Abstract
Introduction
Epithelial cell adhesion molecule (EpCAM) is a surface glycoprotein highly differentially expressed in many epithelial malignancies. The goal of this study was to evaluate the expression of EpCAM and the potential of MT201 (adecatumumab), a human monoclonal antibody targeting EpCAM, against multiple primary cervical carcinoma cell lines.
Methods
Epithelial cell adhesion molecule expression was evaluated by real-time polymerase chain reaction and flow cytometry in a total of 8 primary cervical cancer cell lines. Sensitivity to MT201-mediated cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity was tested in standard 4-hour 51Cr release assays. To investigate the effect of interleukin-2 (IL-2) on MT201-mediated ADCC, 4-hour 51Cr release assays were also conducted in the presence of low doses of IL-2.
Results
High messenger RNA expression by real-time polymerase chain reaction and high EpCAM surface expression by flow cytometry were detected in 4 (50%) of 8 primary cervical carcinoma cell lines. With no exception, the primary cell lines derived from clinically aggressive tumors showed EpCAM overexpression. Whereas these cell lines were highly resistant to complement-dependent cytotoxicity and natural killer (NK)-dependent cytotoxicity in vitro (range of killing, 4%–19%), EpCAM-positive cell lines showed high sensitivity to MT201-mediated ADCC (range of killing, 23%–59%). Incubation with IL-2 in addition to MT201 significantly increased the cytotoxic activity against EpCAM-positive cervical cancer cell lines (P = 0.007). Addition of human serum also further increased the MT201-mediated killing of EpCAM-positive cell lines (P = 0.03).
Conclusions
Epithelial cell adhesion molecule is highly expressed in primary cervical carcinoma cell lines, and these biologically aggressive tumors are highly sensitive to MT201-mediated cytotoxicity in vitro. MT201 may represent a novel, potentially highly effective treatment option for patients with cervical carcinoma, especially for those with advanced, recurrent, or metastatic disease refractory to standard salvage therapy.
Keywords: Cervical cancer, EpCAM, MT201, Immunotherapy, Adecatumumab
Cervical cancer remains the second most common gynecologic cancer worldwide.1,2 In the United States, approximately 11,270 new cases of cervical cancer and 4070 deaths from cervical cancer are estimated3 for 2009. Although cervical cancer is, to a large extent, a preventable disease, it remains an important health problem for women, especially in underserved and minority groups in industrially developed nations.1 Whereas patients with early-stage disease have an excellent prognosis, the recurrence rate for patients with locally advanced disease is 50% to 70%.1,2 Effective treatment options for advanced and recurrent cervical cancer are very limited.2,4 Cisplatin-based chemotherapy is most frequently used and yields a response rate of 20% to 30%.4 Unfortunately, most of these responses are short in duration, and patients with recurrent or metastatic disease have a 1-year survival rate of 10% to 15%.2 Novel therapeutic strategies effective against recurrence/metastatic disease refractory to chemotherapy remain desperately needed.
Epithelial cell adhesion molecule (EpCAM), also referred to as EGP-40, Trop-1, 17-1A, KSA, KS1/4, AUA1, GA733-2, or CD326, has been shown to be overexpressed in several gynecologic malignancies, including uterine and ovarian cancer.5 Epithelial cell adhesion molecule is a 40-kd surface glycoprotein that was first discovered in 1979.6 The EpCAM gene is located on chromosome 4q and contains 9 exons. It consists of an extracellular domain with 2 epidermal growth factor–like repeats, a transmembrane domain, and a short cytoplasmic domain.6 Epithelial cell adhesion molecule is expressed at low levels on the basolateral and intercellular surface of simple, pseudostratified, and transitional epithelia, including most epithelial tissues in the female genital tract.7 The homogeneous distribution of EpCAM on the tumor cell, its glycosylation, and the level of expression help differentiate tumor from normal cells.8 Indeed, most neoplastic epithelial cells overexpress EpCAM as do 85% of adenocarcinomas and 72% of squamous cell carcinomas.6,9 El-Sahwi et al5 reported overexpression of EpCAM in a large number of uterine serous papillary carcinomas. Epithelial cell adhesion molecule is believed to contribute to signaling, cell migration, proliferation, and differentiation.9 It promotes cell adhesion via a calcium-independent mechanism, and formation of EpCAM-mediated adhesions has a negative regulatory effect on adhesions conferred by cadherins.6,10 Consistent with this view, EpCAM silencing with small inhibitory RNA may lead to a reduction in cell proliferation, migration, and invasion.11 In cervical squamous epithelium, EpCAM expression is associated with abnormal proliferation.12
Importantly, because of its localization on the cell surface of carcinomas, EpCAM is an attractive target for immunotherapy.6 Edrecolomab (Panorex), a chimeric murine anti-EpCAM IgG2a antibody, was shown to improve overall and disease-free survival in patients with Duke C colon cancer with minimal residual disease compared with best supportive care.13 It subsequently gained approval in Germany as an adjuvant monotherapy in the treatment of colon carcinoma and was taken off the market only after the introduction of 5-fluorouracil with leucovorin in colon carcinoma led to even better survival results.
