Abstract
Introduction
Although ropivacaine and fentanyl are commonly administered intra-articularly after knee or shoulder arthroscopy for postoperative pain control, there are no studies investigating the toxicity of ropivacaine and fentanyl on human fibroblasts (hF).
Material and methods
Human fibroblasts were seeded in monolayer triple flasks at a density of 104 cells/cm2 and plated into 96 plates at a density of 5000 cells per well. After fully aspirating the culture medium 200 µl of ropivacaine or fentanyl in its corresponding concentration or culture medium only was added to each well. After 30 min ropivacaine or fentanyl was removed and fresh culture medium was added. Fibroblast mitochondrial activity and apoptosis marker level were evaluated after 1 h, 24 h and 7 days.
Results
We found a significant decrease in mitochondrial activity after 7 days when exposed to 0.5% ropivacaine. Mitochondrial activity after 1 h, 24 h and 7 days was significantly decreased when fibroblasts were exposed to 0.05% fentanyl. Also, a significant decrease in mitochondrial activity was observed 1 h after exposure to 0.025% fentanyl. Cell viability remained unchanged at any other point in time. A significant increase of caspase-3, as a marker of apoptosis, was only present after exposure to 0.5% ropivacaine after 7 days.
Conclusions
These data suggest that both drugs have a concentration-dependent effect on mitochondrial activity in hF in vitro. This effect is more pronounced with fentanyl. Because the cytotoxicity of fentanyl, without the anticipated increase of caspase-3 as an apoptosis marker, remains unclear, we cannot support fentanyl as an alternative to ropivacaine.
Keywords: postoperative intraarticular anaesthesia, postoperative pain control
Introduction
Postoperative pain management following knee or shoulder arthroscopy can be a challenge. Intra-articular injection of local anaesthetics is regularly used to enhance postoperative analgesia following arthroscopy and has been shown to offer effective and safe postoperative pain relief [1–3]. At the present time, bupivacaine is probably the best studied and most commonly used local anaesthetic for this purpose [4]. However, several recent studies have demonstrated that even a brief exposure to bupivacaine may result in chondrotoxicity [3, 5–8] leading to chondrolysis [7, 8], a devastating condition of rapid chondrocyte death over a short period of time which ultimately leads to cartilage damage and progressive joint degeneration.
Promising alternatives to amide-type local anaesthetics are opioids such as morphine or fentanyl. As demonstrated in several studies, endogenous as well as exogenous opioid agonists have peripheral antinociceptive effects in inflamed tissue by interacting with κ, δ and mµ receptors and have furthermore been used for intra-articular analgesia with favourable outcomes [9–14].
Previous studies focused on the impact of local anaesthetics on chondrocytes. Since fibroblasts play a crucial role in tissue healing and might be aggrieved by the injected analgesic agent, we were interested in the effects on human fibroblasts. Only a few studies exist evaluating the effects of local anaesthetics [1, 15] on fibroblasts using analgesic concentrations as applied in clinical settings. Until now there are no studies focusing on the effects of opioids on human fibroblasts. We chose to investigate the viability of human fibroblasts after exposure to ropivacaine, being the less toxic local anaesthetic of the amide group [3], and fentanyl.
The purpose of this study was to investigate possible cytotoxic effects of ropivacaine and fentanyl on human fibroblasts in vitro.
Material and methods
Drug exposure and proliferation of fibroblasts
Human fibroblasts (Provitro, Berlin, Germany) were seeded in monolayer triple flasks at a density of 104 cells/cm2 in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, USA) containing 10% fetal calf serum (PAA, Pasching, Austria) and 50 U/ml penicillin/streptomycin (Sigma-Aldrich, Germany). Twenty-four hours before experimental treatment, cells were visually checked under phase microscopy to ensure a proper cell morphology consistent with differentiated fibroblasts. Cells were then replated into 96-well plates at a density of 5000 cells per well.
