Abstract
Introduction
The determination of goitre prevalence in children by ultrasonography is an important tool for considering iodine deficiency disorders. Our objective was to describe measurements of thyroid volumes by ultrasonography in Egyptian South Sinai schoolchildren and compare these with the WHO/International Council for the Control of Iodine Deficiency Disorders normative thyroid volume criteria (WHO/ICCIDD).
Material and methods
Cross-sectional thyroid ultrasonographic data of 719 schoolchildren (339 boys and 380 girls), aged 6-12 years from five cities in South Sinai (El Tur (T), Abu Redis (R), Ras Sudr (S), Saint Katherine (SK), and Nwebaa (N)). Age/sex and body surface area/sex specific upper limits (97th percentile) of normal thyroid volume were derived and urinary iodine (UI) was measured.
Results
The median value of urinary iodine was 150 µg/l. Comparing WHO/ICCIDD thyroid volume references to Egyptian South Sinai schoolchildren resulted in goitre prevalence of 10.6% using age/sex specific and 13.48% using body surface area/sex specific cut-off values. The prevalence of goitre was 20.0% in S, 16.3% in R, 10.8% in N, 9.9% in T, and 10.5% in SC. Upper limits of normal (97th percentile) thyroid volume from South Sinai schoolchildren calculated using BSA, sex, and age were higher than the corresponding WHO/ICCIDD.
Conclusions
Prevalence of goitre is high in South Sinai schoolchildren. The body surface area reference should be preferred to the reference based on age. South Sinai schoolchildren had larger thyroids than WHO/ICCIDD thyroid volumes, perhaps due to hard polluted water with a high fluorine level.
Keywords: South Sinai, thyroid, goitre, iodine, ultrasonography
Introduction
Iodine is an essential trace element for the synthesis of thyroid hormone. Normal human growth and development is dependent upon an adequate supply of thyroid hormone. The essential requirement for normal growth is only 100–150 g/day, but the optimal intake for adults is about 200–300 g daily [1]. The principal source of iodine for human consumption is food. The highest iodine content is found in fish and, to a lesser extent, in milk, eggs, and meat [1]. The most significant and devastating consequences of iodine deficiency are its serious effects on physical and mental development of children [2]. According to the WHO, it is estimated that 285 million school-age children live in countries with significant iodine deficiency and are at risk of its complications [3].
The World Health Organization/International Council for the Control of Iodine Deficiency Disorders (WHO/ICCIDD) adopted a thyroid volume reference in 1997 [4]. However, thyroid volumes from European children from whom the WHO reference data are derived [4] are larger than those in iodine sufficient children from the USA [5], Malaysia [6], Iran [7], Switzerland [8], and Germany [9]. Accordingly, the WHO/ICCIDD conducted thyroid volume studies in 6-12-year-old schoolchildren by ultrasound from iodine sufficient areas of six countries on each continent to establish an international reference for general use [10]. Sex-specific upper normal limits of thyroid volume (the 97th percentile) were provided based on age and body surface area (BSA). In areas with malnutrition, such as Bangladesh, the BSA reference should be preferred to the reference based on age [5], whereas results from US children indicated that thyroid volume reference based on weight alone would perform as well as the one based on BSA [5]. Alternatively, the reference value of thyroid volume in Japanese schoolchildren is rather similar to new WHO/ICCIDD reference values and might be applicable to countries in the Far East [11].
Prevention and control of iodine deficiency disorders can be easily achieved by appropriate iodine supplementation [12–14]. Iodized salt, iodized oil, iodized bread, and iodized water can be used. Salt iodination is most frequently used for iodine prophylaxis, mainly in the form of potassium iodide [1].
In Egypt, endemic goitre and low urinary iodine concentration have been reported in several regions [15–17], where thyroid palpation was the standard method for determining thyroid size. However, since progress is made towards elimination of iodine deficiency disorders, ultrasonographic measurement of thyroid volume is preferable to inspection and palpation for determination of goitre prevalence [4].
The objective of this study was to describe thyroid volumes measured by ultrasonography in South-Sinai schoolchildren and compare these with the WHO/International Council for the Control of Iodine Deficiency Disorders recommended reference.