Adecatumumab (MT201) is a fully human, recombinant monoclonal anti-EpCAM antibody that acts mainly through antibody-dependent cellular cytotoxicity (ADCC).9,14 Compared with the murine antibody edrecolomab, MT201 shows a longer half-life and reduced immunogenicity.6 Unlike other murine high-affinity anti-EpCAM antibodies, adecatumumab is a low- to intermediate-affinity antibody.14 The high-affinity antibodies were associated with significant toxicities in phase I clinical trials.14 Adecatumumab, however, seems to be well tolerated and has been evaluated in phase II trials as a single agent in metastatic breast and early-stage prostate cancer, and in a phase I trial in combination with taxotere.15–17 Currently, another phase II trial assessing MT201 in patients with completely resected liver metastases from colorectal cancer is ongoing. This antibody also has high in vitro cytotoxic activity against uterine serous papillary carcinoma, a biologically aggressive subtype of endometrial cancer.5
In this investigation, we evaluated EpCAM’s potential value as a novel target against cervical cancer by studying its expression at both messenger RNA (mRNA) and protein level in multiple primary cervical cancer cell lines.
MATERIALS AND METHODS
Establishment of Cervical Cancer Cell Lines
Study approval was obtained from the institutional review board, and all patients signed an informed consent form according to institutional guidelines. A total of 8 primary cervical cancer cell lines were established after sterile processing of samples from surgical biopsies as previously described.18 With the exception of CVX-2 and CVX-10 cervical cancer cell lines, which were derived from biopsies obtained from a recurrent carcinoma (ie, CVX-2) or a metastatic (ie, CVX-10) site of disease (ie, lymph node), all other cell lines were established from biopsies collected from the primary tumor (ie, stage IB cervical cancer). Only cell cultures composed of at least 99% epithelial cells were retained for flow cytometry experiments.
Quantitative Real-Time Polymerase Chain Reaction
RNA isolation from a total of 8 primary cervical carcinoma cell lines was performed using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. Quantitative polymerase chain reaction (PCR) was done with a 7500 Real-Time PCR System using the manufacturer’s recommended protocol (Applied Biosystems, Foster City, Calif) to evaluate expression of EpCAM in all samples. Each reaction was run in duplicate. The primers and probe for EpCAM (TACSTD1) were obtained from Applied Biosystems (Hs00158980_m1). The comparative threshold cycle (CT) method (Applied Biosystems) was used to determine gene expression in each sample relative to the value observed in the lowest nonmalignant cervical epithelial cell sample, using glyceraldehyde-3-phosphate dehydrogenase (assay ID Hs99999905_m1) RNA as internal controls.
Flow Cytometry
Human recombinant IgG1 anti-EpCAM monoclonal antibody (mAb) MT201 (Micromet AG) was used for flow cytometry studies. Briefly, freshly established cervical tumor cell lines obtained from the aforementioned patients were stained with MT201 (Micromet AG). The chimeric anti-CD20 mAb rituximab (Rituxan, Genentech, San Francisco, Calif) was used as a control. A goat antihuman F(ab′)2 immunoglobulin (BioSource International, Camarillo, Calif) was used as a secondary reagent. Analysis was conducted with a FACScalibur instrument, using the Cell Quest software (Beckton Dickinson, Franklin Lakes, NJ).
ADCC Measurement
A standard 4-hour chromium (51Cr) release assay was performed to measure the cytotoxic reactivity of Ficoll-Hypaque separated peripheral blood lymphocytes (PBLs) obtained from several healthy donors against representative cervical cancer cell lines. In most of the experiments, the release of 51Cr from the target cells was measured as evidence of tumor cell lysis after exposure of tumor cells to an MT201 concentration of 5 μg/mL (Micromet AG). Controls included the incubation of target cells alone or with PBL or mAb separately. The chimeric anti-CD20 mAb rituximab (Rituxan, Genentech) was used as isotype control for MT201 in all bioassays. Antibody-dependent cellular cytotoxicity was calculated as the percentage of killing of target cells observed with MT201 plus effector cells compared with 51Cr release from target cells incubated alone.
Interleukin-2 Enhancement of ADCC
To investigate the effect of interleukin-2 (IL-2) on MT201-mediated ADCC, effector PBLs were incubated for 4 hours at 37°C at a final concentration of IL-2 (Aldesleukin; Chiron Therapeutics, Emeryville, Calif) ranging from 50 to 100 IU/mL in 96-well microtiter plates. Target cells were primary cervical cell lines exposed to MT201 (concentration, 5 μg/mL; Micromet AG), whereas the controls included the incubation of target cells alone or with PBLs in the presence or absence of IL-2 or mAb, respectively. Rituximab was used as an isotype control mAb.