Cell cultures were divided into subgroups, each subgroup consisting of 6 wells with 5000 cells per well: 0.5%, 0.25%, 0.125% ropivacaine, 0.05%, 0.025% and 0.0125% fentanyl. A DMEM-only (10% fetal calf serum (PAA, Pasching, Austria) and 50 U/ ml penicillin/streptomycin (Sigma-Aldrich, Germany)) group served as a control (Table I). This protocol was similar to the one described by Piper et al. [3]. In short, culture medium (DMEM) was fully aspirated; 200 µl of ropivacaine or fentanyl in its corresponding concentration or DMEM only (control group) was added to each well. Cells were incubated in 5% CO2 at 37°C for 30 min. After 30 min the treatment solution (ropivacaine or fentanyl) was removed and fresh culture medium was added. The same protocol was applied to all samples.
Table I.
Study set-up. At each point of time and for each dilution we had 6 × 5000 cells
| Drug | Dilution [%] | Point of time | ||
|---|---|---|---|---|
| 1 h | 24 h | 7 days | ||
| Ropivacaine | 0.5 | 6 × 5000 cells | 6 × 5000 cells | 6 × 5000 cells |
| 0.25 | 6 × 5000 cells | 6 × 5000 cells | 6 × 5000 cells | |
| 0.125 | 6 × 5000 cells | 6 × 5000 cells | 6 × 5000 cells | |
| Fentanyl | 0.05 | 6 × 5000 cells | 6 × 5000 cells | 6 × 5000 cells |
| 0.025 | 6 × 5000 cells | 6 × 5000 cells | 6 × 5000 cells | |
| 0.0125 | 6 × 5000 cells | 6 × 5000 cells | 6 × 5000 cells | |
| DMEM | 6 × 5000 cells | 6 × 5000 cells | 6 × 5000 cells | |
Samples were returned to the incubator and mitochondrial activity was evaluated after 1 h, 24 h and 7 days using a WST-1 assay [16] (Cell Proliferation Reagent WST-1, Roche, Germany). Medium was replaced with DMEM with 10% WST-1 agent. Samples were returned to the incubator for another 2.5 h. The WST-1 assay is a colorimetric test based on cleavage of the tetrazolium salt WST-1 by mitochondrial dehydrogenases in viable cells into orange formazan. The level of orange formazan produced increases when mitochondrial activity increases and can be quantified using an Elisa-Reader (MWG-Biotech, Ebersberg, Germany) at 450 nm, with a reference wavelength at 690 nm.
Gene expression of apoptosis-specific proteins
In a LightCycler (Roche Applied Science, Mannheim, Germany) the following primers were used: glyceraldehyde-3-phosphate-dehydrogenase (GAPDH): sense: 5‘-TGC ACC ACC AAC TGC TTA GC-3‘; antisense: 5‘-GGC ATG GAC TGT GGT CAT GAG-3‘ [17]. The primers for the apoptosis-specific proteins, caspase-3 and PARP-1, were purchased from Search-LC (Heidelberg, Germany); the sequences were not provided by the company. Total RNA was directly isolated from fresh fibroblasts using the RNeasy Mini Kit (Qiagen, Hilden, Germany). For cDNA synthesis, 1 µg of total RNA was reverse-transcribed using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche Applied Science). Amplification reactions were performed using a LightCycler FastStart DNA Master SYBR Green I Kit (Roche Applied Science). One µl of the 1/10 diluted sample was used for the quantitative two-step PCR. The following protocols were used: GAPDH and caspase-3 (a 10-min step at 95°C, followed by 40 cycles of 60 s at 60°C and 15 s at 72°C); and PARP-1 (a 10-min step at 94°C, followed by 40 cycles of 60 s at 60°C and 10 s at 72°C). Reactions were performed twice and relative quantification of the target gene expression was generated normalizing to GAPDH.