Material and methods
Subjects
Schoolchildren aged 6-12 years in grades 1-6 were recruited from all elementary schools in 5 cities in South Sinai [El Tur (T), Abu Redis (R), Ras Sudr (S), Saint Katherine (SK), and Nwebaa (N)]. Written consent was obtained from the community school boards. All students available on the day of ultrasonography examination in each grade were recruited. Data were collected during 2009. Ethical approval for the study was obtained from the Ethical Committee of the National Research Centre.
Methods
Weights and standing heights were collected. The BSA (m2) was calculated by using the formula: BSA = weight [kg]0.425 × height [cm]0.725 × 71.84 × 10-4 [18]. Ultrasound volume was measured according to Brunn et al. [19] using Falco 100 Human portable ultrasound units with a standard 5.0 MHz transducer manufactured by Biosound Esaote Pie Medical (Genoa, Italy). The volume of each lobe was calculated by the formula: V [ml] = 0.000479 × length × width × thickness [mm]. The thyroid volume was the sum of the volumes of both lobes. The volume of the isthmus was not included [11]. Thyroid glands were classified into normal or enlarged using the new WHO references, thyroid volume-for-age and thyroid volume-for-BSA [11]. Thyroid volumes greater than the 97th percentile were considered abnormally enlarged and those less than or equal to the 97th percentile as normal. Urine samples from 87 children were randomly selected from different cities of South Sinai to measure urinary iodine excretion by the catalytic method [20]. WHO/UNICEF/ICCIDD-recommended criteria have been used to classify a population's severity of iodine deficiency disorders (IDD) based on schoolchildren [21, 22]. The epidemiological criteria for assessing iodine nutrition are based on median UI and are as follows: mild deficiency, 50–99 µg/l; moderate deficiency, 20–49 µg/l; and severe deficiency, < 20 µg/l [11].
Statistical analysis
Statistical analysis was performed using the SPSS for Windows statistical software package version 15 (SPSS Inc., Chicago, IL, USA). Means and standard deviations of thyroid volume were used as parameters to fit a normal distribution and 97th (P97) percentiles were calculated based on the standard normal distribution. Independent sample t-test was used to compare between cases and controls and between the different groups. A p-value of less than 0.05 was considered statistically significant. Curves of the P97 thyroid volumes against age and BSA were constructed and smoothed using regression.
Results
Descriptive data and urinary iodine level of the study group
A total of 718 students were included in the study from 8 schools all over South Sinai in 5 cities [El Tur (T), Abu Redis (R), Ras Sudr (S), Saint Katherine (SK), and Nwebaa (N)]. The sample included 338 males and 380 females aged 6-12 years. Mean age (SD) was 8.7 (1.92) for males and 9.0 (1.88) for females. Characteristics of the subjects with goitre by age and sex are shown in Table I. No difference was found between males and females in each age group studied. The number of children with thyroid nodules was 8 children (5 males and 3 females). The median UI (range) of 284 children randomly selected from different schools in the 5 cities from South Sinai was 150 µg/l (18-380 µg/l). Median urinary iodine concentrations of children with and without goitre were 126 µg/l and 174 µg/l, respectively, and the difference was not significant (p = 0.087).
Table I.