Test for Complement-Mediated Target Cell Lysis and γ-Immunoglobulin Inhibition
A standard 4-hour chromium (51Cr) release assay identical to those used for ADCC assays was used, except that human serum in a dilution of 1:2 was added in place of the effector cells. This human serum was used as a source of complement to test for complement-mediated target cell lysis. To test for the possible inhibition of ADCC against cervical cancer cell lines by physiological human serum concentrations of γ-immunoglobulin, heat-inactivated (56°C for 60 minutes) human serum was diluted 1:2 before being added in the presence or absence of effector PBL. In some experiments, non-heat-inactivated human serum (diluted 1:2) was added in the presence of effector PBL. Controls included the incubation of target cells alone or with either lymphocytes or mAb separately. Rituximab was used as isotype control mAb.
Statistical Analysis
For quantitative real-time (RT) PCR data, the right skewing was removed by taking copy/number ratios relative to the lowest-expressing healthy cervical cell sample (“relative copy number”), log2-transforming them to ΔCTs, and comparing the results via unequal-variance t test for cervical carcinoma versus healthy cervical difference. Differences in EpCAM expression by flow cytometry were analyzed by the unpaired t test, and P <0.05 among samples was considered to be significant. Kruskal-Wallis test and χ2 analysis was used to evaluate differences in MT201-induced ADCC levels in primary tumor cell lines. Statistical analysis was performed using SPSS version 15 (SPSS, Chicago, Ill).
RESULTS
EpCAM Transcript Levels in Cervical Carcinomas
A total of 8 primary cervical cancer cell lines were tested for EpCAM expression by quantitative RT-PCR. Table 1 shows the histopathological characteristics of the patients with cervical cancer. Seven squamous cell carcinomas and one adenocarcinoma were included. Five tumors were human papillomavirus (HPV)-16 positive, 2 were HPV-18 positive, and one tumor was HPV-45 positive (Table 1). Two tumors (ie, CVX-2 and CVX-10) were established from patients with metastatic and/or recurrent disease. Of the 8 tumors tested, 4 carcinomas (3 HPV-16 positive and one HPV-18 positive) showed high mRNA copy numbers ranging from 921 to 1882 (Table 2). In contrast, low EpCAM expression by quantitative RT-PCR was detected in 4 cervical cancer cell lines (2 HPV-16 positive, 1 HPV-18 positive, and 1 HPV-45 positive), with mRNA copy numbers ranging from 2 to 128 (Table 2). The difference between cervical cancer cell lines with low and high EpCAM expression was statistically significant (P < 0.0001). There was also a statistically significant difference in EpCAM expression by quantitative RT-PCR between EpCAM-positive cervical cancer cells and normal epithelial cells (the latter ranging from 1 to 32 mRNA copy numbers; P = 0.002, data not shown). Both cell lines derived from clinically aggressive tumors (ie, metastatic and/or recurrent disease) had a high EpCAM expression (ranging from 1393.7 to 1881.5 mRNA copy numbers; Table 2).
TABLE 1.
Patients’ characteristics
| Cell Line | Histology | HPV Status | Age (Years) | Race | Stage | Grade | LVI |
|---|---|---|---|---|---|---|---|
| CVX-1 | SCC | 45 | 57 | AA | IB | 2 | Yes |
| CVX-2 | SCC | 18 | 42 | W | Recurrence | 3 | — |
| CVX-3 | SCC | 16 | 70 | W | IB | 2 | Yes |
| CVX-4 | SCC | 16 | 40 | W | IB | 3 | Yes |
| CVX-6 | ACA | 18 | 45 | W | IB | 3 | No |
| CVX-7 | SCC | 16 | 26 | W | IB | 3 | Yes |
| CVX-8 | SCC | 16 | 23 | W | IB | 3 | Yes |
| CVX-10 | SCC | 16 | 39 | W | IIA* | 3 | Yes |
Biopsy collected from a metastatic pelvic lymph node.
AA, African American; ACA, adenocarcinoma; LVI, lymphovascular invasion; SCC, squamous cell carcinoma; W, white.
TABLE 2.
EpCAM mRNA and surface protein expression in cervical cancer cell lines
| Cell Line | Flow Cytometry
|
RT-PCR
|
|
|---|---|---|---|
| MFI (SD) | Cells (SD) (%) | mRNA Copy No. | |
| CVX-1 | 35.0 (7.8) | 54.5 (2.6) | 38.4 |
| CVX-2 | 63.0 (21.7) | 100.0 (0.0) | 1393.7 |
| CVX-3 | 37.3 (9.3) | 33.2 (6.4) | 9.4 |
| CVX-4 | 21.1 (5.2) | 38.2 (20.0) | 2.1 |
| CVX-6 | 25.6 (2.1) | 6.6 (0.6) | 128.4 |
| CVX-7 | 62.0 (33.5) | 99.5 (1.0) | 1494.0 |
| CVX-8 | 43.1 (11.2) | 75.6 (9.7) | 921.0 |
| CVX-10 | 42.9 (0.4) | 84. 7 (2.5) | 1881.5 |
MFI, mean fluorescence intensity.