Statistical analysis
For statistical comparison of cell viability and apoptosis marker, data were analysed by one-way analysis of variance (ANOVA) followed by Bonferroni t-test (Prism, GraphPad Software Inc, San Diego, California). A probability of p < 0.05 was considered statistically significant.
Results
Mitochondrial activity
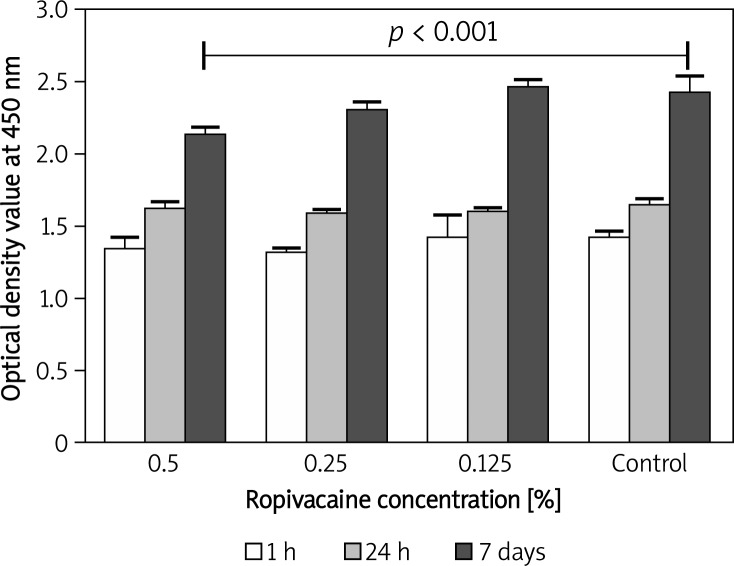
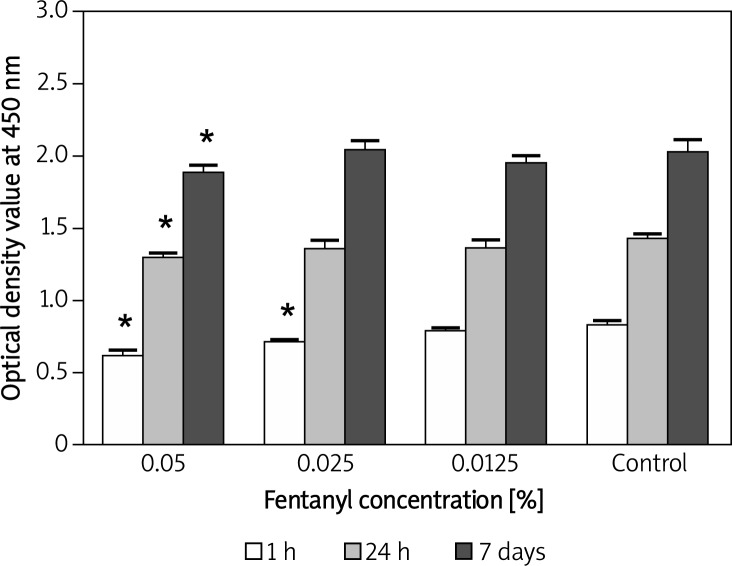
Mitochondrial activity of fibroblasts after 30-minute exposure to 0.5% ropivacaine was significantly (p < 0.001) decreased after 7 days when compared with the control group (Figure 1). There was no aberration at earlier times and mitochondrial activity of human fibroblasts after exposure to 0.25% and 0.125% ropivacaine was similar to that of the DMEM-only control group for 60 min, 24 h and 7 days (p > 0.05). As shown in Figure 2, exposure of 0.05% fentanyl to human fibroblasts induced a significant (p < 0.001) decrease of mitochondrial activity at each tested time point (1 h, 24 h, 7 days). In addition, activity in the 0.025% fentanyl group was significantly lower when compared to the control group after 1 h.
Figure 1.
Bar chart with standard deviation showing a time and concentration-dependent effect on human fibroblast mitochondrial activity after 30- minute exposure to ropivacaine. A significant difference in mitochondrial activity was only detectable after 7 days in the 0.5% ropivacaine group
Figure 2.