Characteristics of subjects with goitre by age and sex
| Age [years] | Sex | N | BSA* [m2] (mean) | Thyroid volume [ml] | No. of subjects with goitre (%) | ||
|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Median | |||||
| 6 | F | 8 | 0.7 | 4.0 ±0.8 | 3.2-5.8 | 3.1 | 8/58 (13.8) |
| M | 12 | 0.7 | 4.1 ±1.0 | 3.3-6.6 | 3.81 | 12/68 (17.6) | |
| 7 | F | 7 | 0.8 | 4.3 ±0.8 | 3.5-5.7 | 4.3 | 7/58 (12.0) |
| M | 8 | 0.8 | 4.6 ±1.1 | 3.5-6.4 | 4.34 | 8/54 (14.8) | |
| 8 | F | 4 | 0.9 | 4.8 ±1.2 | 3.9-5.8 | 4.8 | 4/49 (8.0) |
| M | 9 | 0.9 | 5.2 ±1.1 | 4.0-6.4 | 4.7 | 9/66 (13.6) | |
| 9 | F | 5 | 1.0 | 5.9 ±1.3 | 4.5-7.7 | 4.9 | 5/52 (9.6) |
| M | 6 | 1.0 | 5.7 ±1.0 | 4.6-7.0 | 5.2 | 6/35 (17.0) | |
| 10 | F | 10 | 1.1 | 6.5 ±1.3 | 5.2-8.3 | 6.2 | 10/60 (16.7) |
| M | 6 | 1.1 | 6.2 ±1.2 | 5.0-8.1 | 5.9 | 6/38 (15.8) | |
| 11 | F | 8 | 1.2 | 7.1 ±1.6 | 5.5-11.0 | 7.0 | 8/56 (14.3) |
| M | 6 | 1.2 | 6.9 ±1.7 | 6.8-10.9 | 7.8 | 6/46 (13.0) | |
| 12 | F | 5 | 1.3 | 8.0 ±2.1 | 6.2-11.0 | 8.0 | 5/49 (10.2) |
| M | 4 | 1.3 | 8.2 ±1.8 | 6.5-11.2 | 7.9 | 4/26 (15.4) | |
| Total | 98 | ||||||
| F/M | 47/51 | ||||||
Body surface area. No difference was found between males and females in each age group
Goitre prevalence in the study group
The goitre prevalence in our sample using the P97 of the current WHO/ICCIDD recommended cut-off values was 13.6% (15.1% for males and 12.4% for females). The distribution of goitre in the 5 cities was 20.0% in S, 16.3% in R, 10.8% in N, 9.9% in T, and 10.5% in SC (Table II). The number of students with goitre in both sexes based on age was 76 with a prevalence of goitre of 10.6%, whereas the number of students with goitre in both sexes based on BSA was 96 with a prevalence of goitre of 13.4% (Table I). There was no gender difference in thyroid volume at any age or BSA (data not shown). The mean (SD) thyroid volumes in different age and BSA groups are shown in Table III.
Table II.
Mean ± SD of thyroid volumes by sex and prevalence of goitre based on age and body surface area (BSA) in 5 cities in Egyptian South Sinai schoolchildren
| City | Males | Females | Total both sexes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Thyroid volume [ml] | Number of | Total | n | Thyroid volume [ml] | Number of | Total | ||||
| goitres by age | goitres by BSA | goitres by age | goitres by BSA | ||||||||
| T | 88 | 3.1 ±2.0 | 8 | 13 | 13 (14.7%) | 124 | 2.4 ±1.2 | 8 | 6 | 8 (6.5%) | 21/212 (9.9%) |
| R | 84 | 2.7 ±1.2 | 9 | 15 | 15 (17.9%) | 100 | 3.1 ±1.6 | 14 | 15 | 15 (15%) | 30/184 (16.3%) |
| S | 67 | 3.6 ±1.4 | 13 | 14 | 14 (20.9%) | 68 | 4.1 ±1.6 | 11 | 13 | 13 (19.1%) | 27/135 (20.0%) |
| SK | 33 | 1.7 ±0.8 | 3 | 3 | 3 (9.1%) | 24 | 1.6 ±0.8 | 1 | 3 | 3 (12.5%) | 6/57 (10.5%) |
| N | 66 | 2.3 ±1.1 | 5 | 6 | 6 (9.1%) | 64 | 2.8 ±2.1 | 4 | 8 | 8 (12.5%) | 14/130 (10.8%) |
| Total | 338 | 3.5 ±1.9 | 38 | 51 | 51 (15.1%) | 380 | 3.2 ±1.9 | 38 | 45 | 47 (12.4%) | 98/718 (13.6%) |
T (El Tur), R (Abu Redis), S (Ras Sudr), SK (Saint Katherine), N (Nwebaa)
Table III.
Mean ± SD of thyroid volumes measured by ultrasonography according to age and BSA (sexes combined)
| Age [years] | n | Thyroid volume [ml] | BSA | n | Thyroid volume [ml] |
|---|---|---|---|---|---|
| 6 | 126 | 2.1 ±1.1 | 0.6 | 6 | 2.0 ±0.6 |
| 7 | 112 | 2.2 ±1.0 | 0.7 | 76 | 2.1 ±0.9 |
| 8 | 117 | 2.5 ±1.1 | 0.8 | 170 | 2.4 ±1.2 |
| 9 | 88 | 2.9 ±1.3 | 0.9 | 162 | 2.6 ±1.1 |
| 10 | 98 | 3.6 ±1.7 | 1.0 | 129 | 3.1 ±1.5 |
| 11 | 102 | 3.8±2.2 | 1.1 | 92 | 3.9 ±2.1 |
| 12 | 75 | 4.5±2.2 | 1.2 1.3 |
49 32 |
4.6 ±2.6 4.1 ±1.7 |
Age/sex and BSA/sex specific P97 curves of thyroid volume
Figure 1 and Table IV compare our age/sex specific P97 curve of thyroid volume with the WHO/ICCIDD recommended reference curves [11]. Our age/sex specific P97 curves of thyroid volume are 42% to 55% larger than the WHO/ICCIDD cut-off values [9].