EpCAM Surface Expression by Flow Cytometry in Cervical Carcinomas
To determine whether the high expression of EpCAM mRNA detected by quantitative RT-PCR assays in 4 of our 8 primary cervical cancer cell lines also resulted in high expression of the protein on the surface of tumor cells, we performed flow cytometry on all primary tumors. Four cell lines showed high EpCAM surface expression by flow cytometry (3 HPV-16 positive and 1 HPV-18 positive; Table 2). This is consistent with the mRNA copy numbers measured by quantitative RT-PCR for the same cell lines. The difference in EpCAM surface expression between the cell lines with low and high EpCAM expression was statistically significant (P < 0.0001). The 2 carcinoma cell lines derived from clinically metastatic and/or recurrent tumors showed high surface EpCAM expression.
Cervical Carcinoma Cell Lines Are Highly Resistant to NK Cell Activity but Sensitive to MT201-Mediated ADCC
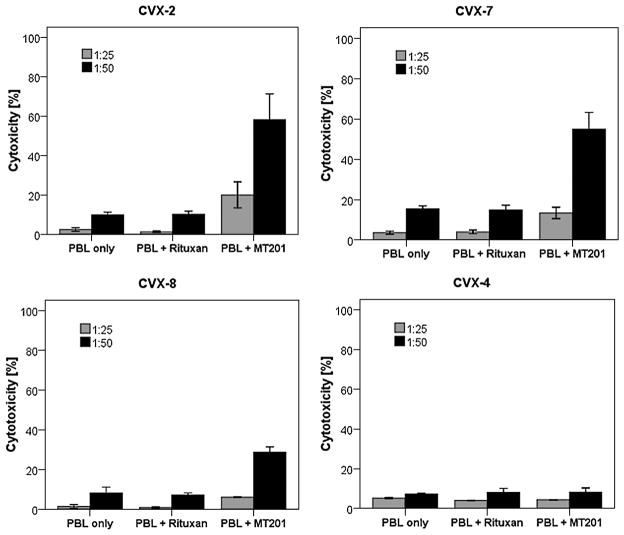
Five representative primary cervical cancer cell lines were evaluated for their sensitivity to natural killer (NK) cell-mediated cytotoxicity. These cell lines were exposed to PBL collected from multiple healthy donors, and the cytotoxicity was measured using a standard 4-hour 51Cr release assay. Using dose titration experiments with different doses of MT201, killing of the cervical cancer cells was found to plateau at an MT201 concentration of 5 μg/mL (data not shown). This dose was therefore used in all experiments that followed. Cervical cancer cell lines were found to be highly resistant to NK cell–mediated killing with exposure to PBL at an effector/target ratio of 25:1 and 50:1 (mean killing [SD] = 6.7% [5.5%]) in the absence of MT201 (Table 3). In contrast, significant killing was observed against EpCAM-positive cell lines after incubation with MT201 to mediate ADCC (range of killing, 23%–59%; mean killing [SD], 28% [24%]; Fig. 1, Table 3). Significant killing was also shown in clinically aggressive cervical cancer cell lines (range of killing, 17.8%–83.2%, mean killing [SD], 44.3% [28.8%]). As expected, EpCAM-negative cell lines were resistant to MT201 (Fig. 1). These experiments were repeated a minimum of 2 times per cervical cancer cell line. These results are in good agreement with the EpCAM expression data by quantitative RT-PCR and flow cytometry (Table 2). All cell lines were highly resistant to incubation with rituximab as isotype control antibody in the presence of PBL (Table 3, Fig. 1).
TABLE 3.
MT201-dependent cytotoxicity results in cervical carcinoma cell lines
| Cell Line | EpCAM Overexpression | Killing (%)
|
|||
|---|---|---|---|---|---|
| Control (SD) | Rituximab (SD) | MT201 (SD) | P* | ||
| CVX-4 | 0 | 7.3 (0.5) | 8.1 (2.9) | 8.2 (2.9) | 0.867 |
| CVX-2 | + | 10.0 (3.3) | 10.2 (3.9) | 59.2 (29.1) | 0.005 |
| CVX-7 | + | 15.5 (3.5) | 14.9 (5.8) | 54.9 (20.1) | 0.012 |
| CVX-8 | + | 8.2 (5.1) | 7.2 (1.6) | 28.8 (4.5) | 0.049 |
| CVX-10 | + | 6.0 (1.3) | 7.6 (0.8) | 22.6 (4.2) | 0.038 |
| Mean† | 6.7 (5.5) | 6.6 (5.7) | 29.7 (24.3) | <0.0001 | |
Cytotoxicity results in EpCAM-positive cell lines versus controls by Kruskal-Wallis test.
Mean of 24 cytotoxicity experiments, EpCAM-positive cell lines only.
FIGURE 1.
Representative cytotoxicity experiments using MT201 against CVX-2, CVX-7, CVX-8, and CVX-4 primary cell lines. High levels of MT201-induced cytotoxicity were evident against CVX-2, CVX-7, and CVX-8 primary cell lines expressing high levels of EpCAM. In contrast, negligible cytotoxicity was detectable against CVX-4 (ie, a low EpCAM expressor cell line). In all the cell lines tested, no significant cytotoxicity was detected in the absence of MT201 or in the presence of rituximab isotype control mAb.