Bar chart with standard deviation showing a time and concentration-dependent effect on human fibroblast mitochondrial activity after 30- minute exposure to fentanyl. A significant difference in mitochondrial activity was detectable after 1 h, 24 h and 7 days in the 0.05% group and after 1 hour in the 0.025% group (*p < 0.05 vs. DMEM-only)
Apoptosis rate
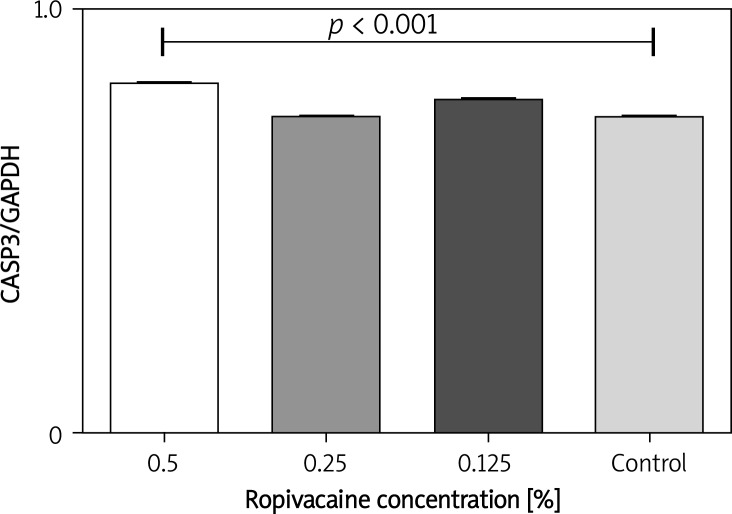
To assess the induction of apoptosis, the levels of caspase-3 and PARP-1 were evaluated at each point of time (1 h, 24 h, 7 days). A significantly (p < 0.05) higher level of caspase-3 was only found in fibroblasts 7 days after treatment with 0.5% ropivacaine (Figure 3). The level of PARP-1 at that moment was not significantly different from the control group. There were no significant differences in apoptosis marker levels in any of the groups treated with fentanyl.
Figure 3.
Bar chart with standard deviation demonstrating significant elevation of caspase-3 after 7 days when human fibroblasts were exposed to 0.5% ropivacaine for 30 min
Discussion
Postoperative pain management following knee or shoulder arthroscopy can be a challenge of its own. Intra-articular injection of local anaesthetics is regularly used to enhance postoperative analgesia following arthroscopy and has been shown to offer effective and safe postoperative pain relief [1–3]. However, several studies and case reports have described cytotoxic effects of local anaesthetics on chondrocytes [3, 5, 7, 18]. Previous studies focused on the impact of local anaesthetics on chondrocytes although fibroblasts play a crucial role in tissue healing and might be aggrieved by the injected analgesic agent as well. This study was conducted because only sparse data exist on the effect of ropivacaine on human fibroblasts [1, 15] and until now no data exist on the effect of fentanyl on human fibroblasts.
As a natural part of the process of tissue regeneration, fibroblasts play an important role in the proliferative phase as they migrate from normal tissue into the wound area to represent the most common cells of connective tissue. In addition to chondroblasts and osteoblasts, they are the primary producers of collagen. Fibroblasts furthermore synthesize the ground substance of the extracellular matrix in which the cells of the connective tissue and the different types of fibres are embedded and express adhesion molecules and fibroblastic markers, and secrete growth factors and cytokines [19]. Due to the crucial role of fibroblasts in the complex process of tissue healing, we were interested if and how these cells are affected by drugs that are commonly used for intra-articular analgesia since the main focus in previous studies were chondrocytes.
We chose ropivacaine as an amide-type local anaesthetic because it has been shown to be as effective as bupivacaine but less toxic and furthermore is widely used in musculoskeletal medicine [1, 3, 20]. Fentanyl on the other hand has been used for intra-articular analgesia with favourable outcomes [9, 10, 12] but toxicity to fibroblasts has never been investigated.
