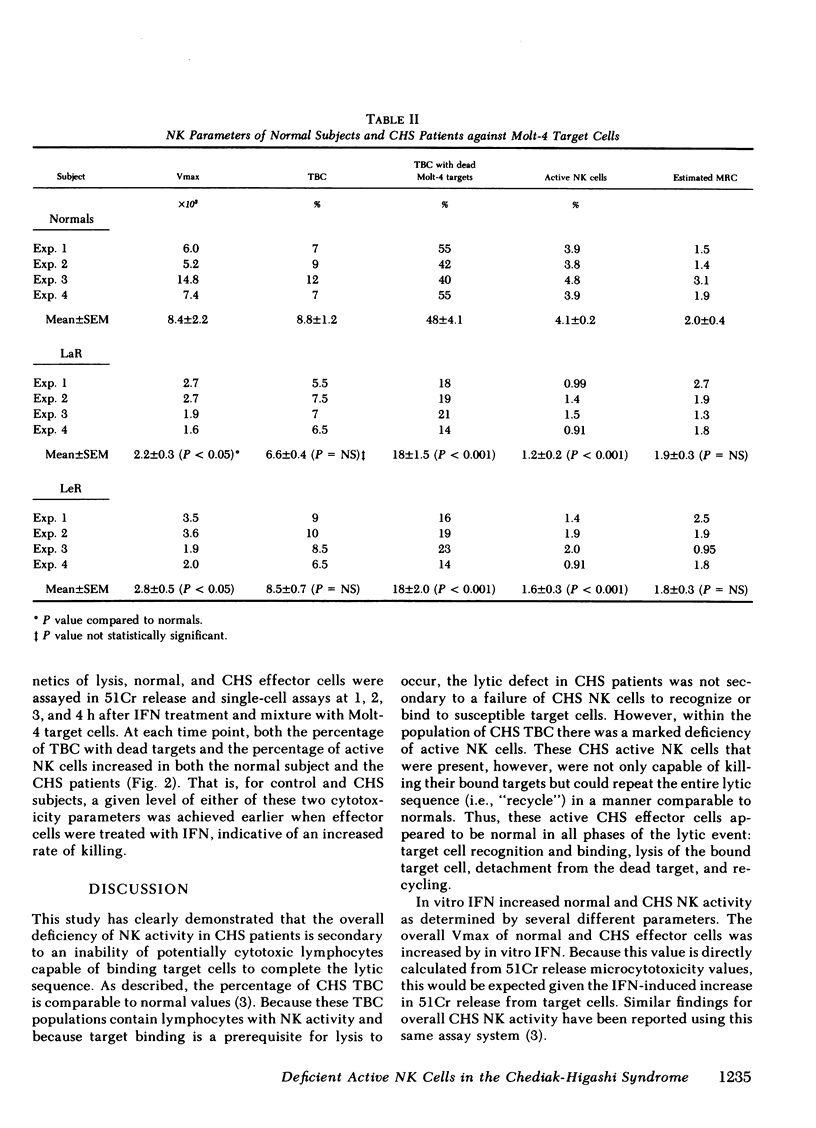
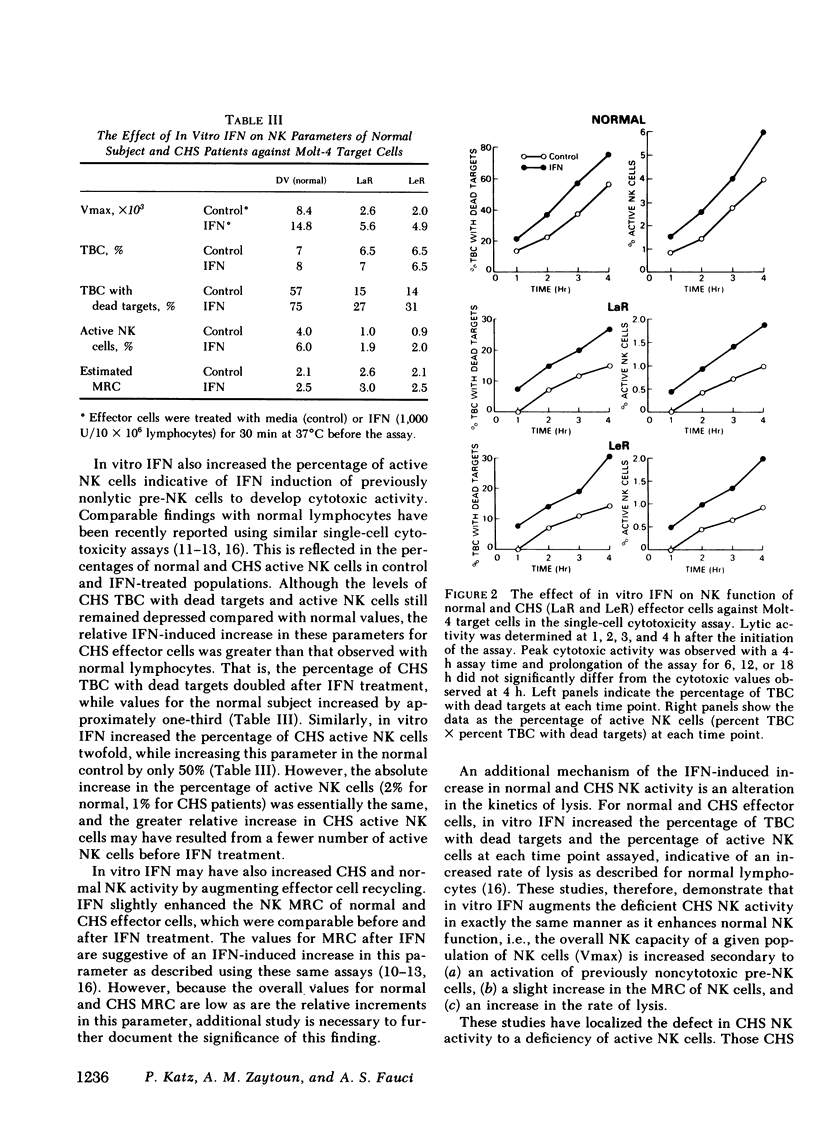
This study investigated the defective natural killer (NK) cell activity in two patients with the Chediak-Higashi syndrome (CHS) using both a standard 51-chromium release microcytoxicity and a single cell-in-agarose assay against K562 and Molt-4 target cells. CHS patients were deficient in overall maximum NK capacity, but had normal percentages of potentially cytotoxic target bindng cells. the relative number of TBC that could kill bound targets (i.e., "active" NK cells) was significantly depressed in CHS patients when compared with normal controls. The diminished CHS active NK cells that were present, however, were capable of recycling and lysing multiple target cells during the assay period. In vitro interferon (INF) treatment of normal and CHS effector cells did not alter target cell binding, but did increase the maximum NK capacity, percentage of active NK cells and the maximum recycling capacity, as well as the rate of lysis. These studies indicate that the depression of NK activity in patients with CHS is secondary to a deficiency of active NK cells. The CHS active NK cells that are present, however, are capable of normal target lysis and recycling. Potentially cytotoxic pre-NK cells, which can bind but not kill target cells, can be activated by in vitro IFN to develop lytic activity. Thus, IFN treatment may be of potential benefit to the immune surveillance network of CHS patients by activating a population of pre-NK cells to express their cytotoxic potential.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume R. S., Wolff S. M. The Chediak-Higashi syndrome: studies in four patients and a review of the literature. Medicine (Baltimore) 1972 Jul;51(4):247–280. [PubMed] [Google Scholar]
