Abstract
Introduction
Regulatory T cells (Tregs, CD4 + CD25high Foxp3+) play a crucial role in allergy and other inflammatory diseases. However, the isolation of viable Tregs on the basis of intracellular expression of specific Forkhead Box Protein P3 (Foxp3) is difficult. In this study we checked if the expression of IL-7 receptor (CD127) on the Tregs could be a useful marker for isolation of viable Treg Foxp3+ cells.
Material and methods
Twenty-five patients sensitized to grass pollen with allergic rhinitis (AR) and ten healthy subjects were included. We compared Foxp3 expression in different CD4+ T cell subsets by flow cytometry and we assessed the relationship between the expression of Foxp3 and CD127 within regulatory T cells.
Results
Within the CD4+ lymphocytes 3.68 ±2.0% showed expression of Foxp3, 51.82 ±8.03% of CD4+CD25high were Foxp3 positive (Foxp3+), whereas 82.12 ±5.4% of CD4+CD25highCD127low were Foxp3+. High intracellular expression of Foxp3 correlated with low superficial CD127 expression (r = 0.42, p = 0.017). There were no significant differences regarding the analysed markers between AR patients and healthy controls.
Conclusions
Regulatory T cells may be purified from the fresh peripheral blood as viable regulatory Foxp3 bright cells using CD4, high expression of CD25 and low expression of CD127 antigen.
Keywords: allergy, cytokines, inflammation, rhinitis, regulatory lymphocytes
Introduction
Regulatory T CD4 cells (Tregs), expressing transcription factor Foxp3, play a critical role in the maintenance of immunological tolerance and control of autoimmunity and effectively suppress allergic inflammation. T CD4+ are a heterogeneous group of cells in phenotype and function [1]. It was demonstrated that CD25 is a crucial surface antigen for the regulatory T CD4+ subset, especially cells expressing the highest levels of CD25 (CD25high), which have suppressive activity [1, 2]. Furthermore, particularly the T CD4+CD25high subset includes transcription factor Foxp3, which plays a crucial role in suppressive and modulatory mechanisms [1, 2].
Interleukin-7, which is constitutively produced especially by stromal cells from the thymus and bone marrow [3], is essential for T lymphocyte development and function. It is crucial for the maturation and differentiation of memory CD4+ and CD8+ T cells and for the survival and homeostatic proliferation of naive T cells [4]. The biological effects of IL-7 are mediated via the IL-7 receptor (IL-7R, CD127). The receptor for IL-7 is expressed on naive T CD4 and CD8 cells, and memory CD4+ as well as CD8+ cells, but is downregulated on all human T cells after activation [4].
In this study, we assessed the expression of Foxp3 separately in T CD4+, T CD4+CD25high and T CD4+CD25highCD127low cells both in healthy and AR patients. We also assessed whether a low CD127 expression level correlates with higher expression of Foxp3 and if CD127 could be a useful additional marker for isolation of viable Tregs from the peripheral blood.
Material and methods
Patients
Thirty-five individuals (20 female, 15 male; 25 patients with allergic rhinitis and 10 healthy) were included in the study. Allergic rhinitis was diagnosed on the basis of a positive history, a skin prick test positive for grass pollen (mixture for five common grasses, Allergopharma®, Reinbek, Germany), specific serum IgE for grass pollen (> 1.5 international units per millilitre (UniCap®, Pharmacia & Upjohn, Diagnostics AB) and positive result of a conjunctival provocation test with grass pollen allergen extracts (Allergopharma®, Reinbek, Germany). Patients suffering from persistent allergic rhinitis, asthma, upper respiratory tract infection during the 6 weeks preceding the study, neoplastic disease, autoimmunological disease (e.g. diabetes type I, systemic lupus erythematosus), current smokers, patients with tuberculosis, chronic sinusitis, systemic glucocorticosteroid treatment, immunosuppressive treatment, chronic obstructive pulmonary disease (COPD), heart failure, uncontrolled hypertension as well as pregnant and breast feeding women were all excluded from the study. In addition the healthy individuals included as a control were non-atopic and had no symptoms of allergy.
All patients discontinued their medications, including topical and oral antihistamines and topical corticosteroids, for at least 4 weeks before the visit.
The study was approved by the local Ethical Committee of the Medical University of Lodz and written informed consent was obtained from the patients.
Outside the pollen season, after the initial inclusion and exclusion criteria evaluation, a blood sample (20 ml) was drawn from all participants.