The most important finding of the present study was that both drugs have a detrimental effect on human fibroblasts, depending on the time and concentration. This effect was more distinct in fentanyl.
We chose to determine fibroblast viability by quantifying mitochondrial activity [21, 22]. Furthermore, we evaluated the expression of apoptosis markers such as caspase-3 and Parp-1 to elucidate drug-induced initiation or execution of apoptosis [21].
In our experiment, we noted a significant (p < 0.001) decrease of mitochondrial activity 7 days after exposure to 0.5% ropivacaine when compared to the control group. Previous studies have found a concentration-dependent adverse effect of ropivacaine on mitochondrial activity and cell proliferation in human fibroblasts [1, 23]. At the same point of time, we found a significant increase of caspase-3 as a marker of apoptosis in this same group. Fedder et al. [1] were able to demonstrate the same increase in caspase-3 3 days after exposure to ropivacaine.
On the other hand, fibroblast mitochondrial activity was already significantly (p < 0.001) decreased when exposed to 0.05% fentanyl after 1 h. This effect was also measurable after 24 h and 7 days. Furthermore, we also noted a significant (p < 0.001) decrease of mitochondrial activity 1 h after exposure to 0.025% fentanyl. Since apoptosis markers were not increased at any time, one might assume that fentanyl has a quick but not very powerful effect on human fibroblasts, not leading to apoptosis. This hypothesis can be supported by Haasters et al. [24], who did not find a significant difference in apoptosis markers after exposing hamstring-derived progenitor cells to ropivacaine when compared with a control group. But on the other hand, the hypothesis can be dismissed by some studies that postulate caspase-independent mechanisms of cell death due to changes in intracellular Ca2 + dysregulation [25–28].
Either way, the results of the present study demonstrated that 30-minute exposure to 0.5% ropivacaine in vitro causes a decrease of fibroblast mitochondrial activity and an increase of caspase-3 in the highest concentration after 7 days whereas fentanyl in any concentration decreases mitochondrial activity right away.
However, there are some limitations of the present study. First of all, this in vitro model cannot represent postoperative intra-articular conditions such as postoperative bleeding, active blood supply, cell metabolic activities or the amount of synovial fluid. Furthermore, because it is an in vitro model, the observations cannot necessarily be applied directly in vivo. Nevertheless, the standardized in vitro setup provided highly reproducible results in terms of substance-specific effects on cell viability, metabolic activity, and induction of apoptosis. The consequences of intra-articular application of ropivacaine and fentanyl on fibroblasts require further investigation. Further studies have to evaluate these effects probably even closer to the cell membrane. One option is to determine the expression of inducible nitric oxide synthase (iNOS), which begins to synthesize a great quantity of nitrogen oxide shortly after induction. Overproduction of nitric oxygen, a free radical, damages cellular components, including lipids, which results in declining physiological function and cell death [29, 30]. In addition, the results of our study should be clarified in an animal model.
In conclusion, these data suggest that both drugs do have a concentration-dependent effect on mitochondrial activity in human fibroblasts in vitro in varying degrees. Because of the immediate effects of fentanyl on mitochondrial activity but without the anticipated increase of caspase-3 as an apoptosis marker, the toxicity remains unclear. Therefore, we cannot support fentanyl as an alternative to ropivacaine. Further studies will still have to prove the benefit of opioids in intra-articular analgesia.