Figure 1.
Comparison of the age/sex specific WHO/ICCIDD upper limits with changes in thyroid volume, by sex and age, in Egyptian South Sinai school-children (sexes combined) (P97 = 97th percentile, i.e. upper limit of normal)
Table IV.
Comparison of median and P97 (97th percentile) of thyroid volumes measured by ultrasonography according to age (sexes combined) with the 2003 WHO/ICCIDD* international reference values
| Age | Total | Boys | Girls | |||
|---|---|---|---|---|---|---|
| Median | P97 | Median* | P97* | Median* | P97* | |
| 6 | 1.9 | 4.5 | 1.60 | 2.91 | 1.57 | 2.84 |
| 7 | 2.0 | 4.8 | 1.80 | 3.29 | 1.81 | 3.26 |
| 8 | 2.3 | 5.5 | 2.03 | 3.71 | 2.08 | 3.76 |
| 9 | 2.7 | 6.2 | 2.30 | 4.19 | 2.40 | 4.32 |
| 10 | 3.3 | 7.0 | 2.59 | 4.73 | 2.76 | 4.98 |
| 11 | 3.5 | 7.8 | 2.92 | 5.34 | 3.17 | 5.73 |
| 12 | 4.1 | 8.6 | 3.30 | 6.03 | 3.65 | 6.59 |
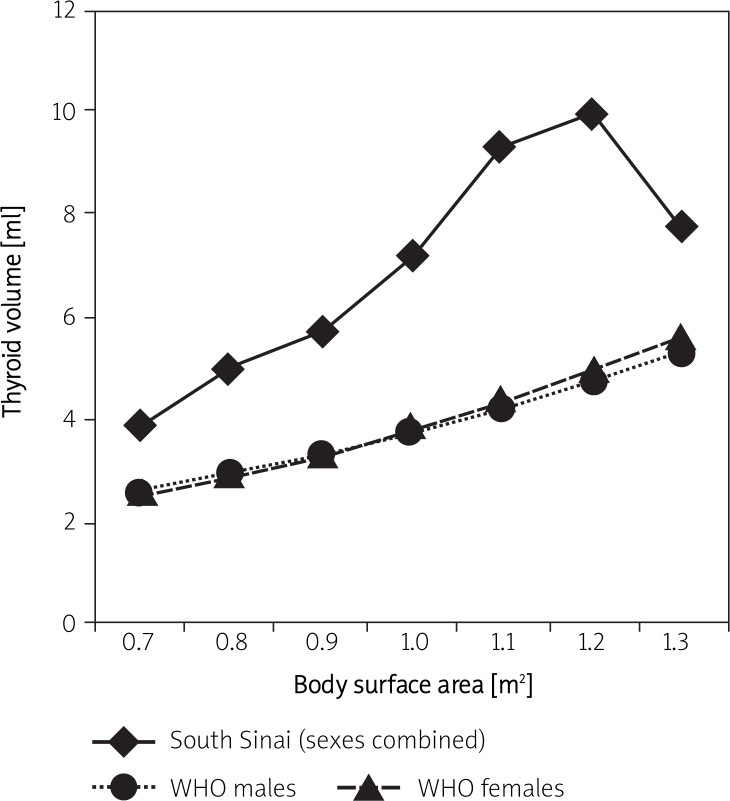
Figure 2 and Table V compare our BSA/sex specific P97 curve of thyroid volume with the WHO/ICCIDD recommended reference curves [11]. Our BSA/sex specific P97 curves of thyroid volume are 49% to 121% larger than the WHO/ICCIDD cut-off values [9]. In contrast, our age and BSA/sex specific P97 curves of thyroid volume are 20-40% lower than the previous European children dependent WHO/ICCIDD recommended reference cut-off values published in 1997 [3].