IL-2 Enhancement of ADCC Against Cervical Carcinoma
To investigate the effect of IL-2 in combination with MT201 (5 μg/mL) on ADCC against cervical carcinoma cell lines, PBL from healthy donors were incubated with 50 to 100 IU of IL-2 per milliliter for 4 hours. As representatively shown in Figure 2, MT201-mediated ADCC was significantly increased in the presence of IL-2 in all the primary cell lines tested (ie, CVX-2 and CVX-7; P = 0.007). Although the stimulation of PBL with IL-2 led to a significantly higher ADCC in the presence of MT201, it did not significantly increase tumor killing in the absence of MT201 or in the presence of the rituximab isotype control antibody and PBL (P = 0.916; Fig. 2).
FIGURE 2.
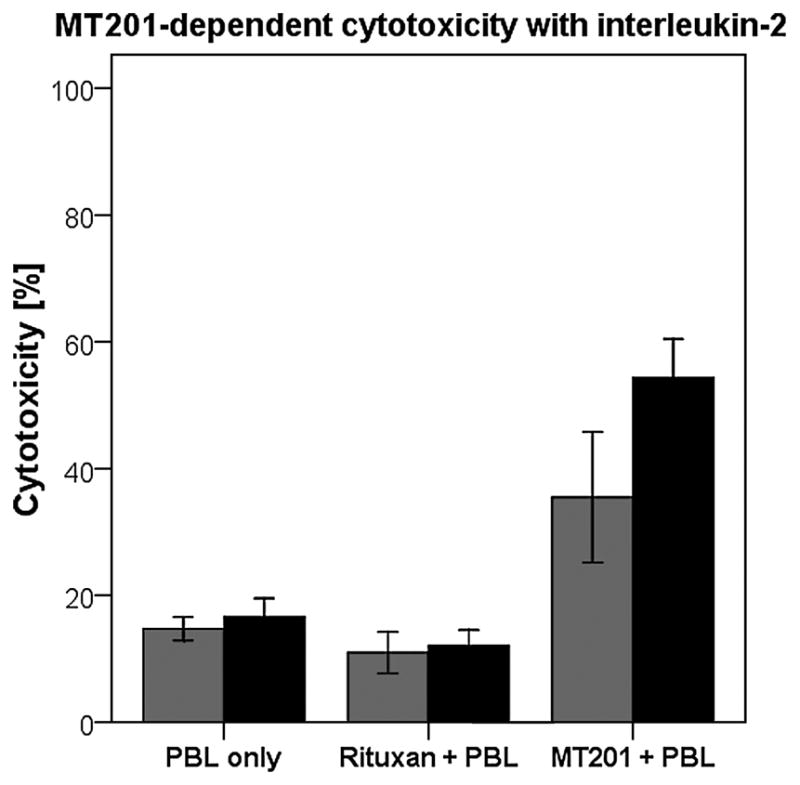
Effect of low doses of IL-2 in combination with MT201 (5 μg/mL) on ADCC against 2 primary cervical cancer cell lines overexpressing EpCAM (ie, CVX-2 and CVX-7: effectors to target ratio 25:1). Peripheral blood lymphocytes from healthy donors were incubated for 4 hours in the presence of 100 IU/mL of IL-2. MT201-mediated ADCC was significantly increased in the presence of low doses of IL-2. No significant increase in cytotoxicity was detected after a 4-hour IL-2 treatment in the absence of MT201 or in the presence of the rituximab isotype control mAb. Gray bars, no IL-2; black bars, IL-2.
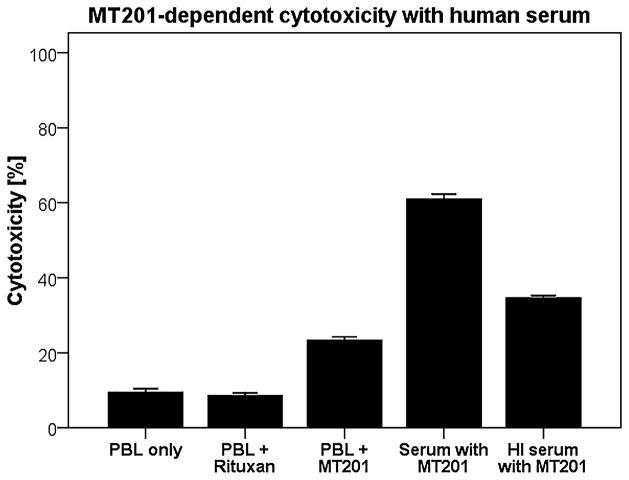
Effect of Complement and Physiological Concentrations of IgG on MT201-Mediated ADCC
To evaluate the effect of complement on the MT201-mediated ADCC and its potential inhibition by physiological serum IgG concentrations, human serum diluted 1:2 (with and without heat inactivation) was added to the cervical cancer cell lines during standard 4-hour 51Cr release assays. In the representative EpCAM-positive cell lines studied (ie, CVX-2 and CVX-7), significantly increased killing after incubation with physiological concentrations of IgG (serum diluted 1:2) in the presence of PBL compared with incubation without serum was noted (P = 0.03, Fig. 3). In contrast, after addition of heat-inactivated serum (diluted 1:2), the MT201-mediated killing consistently decreased to levels of MT201-mediated killing without serum (Fig. 3).