References
- 1.Fedder C, Beck-Schimmer B, Aguirre J, et al. In vitro exposure of human fibroblasts to local anaesthetics impairs cell growth. Clin Exp Immunol. 2010;162:280–8. doi: 10.1111/j.1365-2249.2010.04252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs TF, Vansintjan PS, Roels N, et al. The effect of Lidocaine on the viability of cultivated mature human cartilage cells: an in vitro study. Knee Surg Sports Traumatol Arthrosc. 2011;19:1206–13. doi: 10.1007/s00167-011-1420-5. [DOI] [PubMed] [Google Scholar]
- 3.Piper SL, Kim HT. Comparison of ropivacaine and bupivacaine toxicity in human articular chondrocytes. J Bone Joint Surg Am. 2008;90:986–91. doi: 10.2106/JBJS.G.01033. [DOI] [PubMed] [Google Scholar]
- 4.Moiniche S, Mikkelsen S, Wetterslev J, Dahl JB. A systematic review of intra-articular local anesthesia for postoperative pain relief after arthroscopic knee surgery. Reg Anesth Pain Med. 1999;24:430–7. doi: 10.1016/s1098-7339(99)90010-x. [DOI] [PubMed] [Google Scholar]
- 5.Chu CR, Izzo NJ, Papas NE, Fu FH. In vitro exposure to 0.5% bupivacaine is cytotoxic to bovine articular chondrocytes. Arthroscopy. 2006;22:693–9. doi: 10.1016/j.arthro.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Dogan N, Erdem AF, Erman Z, Kizilkaya M. The effects of bupivacaine and neostigmine on articular cartilage and synovium in the rabbit knee joint. J Int Med Res. 2004;32:513–9. doi: 10.1177/147323000403200509. [DOI] [PubMed] [Google Scholar]
- 7.Hansen BP, Beck CL, Beck EP, Townsley RW. Postarthroscopic glenohumeral chondrolysis. Am J Sports Med. 2007;35:1628–34. doi: 10.1177/0363546507304136. [DOI] [PubMed] [Google Scholar]
- 8.Petty DH, Jazrawi LM, Estrada LS, Andrews JR. Glenohumeral chondrolysis after shoulder arthroscopy: case reports and review of the literature. Am J Sports Med. 2004;32:509–15. doi: 10.1177/0363546503262176. [DOI] [PubMed] [Google Scholar]
- 9.Kalso E, Tramer MR, Carroll D, McQuay HJ, Moore RA. Pain relief from intra-articular morphine after knee surgery: a qualitative systematic review. Pain. 1997;71:127–34. doi: 10.1016/s0304-3959(97)03344-7. [DOI] [PubMed] [Google Scholar]
- 10.Kazemi AP, Rezazadeh S, Gharacheh HR. Pain relief after arthroscopic knee surgery: intraarticular sufentanil vs morphine. Middle East J Anesthesiol. 2004;17:1099–112. [PubMed] [Google Scholar]
- 11.Kizilkaya M, Yildirim OS, Dogan N, Kursad H, Okur A. Analgesic effects of intraarticular sufentanil and sufentanil plus methylprednisolone after arthroscopic knee surgery. Anesth Analg. 2004;98:1062–5. doi: 10.1213/01.ANE.0000103185.18333.68. [DOI] [PubMed] [Google Scholar]
- 12.Vranken JH, Vissers KC, de Jongh R, Heylen R. Intraarticular sufentanil administration facilitates recovery after day-case knee arthroscopy. Anesth Analg. 2001;92:625–8. doi: 10.1097/00000539-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Stein C, Comisel K, Haimerl E, et al. Analgesic effect of intraarticular morphine after arthroscopic knee surgery. N Engl J Med. 1991;325:1123–6. doi: 10.1056/NEJM199110173251602. [DOI] [PubMed] [Google Scholar]
- 14.Stein C, Millan MJ, Yassouridis A, Herz A. Antinociceptive effects of mu- and kappa-agonists in inflammation are enhanced by a peripheral opioid receptor-specific mechanism. Eur J Pharmacol. 1988;155:255–64. doi: 10.1016/0014-2999(88)90511-0. [DOI] [PubMed] [Google Scholar]