Figure 2.
Comparison of the BSA/sex specific WHO/ICCIDD upper limits with changes in thyroid volume, by sex and age, in Egyptian South Sinai school-children (sexes combined) (P97 = 97th percentile, i.e. upper limit of normal)
Table V.
Comparison of median and P97 (97th percentile) of thyroid volumes measured by ultrasonography according to BSA (sexes combined) with the 2003 WHO/ICCIDD* international reference values
| BSA | Total | Boys | Girls | |||
|---|---|---|---|---|---|---|
| Median | P97 | Median* | P97* | Median* | P97* | |
| 0.6 | 1.9 | 2.2 | ||||
| 0.7 | 1.9 | 3.9 | 1.47 | 2.62 | 1.46 | 2.56 |
| 0.8 | 2.1 | 5.1 | 1.66 | 2.95 | 1.67 | 2.91 |
| 0.9 | 2.4 | 5.7 | 1.86 | 3.32 | 1.9 | 3.32 |
| 1.0 | 2.7 | 7.2 | 2.1 | 3.73 | 2.17 | 3.79 |
| 1.1 | 3.5 | 9.3 | 2.36 | 4.2 | 2.47 | 4.32 |
| 1.2 | 4.1 | 9.9 | 2.65 | 4.73 | 2.82 | 4.92 |
| 1.3 | 3.5 | 7.7 | 2.99 | 5.32 | 3.21 | 5.61 |
Discussion
Endemic iodine deficiency is defined by the goitre prevalence and the median iodine concentration in a population. According to WHO, a region is considered endemic if more than 5% of schoolchildren have goitre or thyroid enlargement [21]. Thyroid ultrasound, along with measurement of urinary iodide levels, has been recommended by WHO/ ICCIDD for monitoring the sustained impact of iodine deficiency control programs through universal salt iodisation. Since their introduction in 1997, the recommended normative values for thyroid volume in children have been revised on two occasions. The validity of the 1997 WHO/ICCIDD recommended values was challenged by a number of studies, including surveys using a ThyroMobil [22]. A new set of international reference values for thyroid volume assessed by ultrasound examination, based on studies of children living in areas of long-term iodine sufficiency, was released in 2003 [11]. The thyroid volume results in our study were compared with these new international reference values. The Egyptian South Sinai schoolchildren in this study had larger thyroid volumes than the iodine-sufficient children from which the WHO/ICCIDD reference data are derived [11]. In contrast, our age and BSA/sex specific P97 curves of thyroid volume are 20-40% lower than the previous European children dependent WHO/ICCIDD recommended reference cut-off values [3]. We compared our thyroid volume results with the updated normal age/sex specific values and 12.8% of children had an enlarged thyroid gland. However, using body surface area/sex specific cut-off values for the same children resulted in a goitre prevalence of 20.8%. This is considered moderate iodine deficiency according to WHO/UNICEF/ICCIDD-recommended criteria [21, 23]. It is recommended that thyroid volume based on BSA should be used in areas with malnutrition [3, 24]. Since BSA is the best predictor of thyroid volume, at least in Egyptian children, we would advise that it should always be used, regardless of the nutritional status of the population when both weight and height are available.
It is known that genetic predisposition and environmental factors are involved in the regulation of thyroid volume [25]. In iodine-deficient areas the effect of iodine deficiency is the most important determinant, while in an iodine sufficient area the effect of dietary habits and genetic differences in growth and development on thyroid volume is reported in children [26]. Prevalence of goitre in Egypt has not changed much in the past few decades. In 1995, 1996, and 1997, the rate was 13.5%, 19.6%, and 19.4% in Cairo, Upper Egypt, and Alexandria, respectively [25, 27]. After implementation of the Universal Salt Iodization Program, goitre prevalence was 21.4%, 57.5%, and 31.9% and 60.1% in Cairo [28], New Valley (a desert oasis) [16, 29], and in two Delta Governorates [17], respectively. Thus, the prevalence of goitre is still high despite the implementation of the Universal Salt Iodization Program in Egypt. The problem of goitre is also prevalent in other Eastern Mediterranean countries. In 2004, WHO/UNICEF/ICCIDD studied the prevalence of goitre in the Eastern Mediterranean area where the highest rate was detected in Syria (70%) and the lowest in Tunisia (0.58%) [30].