FIGURE 3.
Pooled representative cytotoxicity experiments adding human serum to MT201 against 2 primary cervical cancer cell lines overexpressing EpCAM (ie, CVX-2 and CVX-7: effectors to target ratio 25:1). Cervical cancer cell lines were challenged by diluted (1:2) serum (with or without heat inactivation) in the presence of the effector cells and MT201 to standard 4-hour51Cr release assays. Addition of untreated serum (diluted 1:2) to PBL in the presence of MT201 consistently increased MT201-mediated cytotoxicity against CVX-2 and CVX-7 (P < 0.05). Addition of heat-inactivated serum (diluted 1:2) to PBL in the presence of MT201 reduced the degree of ADCC achieved in the presence of MT201 + serum against CVX-2 and CVX-7.
DISCUSSION
The management of disseminated carcinoma of the cervix that is no longer amenable to control with surgery or radiation therapy has not improved significantly with the advent of modern chemotherapy.2 The 1-year survival rate remains between 10% and 15%.2 Thus, the development of novel, target-specific, and effective treatment modalities against chemotherapy-refractory cervical cancer remains desperately needed.
Epithelial cell adhesion molecule, an adhesion molecule found highly differentially expressed in gynecologic cancers by our research group and others may represent a potentially effective target for the immunotherapy of chemotherapy-resistant and biologically aggressive cervical disease.5,18,19 Although EpCAM was initially assumed to be mainly involved in cell adhesion, it is now clear that it also plays a role in carcinogenesis via the upregulation of oncogenes such as c-myc and cell cycle–regulating genes of the cyclin family.20 This finding is further confirmed by the fact that silencing EpCAM leads to a decrease in proliferation and metabolism of tumor cells, suggesting that EpCAM may be an attractive target for immunotherapy.6 Intramembrane proteolysis and shedding of EpCAM’s extracellular domain have been shown to activate EpCAM signaling.7 The extracellular domain, once cleaved, may also function as an agonist to EpCAM-expressing cells.7 Tumor necrosis factor α can down-regulate, and several chemotherapeutic agents can modulate, EpCAM expression.21 EpCAM knockdown reduces the expression of c-myc, cyclin D, and survivin.9 Importantly, EpCAM expression has been described as a marker of progression in cervical dysplasia and squamous cell lung cancer, and its overexpression is an independent prognostic marker for reduced survival in breast and ovarian cancer.6,10,19,22
Our study carefully evaluated EpCAM expression level in primary cervical cancer cell lines and their in vitro sensitivity to adecatumumab (MT201), a novel human mAb targeting EpCAM. We found EpCAM to be overexpressed in 4 of the 8 primary cervical cancer cell lines available to this study, by RT-PCR and by flow cytometry. To our knowledge, our report represents the first evaluation of EpCAM expression in primary cervical cancer cell lines. In squamous cervical epithelium, EpCAM expression has been shown to be associated with an increased proliferative activity and loss of tissue-specific marker for terminal differentiation, suggesting that the expression of EpCAM may be an early event in cervical carcinogenesis.12 There are conflicting data regarding a possible correlation between EpCAM expression and tumor stage in different gynecologic tumors.19 Importantly, however, our data demonstrate that both cervical carcinoma cell lines derived from metastatic and/or recurrent tumors showed EpCAM overexpression, making them potential targets for EpCAM-directed immunotherapy.
The cytotoxic activity of MT201 against other gynecologic cancer tumor cells (ie, ovarian cancer) in the presence of PBL has previously been demonstrated by Xiang et al.23 In our study, we extend the results of Xiang et al23 evaluating the cytotoxic potential of MT201 against multiple biologically aggressive cervical cancer cell lines. We found all primary cervical tumor tested showing EpCAM overexpression (ie, CVX-2, CVX-7, CVX-8, and CVX-10) to be highly susceptible to MT201-mediated ADCC in the presence of effector cells regardless of their HPV status. In this regard, it is worth noting that although these cell lines were resistant to NK cell cytotoxic activity (ie, average killing of 7% at an effector/target cell ratio of 50:1), MT201-mediated ADCC resulted in killing up to 83% in 4-hour 51Cr release assays.
For effective cytotoxicity, the effector cells must be able to interact with the antibody at the target site in vivo even in the presence of high concentrations of human IgG. Consistent with the results of El-Sahwi et al5 using MT201 against highly aggressive type II endometrial carcinomas (ie, uterine serous tumors), in this study, we showed that ADCC against cervical cancer cell lines was not inhibited by high concentrations (up to 50%) of human serum. In fact, a significant increase in cytotoxicity was detected in the presence of effector cells and non–heat-inactivated human serum. These data, therefore, suggest that in the presence of effector PBL, human serum may augment MT201-mediated cytotoxicity against cervical cancer cell lines. Moreover, these results indicate that the binding of MT201 to the Fc receptor on mononuclear effector cells is likely to occur in vivo.