- 15.Pescosolido N, Scarsella G, Tafani M, Nebbioso M. Cataract surgery complications: an in vitro model of toxic effects of ropivacaine and lidocaine. Drugs in R&D. 2011;11:303–7. doi: 10.2165/11595120-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birkenmaier C, Redeker J, Sievers B, Melcher C, Jansson V, Mayer-Wagner S. An evaluation of medications commonly used for epidural neurolysis procedures in a human fibroblast cell culture model. Reg Anesth Pain Med. 2011;36:140–4. doi: 10.1097/AAP.0b013e31820d41c4. [DOI] [PubMed] [Google Scholar]
- 17.Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson SL, Buchko JZ, Taillon MR, Ernst MA. Chondrolysis of the glenohumeral joint after infusion of bupivacaine through an intra-articular pain pump catheter: a report of 18 cases. Arthroscopy. 2010;26:451–61. doi: 10.1016/j.arthro.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 19.Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood) 2008;233:109–22. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- 20.Scherb MB, Han SH, Courneya JP, Guyton GP, Schon LC. Effect of bupivacaine on cultured tenocytes. Orthopedics. 2009;32:26. doi: 10.3928/01477447-20090101-19. [DOI] [PubMed] [Google Scholar]
- 21.Unami A, Shinohara Y, Ichikawa T, Baba Y. Biochemical and microarray analyses of bupivacaine-induced apoptosis. J Toxicol Sci. 2003;28:77–94. doi: 10.2131/jts.28.77. [DOI] [PubMed] [Google Scholar]
- 22.Cela O, Piccoli C, Scrima R, et al. Bupivacaine uncouples the mitochondrial oxidative phosphorylation, inhibits respiratory chain complexes I and III and enhances ROS production: results of a study on cell cultures. Mitochondrion. 2010;10:487–96. doi: 10.1016/j.mito.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Martinsson T, Haegerstrand A, Dalsgaard CJ. Ropivacaine and lidocaine inhibit proliferation of non-transformed cultured adult human fibroblasts, endothelial cells and keratinocytes. Agents Actions. 1993;40:78–85. doi: 10.1007/BF01976755. [DOI] [PubMed] [Google Scholar]
- 24.Haasters F, Polzer H, Prall WC, et al. Bupivacaine, ropivacaine, and morphine: comparison of toxicity on human hamstring-derived stem/progenitor cells. Knee Surg Sports Traumatol Arthrosc. 2011;19:2138–44. doi: 10.1007/s00167-011-1564-3. [DOI] [PubMed] [Google Scholar]
- 25.Brun A. Effect of procaine, carbocain and xylocaine on cutaneous muscle in rabbits and mice. Acta Anaesthesiol Scand. 1959;3:59–73. doi: 10.1111/j.1399-6576.1959.tb00008.x. [DOI] [PubMed] [Google Scholar]
- 26.Foster AH, Carlson BM. Myotoxicity of local anesthetics and regeneration of the damaged muscle fibers. Anesth Analg. 1980;59:727–36. [PubMed] [Google Scholar]
- 27.Gomez-Arnau JI, Yanguela J, Gonzalez A, et al. Anaesthesia-related diplopia after cataract surgery. Br J Aanaesth. 2003;90:189–93. doi: 10.1093/bja/aeg029. [DOI] [PubMed] [Google Scholar]
- 28.Zink W, Graf BM. Local anesthetic myotoxicity. Reg Anesth Pain Med. 2004;29:333–40. doi: 10.1016/j.rapm.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Bentz M, Zaouter C, Shi Q, et al. Inhibition of inducible nitric oxide synthase prevents lipid peroxidation in osteoarthritic chondrocytes. J Cell Biochem. 2012;113:2256–67. doi: 10.1002/jcb.24096. [DOI] [PubMed] [Google Scholar]
- 30.Sharpe MA, Ollosson R, Stewart VC, Clark JB. Oxidation of nitric oxide by oxomanganese-salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem J. 2002;366:97–107. doi: 10.1042/BJ20020154. [DOI] [PMC free article] [PubMed] [Google Scholar]