Peripheral blood mononuclear cell preparation
Leukocytes were prepared from the whole blood anticoagulated with heparin. Blood samples (20 ml) were centrifuged (180 xg, 10 min, at room temperature) and the platelet rich plasma was removed. The phase containing cells was collected and cells were suspended in RPMI-1640 medium 1: 1 (Sigma-Aldrich®, Poznań, Poland). The peripheral blood mononuclear leukocyte (PBML) fractions were obtained by density gradient centrifugation (460 xg, 30 min, at room temperature) over Ficoll-Paque (GE Medical Systems®, Warsaw, Poland). The interphase containing PBMLs was collected and suspended in RPMI-1640 medium (up to 15 ml). Samples were centrifuged (400 xg, 10 min, at room temperature) then supernatant was removed and cells were suspended in 1 ml of RPMI-1640 medium. Cells were counted and the density of this suspension was assessed.
Flow cytometry
To assess CD4+CD25highCD127lowFoxP3+ lymphocytes, we used Human Treg Flow Kit (BioLegend®, San Diego, USA). PBMLs were incubated with anti-CD4 PE-Cy5 and CD25 PE and anti-CD127 AlexaFluor 647 (BioLegend®, San Diego, USA) antibodies for 20 min at room temperature in the dark. After washing with phosphate buffered saline (PBS), 20 min incubation with fixation/permeabilisation buffer and then permeabilization buffer (BioLegend®, San Diego, USA) was performed. Next, the cells were washed with PBS and Alexa Fluor 488 anti-human FoxP3 antibodies (BioLegend®, San Diego, USA) were added and cells were incubated for 30 min at room temperature in the dark. After washing with PBS, cells were suspended in 10% CellFix solution (Becton Dickinson®, Warsaw, Poland).
Samples were analysed on FACSCanto™ II and LSR (3-laser, 8-colour benchtop flow cytometer, Becton Dickinson®, MI, USA) and were determined by comparison with cells stained with isotype control. The data acquisition threshold was set on a forward channel to exclude dead cells and debris with very low size. The results were analysed using FACSDiva software (Becton Dickinson®, MI, USA).
Statistical analysis
Data were expressed as mean ± standard error of means (SEM) unless otherwise stated. To assess normality we used the Kolmogorov-Smirnov test. To compare between the AR and healthy group for normally distributed data unpaired t-test whereas for non-parametric data Mann-Whitney tests were used. When more than 2 groups were compared, ANOVA test was used. To assess correlations Spearman test was applied. A p < 0.05 was regarded as statistically significant. Statistica 5.1 PL for Windows software (StatSoft Polska®, Cracow, Poland) was used for analyses.
Results
The study population included 25 rhinitic, non-asthmatic patients (18 with allergic rhinitis (AR), 7 with AR and allergic conjunctivitis) and 10 healthy subjects as a control group. Sensitization to grass pollen was confirmed with skin prick tests, conjunctival provocation tests and specific IgE for grass allergens in the serum. None of the patients had clinically relevant sensitization to perennial allergens. Detailed characteristics of subjects participating in the study are presented in Table I.
Table I.