MT201-mediated tumor cell lysis depends on the presence of effector cells, and CD56-positive lymphocytes have been shown to contribute most to the effect of IgG1-mediated ADCC in vitro as well as in vivo.14,23,24 Interleukin-2 treatment leads to the activation of NK cell cytotoxicity and expansion of the NK cell population within the PBL in vivo.25 Consistent with its immunostimulatory effect on NK-cells, IL-2 has been previously shown to work synergistically with monoclonal antibodies in vivo.26,27 Importantly, contrary to the significant toxicity of high-dose recombinant IL-2 therapy, low-dose IL-2 administered subcutaneously or by continuous infusion has been shown to have high clinical and immunological activity with a relatively benign toxicity profile.25 These findings are of particular interest because a modulation of the number and function of NK cells has been associated with tumor progression in both in vitro and animal models, and, unfortunately, it is commonly recognized as a result of standard treatment modalities of cervical cancer (ie, radiation and/or chemotherapy).27–29 Pretreatment of the PBL with IL-2 can increase the cytotoxicity levels in patients with suppressed ADCC to levels similar to those of normal donors.16,28 Consistent with these data, our in vitro experiments revealed a significant increase of MT201-induced cytotoxicity after the incubation of PBL with IL-2 compared with the cytotoxicity detected in the absence of IL-2. Interleukin-2 seems to enhance the cytotoxic potential of the effector cells in our study. The administration of low doses of IL-2 might therefore be a valid therapeutic option to increase MT201-mediated ADCC in heavily pretreated cervical cancer patients with metastatic/recurrent disease.
There is a strong need for effective novel targeted therapies in the treatment of advanced and recurrent cervical cancer. The location of EpCAM on the cell surface makes it an attractive target for immunotherapy.14,18 In this study, we have demonstrated a significant MT201-mediated killing of primary cell lines derived from metastatic and recurrent cervical cancers for which treatment options are very limited. Interestingly, this killing was independent of the patients’ HPV status. MT201 might therefore represent a fascinating new addition to the treatment of cervical disease refractory to standard treatment modalities. Clinical trials will ultimately determine the validity of this novel therapeutic approach.
Acknowledgments
This work was supported by grants from the Angelo Novicelli, the Berlucchi and the Camillo Golgi Foundation, Brescia, Italy; NIH R01 CA122728-01A2 to AS; and grants 501/A3/3 and 00227557 from the Italian Institute of Health (ISS) to AS. This investigation was also supported by NIH Research Grant CA-16359 from the National Cancer Institute.
Footnotes
DR is an employee of Micromet AG, Munich, Germany. The other authors declare that they have no competing interests.
References
- 1.Wright JD, Nathavithrana R, Lewin SN, et al. Fertility-conserving surgery for young women with stage IA1 cervical cancer: safety and access. Obstet Gynecol. 2010;115:585–590. doi: 10.1097/AOG.0b013e3181d06b68. [DOI] [PubMed] [Google Scholar]
- 2.Pectasides D, Kamposioras K, Papaxoinis G, et al. Chemotherapy for recurrent cervical cancer. Cancer Treat Rev. 2008;34:603–613. doi: 10.1016/j.ctrv.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Del Campo JM, Prat A, Gil-Moreno A, et al. Update on novel therapeutic agents for cervical cancer. Gynecol Oncol. 2008;110(suppl 2):72–76. doi: 10.1016/j.ygyno.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 5.El-Sahwi K, Bellone S, Cocco E, et al. Overexpression of EpCAM in uterine serous papillary carcinoma: implications for EpCAM-specific immunotherapy with human monoclonal antibody adecatumumab (MT201) Mol Cancer Ther. 2010;9:57–66. doi: 10.1158/1535-7163.MCT-09-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong A, Eck SL. EpCAM. A new therapeutic target for an old cancer antigen. Cancer Biol Ther. 2003;2:320–325. doi: 10.4161/cbt.2.4.451. [DOI] [PubMed] [Google Scholar]
- 7.Muenz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627–5629. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- 8.Muenz M, Fellinger K, Hofmann T, et al. Glycosylation is crucial for stability of tumor and cancer stem cell antigen EpCAM. Front Biosci. 2008;13:5195–5201. doi: 10.2741/3075. [DOI] [PubMed] [Google Scholar]