- Bradley T. P., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. III. Evidence that cytotoxic T lymphocytes lyse both antigen-specific and -nonspecific targets pretreated with lectins or periodate. J Immunol. 1981 Jan;126(1):208–213. [PubMed] [Google Scholar]
- Dent P. B., Fish L. A., White L. G., Good R. A. Chediak-Higashi syndrome. Observations on the nature of the associated malignancy. Lab Invest. 1966 Oct;15(10):1634–1642. [PubMed] [Google Scholar]
- Grimm E., Bonavida B. Mechanism of cell-mediated cytotoxicity at the single cell level. I. Estimation of cytotoxic T lymphocyte frequency and relative lytic efficiency. J Immunol. 1979 Dec;123(6):2861–2869. [PubMed] [Google Scholar]
- Haliotis T., Roder J., Klein M., Ortaldo J., Fauci A. S., Herberman R. B. Chédiak-Higashi gene in humans I. Impairment of natural-killer function. J Exp Med. 1980 May 1;151(5):1039–1048. doi: 10.1084/jem.151.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Djeu J., Kay H. D., Ortaldo J. R., Riccardi C., Bonnard G. D., Holden H. T., Fagnani R., Santoni A., Puccetti P. Natural killer cells: characteristics and regulation of activity. Immunol Rev. 1979;44:43–70. doi: 10.1111/j.1600-065x.1979.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Holden H. T. Natural cell-mediated immunity. Adv Cancer Res. 1978;27:305–377. doi: 10.1016/s0065-230x(08)60936-7. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Katz P., Simone C. B., Henkart P. A., Fauci A. S. Mechanisms of antibody-dependent cellular cytotoxicity: the use of effector cells from chronic granulomatous disease patients as investigative probes. J Clin Invest. 1980 Jan;65(1):55–63. doi: 10.1172/JCI109660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Roder J., Haliotis T., Korec S., Jett J. R., Herberman R. B., Katz P., Fauci A. S. Chédiak-Higashi gene in humans. II. The selectivity of the defect in natural-killer and antibody-dependent cell-mediated cytotoxicity function. J Exp Med. 1980 May 1;151(5):1049–1058. doi: 10.1084/jem.151.