Patients’ baseline characteristics
| Variable | Patients with allergic rhinitis | Healthy controls |
|---|---|---|
| Number of subjects | 25 | 10 |
| Sex F: M | 14: 11 | 4: 6 |
| Ethnic origin | Caucasian (100%) | Caucasian (100%) |
| Mean age [years] | 30.2 ±8.6 | 26.3 ±4.1 |
| Duration of allergic rhiniti [years] | 10.4 ±5.5 | – |
Values are presented as mean with the SD
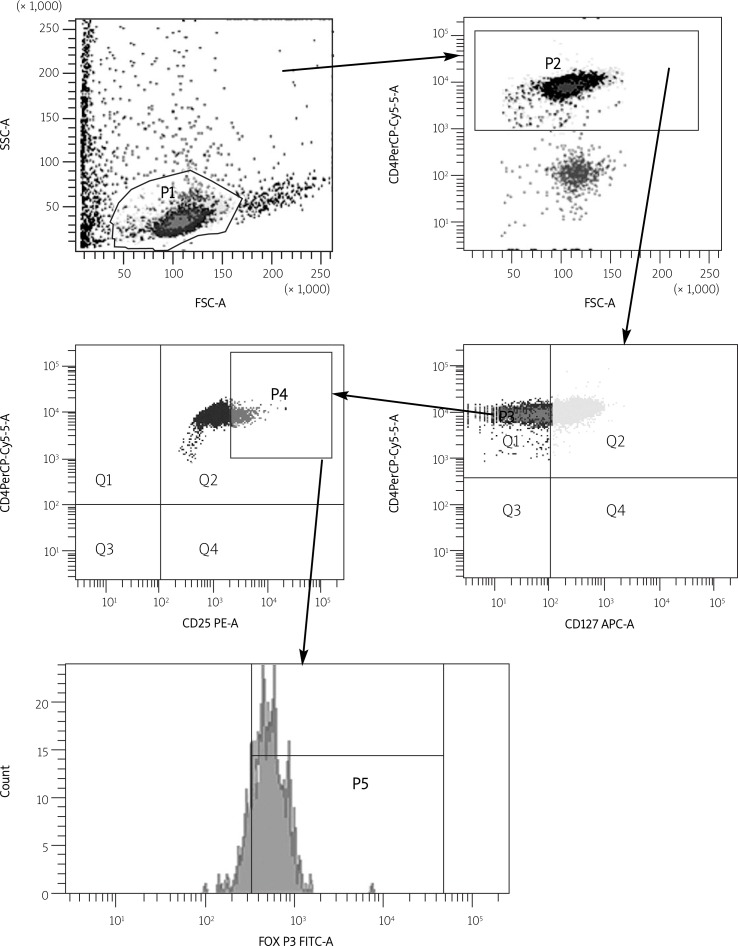
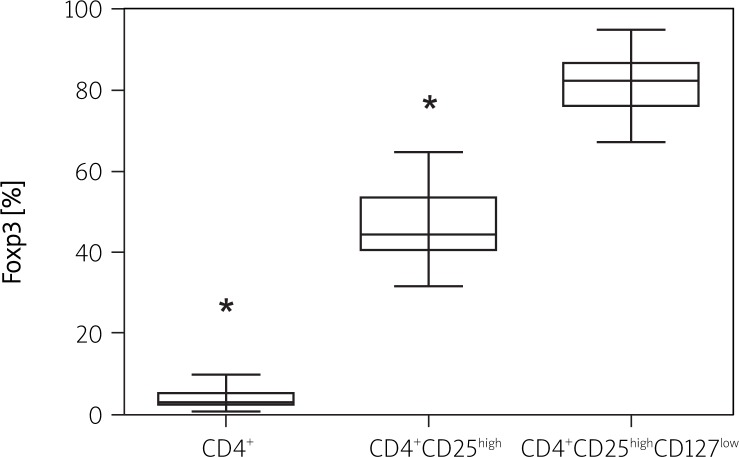
The PBMCs of healthy control and AR patients were stained with fluorescent labelled Abs directed against CD4, CD25, CD127 and Foxp3 and analysed by means of flow cytometry (Figure 1). Within the CD4+ T cell population, a small subset of cells (3.68 ±2.0%) showed high expression of Foxp3. Further phenotypic analysis revealed that 51.82 ±8.03% of CD4+ T cells with coexisting high expression level of CD25 (CD25high) were Foxp3 and 86 ±7.1% of CD4+CD25high cells were with low CD127 expression. The highest expression of Foxp3 was indicated in the CD4 + CD25high subset of T cells where the CD127 expression level was low (82.12 ±5.4%). There were no significant differences regarding the analysed markers between AR patients and healthy controls (Table II, Figure 2).
Figure 1.
Cells staining for CD4+, CD25high, CD127low and FoxP3 expression by flow cytometry analysis
Table II.
CD4+, CD4+CD25high and CD4+CD25highCD127low T cell subsets with high expression of Forkhead Box Protein P3 (FoxP3)
| Patients with AR | Healthy controls | All | |
|---|---|---|---|
| CD4+ | 3.82 ±2.19% | 3.35 ±1.46% | 3.68 ±2.0% |
| CD4+CD25high | 52.76 ±7.74% | 49.5 ±8.67% | 51.82 ±8.03% |
| CD4+CD25highCD127low | 81.42 ±5.1% | 83.86 ±6.1% | 82.12 ±5.4% |
Figure 2.