- 9.Baeuerle PA, Gires O. EpCAM (CD326) finding its role in cancer. Br J Cancer. 2007;96:417–423. doi: 10.1038/sj.bjc.6603494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litvinov SV, Balzar M, Winter MJ, et al. Epithelial cell adhesion molecule (EpCAM) modulates cell-cell interactions mediated by classic cadherins. J Cell Biol. 1997;139:1337–1348. doi: 10.1083/jcb.139.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osta WA, Chen Y, Mikhitarian K, et al. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64:5818–5824. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- 12.Litvinov SV, van Driel W, van Rhijn CM, et al. Expression of Ep-CAM in cervical squamous epithelia correlates with in increased proliferation and the disappearance of markers for terminal differentiation. Am J Pathol. 1996;148:865–875. [PMC free article] [PubMed] [Google Scholar]
- 13.Riethmueller G, Holz E, Schlimok G, et al. Monoclonal antibody therapy for resected Duke’s C colorectal cancer: seven-year outcome of a multicenter randomized trial. J Clin Oncol. 1998;16:1788–1794. doi: 10.1200/JCO.1998.16.5.1788. [DOI] [PubMed] [Google Scholar]
- 14.Naundorf S, Preithner S, Mayer P, et al. In vitro and in vivo activity of MT201, a fully human monoclonal antibody for pancarcinoma treatment. Int J Cancer. 2002;100:101–110. doi: 10.1002/ijc.10443. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt M, Scheulen ME, Dittrich C, et al. An open-label, randomized phase II study of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy in patients with metastatic breast cancer. Ann Oncol. 2010;21:275–282. doi: 10.1093/annonc/mdp314. [DOI] [PubMed] [Google Scholar]
- 16.Marschner N, Rűttinger D, Zugmaier G, et al. Phase II study of the human anti-EpCAM antibody adecatumumab in prostate cancer patients with increasing serum levels of prostate specific antigen after radical prostatectomy. Urol Int. doi: 10.1159/000318055. in press. [DOI] [PubMed] [Google Scholar]
- 17.Sebastian M, Hanusch C, Schmidt M, et al. Safety and antitumor activity of 3 weekly anti-EpCAM antibody adecatumumab in combination with docetaxel for patients with metastatic breast cancer: results of a multicenter phase Ib trial [abstract] J Clin Oncol. 2009;27:s15. [Google Scholar]
- 18.Santin AD, Zhan F, Cané S, et al. Gene expression fingerprint of uterine serous papillary carcinoma: identification of novel molecular markers for uterine serous cancer diagnosis and therapy. Br J Cancer. 2005;92:1561–1573. doi: 10.1038/sj.bjc.6602480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spizzo G, Went P, Dirnhofer S, et al. Overexpression of epithelial cell adhesion molecule (EpCAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol Oncol. 2006;103:483–488. doi: 10.1016/j.ygyno.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 20.Maetzel D, Denzel S, Mack B, et al. Nuclear signaling by tumour-associated antigen EpCAM. Nat Cell Biol. 2009;11:162–171. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- 21.Trzpis M, McLaughlin PMJ, de Leij LMHF, et al. Epithelial cell adhesion molecule. More than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piyathilake CJ, Frost AR, Weiss H, et al. The expression of Ep-CAM (17-1A) in squamous cell cancers of the lung. Hum Pathol. 2000;31:482–487. doi: 10.1053/hp.2000.6711. [DOI] [PubMed] [Google Scholar]
- 23.Xiang W, Wimberger P, Dreier T, et al. Cytotoxic activity of novel human monoclonal antibody MT201 against primary ovarian tumor cells. J Cancer Res Clin Oncol. 2003;129:341–348. doi: 10.1007/s00432-003-0438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preithner S, Elm S, Lippold S, et al. High concentrations of therapeutic IgG1 antibodies are needed to compensate for inhibition of antibody-dependent cellular cytotoxicity by excess endogenous immunoglobulin G. Mol Immunol. 2006;43:1183–1193. doi: 10.1016/j.molimm.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Caligiuri MA, Murray C, Robertson MJ, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91:123–132. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyman O, Surh CD, Sprent J. Potential use of IL-2/anti-IL-2 antibody immune complexes for the treatment of cancer and autoimmune disease. Expert Opin Biol Ther. 2006;6:1323–1331. doi: 10.1517/14712598.6.12.1323. [DOI] [PubMed] [Google Scholar]
- 27.Hoojberg E, Sein JJ, van den Berk PCM, et al. Eradication of large human B cell tumors in nude mice with unconjugated CD20 monoclonal antibodies and interleukin 2. Cancer Res. 1995;55:2627–2634. [PubMed] [Google Scholar]
- 28.Honsik CJ, Jung G, Reisfeld RA. Lymphokine-activated killer cells targeted by monoclonal antibodies to the disialogangliosides GD2 and GD3 specifically lyse human tumor cells of neuroectodermal origin. Proc Natl Acad Sci U S A. 1986;83:7893–7897. doi: 10.1073/pnas.83.20.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santin AD, Hermonat PL, Ravaggi A, et al. Effects of concurrent cisplatinum administration during radiotherapy vs. radiotherapy alone on the immune function of patients with cancer of the uterine cervix. Int J Rad Oncol Biol Phys. 2000;48:997–1006. doi: 10.1016/s0360-3016(00)00769-0. [DOI] [PubMed] [Google Scholar]