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M. Impaired microtubule function correctable by cyclic GMP and cholinergic agonists in the Chediak-Higashi syndrome. Am J Pathol. 1976 Nov;85(2):395–418. [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Rosenthal A. S., Balestra D. J. Abnormal bactericidal, metabolic, and lysosomal functions of Chediak-Higashi Syndrome leukocytes. J Clin Invest. 1972 Mar;51(3):649–665. doi: 10.1172/JCI106854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A., Bonavida B., Targan S. Mode of action of interferon-mediated modulation of natural killer cytotoxic activity: recruitment of pre-NK cells and enhanced kinetics of lysis. J Immunol. 1980 Aug;125(2):479–484. [PubMed] [Google Scholar]
- Targan S. R. The dual interaction of prostaglandin E2 (PGE2) and interferon (IFN) on NK lytic activation: enhanced capacity of effector-target lytic interactions (recycling) and blockage of pre-NK cell recruitment. J Immunol. 1981 Oct;127(4):1424–1428. [PubMed] [Google Scholar]
- Targan S., Britvan L., Dorey F. Activation of human NKCC by moderate exercise: increased frequency of NK cells with enhanced capability of effector--target lytic interactions. Clin Exp Immunol. 1981 Aug;45(2):352–360. [PMC free article] [PubMed] [Google Scholar]
- Targan S., Dorey F. Interferon activation of "pre-spontaneous killer" (pre-SK) cells and alteration in kinetics of lysis of both "pre-SK" and active SK cells. J Immunol. 1980 May;124(5):2157–2161. [PubMed] [Google Scholar]
- Ullberg M., Jondal M. Recycling and target binding capacity of human natural killer cells. J Exp Med. 1981 Mar 1;153(3):615–628. doi: 10.1084/jem.153.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]