Fractions of CD4+, CD4+CD25high and CD4+CD25highCD127low T cells with high FoxP3 expression. *p < 0.0001 for CD4+ vs. CD4+CD25high and CD4+CD25highCD127low as well as CD4+CD25high vs. CD4+CD25highCD127low subsets
Values are presented as mean ± SEM
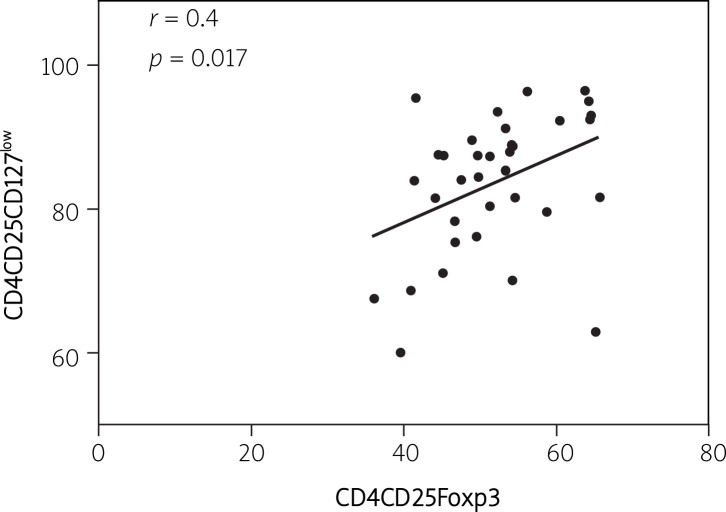
In addition, low expression of CD127 correlated (r = 0.42, p = 0.017) (Figure 3) with high expression of Foxp3 in TCD4+CD25high cells; thus regulatory T Foxp3+ cells in peripheral blood could be detected as CD4+CD25highCD127low cells in healthy individuals and allergic patients.
Figure 3.
Correlation of CD127 and Foxp3 expression in CD4+CD25high Treg cells
Discussion
Regulatory T lymphocytes (Tregs) play a key role in maintaining immune homeostasis and suppress the function of other cells limiting the immune response [1, 5, 6]. Alterations in the function or number of Tregs have been implicated in several autoimmune diseases including diabetes, rheumatoid arthritis, multiple sclerosis and allergy. Regrettably, Tregs, found in a high number in malignant disorders, may attenuate the antitumor response and deteriorate the prognosis.
Regulatory T cells are heterogeneous in phenotype and function and include natural Tregs, adaptive Tregs, Tr1 and Th3 subsets [5, 7, 8]. Natural Tregs originate in the thymus as CD4+ cells with constitutive high expression of CD25 (IL-2 receptor) together with transcription factor Foxp3. Adaptive Tregs originate peripherally from simple CD4+ expressing T cells into CD25 and Foxp3 expressing cells following antigenic stimulation in the presence of TGF-β and IL-10 [2, 7, 8]. Forkhead Box Protein P3 (Foxp3) is a master transcriptional repression factor that is expressed in all CD4+ cells expressing suppressive/regulatory activities. In humans, mutations in the Foxp3 gene result in defective CD4+CD25+ regulatory T cell development and cause severe immunodysregulation, polyendocrinopathy, and enteropathy X-linked syndrome (IPEX) [1, 5, 6, 9]. As a marker of regulatory T cells, Foxp3 is useful to characterise preserved Tregs or to confirm the yield and purity of isolated Tregs. However, Foxp3 staining requires fixation and permeabilisation which facilitate the penetration of molecules into the cells; therefore, it is not suitable for isolating viable Tregs. Today, superficial markers usually used for Treg identification are CD4, CD25, CTLA-4, CD45 RO/RA, and CD39 [1, 2, 5, 7]. However, some of these markers (e.g. cytotoxic T-lymphocyte antigen 4 [CTLA-4] or glucocorticoid-induced tumour necrosis factor receptor [GITR]) are also expressed on effector T cells, which creates a problem in Treg immunophenotyping and isolation. Recently, Groth et al. [10] and Bluestone et al. [11] demonstrated that CD127 (receptor for IL-7) is downregulated on all human T cells after activation and, in contrast with the reported re-expression of CD127 on the majority of peripheral memory and effector T cells, Treg CD4+CD25highFoxp3+ cells remain CD127low [11, 12]. Constitutively reduced expression of CD127 in Tregs may result from the Foxp3 interaction with the CD127 promoter that reduces expression of CD127 in this subset [11]. On the other hand, Foxp3 induced by activation in effector T cells is not sufficient to permanently suppress CD127 expression in these cells and after the initial, post-activation decline of CD127 expression in Teff cells CD127 is reconstituted [12].