The median urinary iodine concentration (UIC) of the study was 150 µg/l, showing adequacy of iodine nutrition; 11.5% of the studied group had UIC below 50 µg/l and 31% had UIC below 100 µg/l. According to WHO/UNICEF/ICCIDD criteria [21, 22], the proportion of the population with UIC less than 100 µg/l should not exceed 50% and those with UIC less than 50 µg/l should not exceed 20%. Our UIC corresponds exactly to those reported earlier in Egypt and from iodine-sufficient areas such as Campania in southern Italy (median UI = 80 µg/l) [31], Switzerland (median UI = 150 µg/l) [32], Malaysia (median UI = 132.8 µg/l) [6], the Netherlands (median UI = 154.4 µg/l) [26], and Australia (median UI = 132.8 µg/l) [33]. However, it is smaller than the Atlanta metropolitan area in the United States (median UI = 282 µg/l) [5] and Japan (median UI = 281.6 µg/l) [11]. Although the exact reason is not clear, lower iodine intake may partially account for the larger thyroids in Egyptian South Sinai schoolchildren.
In the present study, no difference in thyroid volumes was found between males and females. A number of other studies based on ultrasonography in iodine sufficient areas have also found no difference by sex [5, 34]. On the other hand, larger thyroid volume was found in European girls [3]; this may be because either borderline iodine deficiency affects girls more or enlarged thyroid regresses less quickly in girls after iodine intervention.
The prevalence of iodine deficiency is still high in South Sinai despite the implementation of a universal salt iodization program since 1996. In many developing countries including Egypt, despite improvement of salt production and marketing technology, the quality of salt is still poor, incorrectly iodized or spoilt due to excessive exposure to moisture, light, heat, and contaminants [35]. It was previously concluded that iodine nutrition status in Egypt is inadequate although significant improvement in iodized salt utilization has been achieved [29]. However, current iodine adequacy does not explain the increased prevalence of goitre detected in the study. Many factors interfere to affect iodine status, including undernutrition and iron deficiency anaemia. Iron deficiency adversely affects thyroid metabolism and may reduce the efficacy of iodine prophylaxis in areas with endemic goitre and iron supplementation improves the efficacy of iodized salt in goitrous children [36]. Moreover, increased prevalence of goitre has also been related to bacterial pollution of drinking water and excessive fluoride intake. Water supply from wells in Sinai showed in analytical studies the presence of Escherichia coli and excess fluorine content and previous reports demonstrated that the occurrence of goitre in iodine-sufficient areas in Africa is due to fluoride [37].
In conclusion, prevalence of goitre is high in South Sinai schoolchildren. Hard polluted water with a high fluorine level which is the main water supply in these areas could be one of the causes of goitre. We recommend strengthening the monitoring system for the salt iodization program to ensure regular quality control, conducting a periodic survey on a representative sample, and undertaking international education to promote community awareness of the importance of the use of iodized salt and the hazardous effects of inappropriate iodine intake.
Acknowledgments
This document has been produced with the financial assistance of the EU. The contents of the document are the sole responsibility of Professor Dr. Yamamah and can under no circumstances be regarded as reflecting the position of the EU.