In this study we have stained PBMCs to identify CD4+, CD4+CD25high, CD4+CD25high CD127low cells and further we decided to analyse the expression of Foxp3 in the various CD4+ T cell subsets. First, only 3.68 ±2.0% of CD4+ T cells were Foxp3 positive. This is in agreement with O'Connor's study where Tregs constituted 3-5% of peripheral CD4+ cells [13]. Additional staining for CD127 expression in CD4+ lymphocytes revealed that 41.5% of CD4+CD127low cells expressed Foxp3 but only 2.5% from CD4+CD127high T lymphocytes were Foxp3 positive [11]. Furthermore, we have demonstrated that 51.82 ±8.03% of CD4+CD25high cells expressed Foxp3. CD25 is the most important superficial antigen of nTregs but it is upregulated on other cells upon activation. Staining for CD4 and CD25 markers may identify both regulatory T cells and activated effector cells where Foxp3 is present temporarily or is absent. In O'Connor's study 64% of CD4+CD25high cells were Foxp3 positive [13]. Finally, in our study, supplemental staging for CD127 allowed us to identify Foxp3 in 82.12 ±5.4% of CD4+CD25highCD127low cells. This finding was supported by Liu et al., who revealed that 22.9% of CD4+CD25high lymphocytes with high coexpression of CD127 were Foxp3+ whereas 86.6% were Foxp3+ if the CD4+CD25+ cells with low coexpression of CD127 were immunophenotyped [11]. Additionally, they indicated that low CD127 expression correlates inversely with high intracellular Foxp3 expression and CD127 may be a helpful superficial marker for Treg identification and for viable cell isolation [11]. What is more, Tregs isolated on the basis of CD25+CD127low expression displayed all the characteristics of regulatory T cells (expression of Foxp3, CTLA-4, hypoproliferation and ability to suppress proliferation of other T cells) [11]. In our study 86% of CD4+CD25high cells were CD127low and low expression of CD127 correlated with high Foxp3 coexpression. Similarly, in another study [13] 82% of CD4+CD25high cells showed low CD127 expression and additional staining revealed that especially these cell subsets were FoxP3+.
In conclusion, quantitative identification and isolation of viable Tregs is difficult in samples from human patients. Our data demonstrate that human peripheral CD4+CD25high regulatory T cells express lower levels of the IL-7 receptor (CD127) and especially CD4+CD25high CD127low cells were Foxp3+. The data suggest that the CD127 biomarker can be used to more selectively enrich human regulatory T cells for further in vitro and in vivo studies.
Acknowledgments
The study was supported by a grant from the Ministry of Science and Higher Education, Poland, number N402 057 32/1788.
References
- 1.Ozdemir C, Akdis M, Akdis CA. T regulatory cells and their counterparts: masters of immune regulation. Clin Exp Allergy. 2009;39:626–39. doi: 10.1111/j.1365-2222.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 2.Palomares O, Yaman G, Azkur AK, Akkoc T, Akdis M, Akdis CA. Role of Treg in immune regulation of allergic diseases. Eur J Immunol. 2010;40:1232–40. doi: 10.1002/eji.200940045. [DOI] [PubMed] [Google Scholar]
- 3.Sakata T, Iwagami S, Tsuruta Y, et al. Constitutive expression of interleukin-7 mRNA and production of IL-7 by a cloned murine thymic stromal cell line. J Leukoc Bio. 1990;48:205–12. doi: 10.1002/jlb.48.3.205. [DOI] [PubMed] [Google Scholar]
- 4.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 5.Nouri-Aria KT, Durham SR. Regulatory T cells and allergic disease. Inflamm Allergy Drug Targets. 2008;7:237–52. doi: 10.2174/187152808786848405. [DOI] [PubMed] [Google Scholar]
- 6.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 7.Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol. 2009;123:735–46. doi: 10.1016/j.jaci.2009.02.030. [DOI] [PubMed] [Google Scholar]
- 8.Dunham RM, Cervasi B, Brenchley JM, et al. CD127 and CD25 expression defines CD4+ T cell subsets that are differentially depleted during HIV infection. J Immunol. 2008;180:5582–92. doi: 10.4049/jimmunol.180.8.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildin RS, Smyk-Pearson S, Filipovich AH. Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J Med Genet. 2002;8:537–45. doi: 10.1136/jmg.39.8.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allan SE, Crome SQ, Crellin NK, et al. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 13.Hartigan-O'Connor DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]