References
- 1.Hetzel BS. The history of goiter and cretinism. In: Hetzel BS, editor. The story of iodine deficiency: an international challenge in nutrition. New York: Oxford University Press; 1989. pp. 3–20. [Google Scholar]
- 2.WHO-UNICEF-ICCIDD. Global Iodine nutrition IDD Newsletter; 2003. pp. 24–5. [Google Scholar]
- 3.ICCIDD, UNICEF, WHO. 2nd ed. Geneva: WHO; 2001. Assessment of IDD and monitoring their elimination. [Google Scholar]
- 4.WHO/ICCIDD. Recommended normative values for thyroid volume in children aged 6-15 years. Bull World Health Org. 1997;75:95–7. [PMC free article] [PubMed] [Google Scholar]
- 5.Xu F, Sullivan K, Houston R, Zhao J, May W, Maberly G. Thyroid volumes in US and Bangladeshi schoolchildren: comparison with European schoolchildren. Eur J Endocrinol. 1999;140:498–504. doi: 10.1530/eje.0.1400498. [DOI] [PubMed] [Google Scholar]
- 6.Foo LC, Zulfiqar A, Nafikudin M, Fadzil MT, Asmah AS. Local versus WHO/International Council for Control of Iodine Deficiency Disorders-recommended thyroid volume reference in the assessment of iodine deficiency disorders. Eur J Endocrinol. 1999;140:491–7. doi: 10.1530/eje.0.1400491. [DOI] [PubMed] [Google Scholar]
- 7.Azizi F, Delshad H, Mehrabi Y. Thyroid volumes in schoolchildren of Tehran: comparison with European schoolchildren. J Endocrinol Invest. 2001;24:756–62. doi: 10.1007/BF03343924. [DOI] [PubMed] [Google Scholar]
- 8.Hess SY, Zimmermann MB. Thyroid volumes in a national sample of iodine-sufficient swiss school children: comparison with the World Health Organization/International Council for the control of iodine deficiency disorders normative thyroid volume criteria. Eur J Endocrinol. 2000;142:599–603. doi: 10.1530/eje.0.1420599. [DOI] [PubMed] [Google Scholar]
- 9.Rendl J, Juhran N, Reiners C. Thyroid volumes and urinary iodine in German school children. Exp Clin Endocrinol Diabetes. 2001;109:8–12. doi: 10.1055/s-2001-11015. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann MB, Hess SY, Molinari L, et al. New reference values for thyroid volume by ultrasound in iodine-sufficient schoolchildren: a World Health Organization/Nutrition for Health and Development Iodine Deficiency Study Group Report. Am J Clin Nutr. 2004;79:231–7. doi: 10.1093/ajcn/79.2.231. [DOI] [PubMed] [Google Scholar]
- 11.Fuse Y, Saito N, Tsuchiya T, Shishiba Y, Irie M. Smaller thyroid gland volume with high urinary iodine excretion in Japanese schoolchildren: normative reference values in an iodine-sufficient area and comparison with the WHO/ICCIDD reference. Thyroid. 2007;17:145–55. doi: 10.1089/thy.2006.0209. [DOI] [PubMed] [Google Scholar]
- 12.Jukić T, Dabelić N, Rogan SA, et al. The story of the Croatian village of Rude after fifty years of compulsory salt iodination in Croatia. Coll Antropol. 2008;32:1251–4. [PubMed] [Google Scholar]
- 13.Li M, Eastman CJ, Waite KV, et al. Are Australian children iodine deficient? Results of the Australian National Iodine Nutrition Study. Med J Aust. 2006;184:165–9. doi: 10.5694/j.1326-5377.2008.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 14.Erdogan MF, Ağbaht K, Altunsu T, et al. Current iodine status in Turkey. J Endocrinol Invest. 2009;32:617–22. doi: 10.1007/BF03346519. [DOI] [PubMed] [Google Scholar]
- 15.El Sayed NA, Ismail HM, Hussein MA, Kamel AR. Assessment of the prevalence of iodine deficiency disorders among primary school children in Cairo. East Mediterr Health J. 1995;1:55–63. [Google Scholar]
- 16.Yamamah GH, Hassanien MH. Prevalence of goiter among school children of the New Vally. The value of urinary iodine in diagnosis and prognosis. JAC. 1997;8:553–63. [Google Scholar]
- 17.Mansour E, Abdel Raouf RK, El-Nekhily I, Ahmed A, Moharam N. Iodine deficiency among school children in Qualiobia and Demietta Governorates. JAC. 2001;12:683–701. [Google Scholar]
- 18.DuBois D, DuBois EF, Clinical calorimetry. X. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71. [Google Scholar]
- 19.Brunn I, Block U, Ruf J, Bos I, Kunze WP, Scriba PC. Volumetrie der schildrüsenlappen mittels real-time-sonographie. Deutsche Medizinische Wochenschrift. 1981;106:1338–40. doi: 10.1055/s-2008-1070506. [DOI] [PubMed] [Google Scholar]
- 20.Sandell EB, Kolthoff M. Micro determination of iodine by catalytic method. Mikrochim Acta. 1937;1:9–25. [Google Scholar]
- 21.WHO/UNICEF/ICCIDD. Geneva: WHO; 1994. Indicators for assessing iodine deficiency disorders and their control program through salt iodization. [Google Scholar]
- 22.Djokomoeljanto R, Setyawan H, Dramaix M, Hadisaputro S, Soehartono T, Delange F. The ThyroMobil model for standardized evaluation of iodine deficiency disorder control in Indonesia. Thyroid. 2001;11:365–72. doi: 10.1089/10507250152039118. [DOI] [PubMed] [Google Scholar]
- 23.WHO/UNICEF/ICCIDD. Experiences in the prevention, control and elimination of IDD: a regional perspective. East Mediterr Health J. 2004;10:761–70. [PubMed] [Google Scholar]
- 24.Brahmbhatt S, Brahmbhatt RM, Boyages SC. Thyroid ultrasound is the best prevalence indicator for assessment of iodine deficiency disorders: a study in rural/tribal schoolchildren from Gujarat (Western India) Eur J Endocrinol. 2000;143:37–46. doi: 10.1530/eje.0.1430037. [DOI] [PubMed] [Google Scholar]
- 25.Langer P, Tajtáková M, Bohov P, Klimes I. Possible role of genetic factors in thyroid growth rate and in the assessment of upper limit of normal thyroid volume in iodine-replete adolescents. Thyroid. 1999;9:557–62. doi: 10.1089/thy.1999.9.557. [DOI] [PubMed] [Google Scholar]
- 26.Wiersinga WM, Podoba J, Srbecky M, et al. A survey of iodine intake and thyroid volume in Dutch schoolchildren: reference values in an iodine-sufficient area and the effect of puberty. Eur J Endocrinol. 2001;144:595–603. doi: 10.1530/eje.0.1440595. [DOI] [PubMed] [Google Scholar]
- 27.Hamed AM. A study of the prevalence of IDD among primary school children in Alexandria; Alexandria: Alexandria University; 1997. MD thesis. [Google Scholar]
- 28.MOH Egypt, ICCIDD, UNICEF. National urinary iodine survey and USI 2006-2007; 2009. [Google Scholar]
- 29.UNICEF, HIPH. Report on assessment of the prevalence of IDD in New Valley Governorate; Alexandria: High Institute of Public Health; [Google Scholar]
- 30.WHO, UNICEF, ICCIDD. Assessment of iodine deficiency disorders and monitoring their elimination: a guide for program managers; Geneva: WHO/NHD; 2001. [Google Scholar]
- 31.Lupoli G, Russo D, Fittipaldi MR, et al. Evaluation of goiter endemia by ultrasound in schoolchildren in Val Sarmento (Italy) J Endocrinol Invest. 1999;22:503–7. doi: 10.1007/BF03343600. [DOI] [PubMed] [Google Scholar]
- 32.Bürgi H, Portmann L, Podoba J, Vertongen F, Srbecky M. Thyroid volumes and urinary iodine in Swiss school children, 17 years after improved prophylaxis of iodine deficiency. Eur J Endocrinol. 1999;140:104–6. doi: 10.1530/eje.0.1400104. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Eastman CJ, Waite KV, et al. Are Australian children iodine deficient? Results of the Australian National Iodine Nutrition Study. Med J Aust. 2006;184:165–9. doi: 10.5694/j.1326-5377.2008.tb01831.x. [DOI] [PubMed] [Google Scholar]
- 34.Vitti P, Martino E, Aghini-Lombardi F, et al. Thyroid volume measurement by ultrasound in children as a tool for the assessment of mild iodine deficiency. J Clin Endocrinol Metab. 1994;79:600–3. doi: 10.1210/jcem.79.2.8045982. [DOI] [PubMed] [Google Scholar]
- 35.WHO. Assessment and monitoring of Iodine Deficiency Disorders in countries of the Eastern Mediterranean region; Report on Symposium – Workshop in Tehran, Islamic Republic of Iran; 2000. [Google Scholar]
- 36.Hess SY, Zimmermann MB, Adou P, Torresani T, Hurrell RF. Treatment of iron deficiency in goitrous children improves the efficacy of iodized salt in Côte d'Ivoire. Am J Clin Nutr. 2002;75:743–8. doi: 10.1093/ajcn/75.4.743. [DOI] [PubMed] [Google Scholar]
- 37.Jooste PL, Weight MJ, Kriek JA, Louw AJ. Endemic goitre in the absence of iodine deficiency in schoolchildren of the Northern Cape Province of South Africa. Eur J Clin Nutr. 1999;53:8–12. doi: 10.1038/sj.ejcn.1600671. [DOI] [PubMed] [Google Scholar]