Abstract
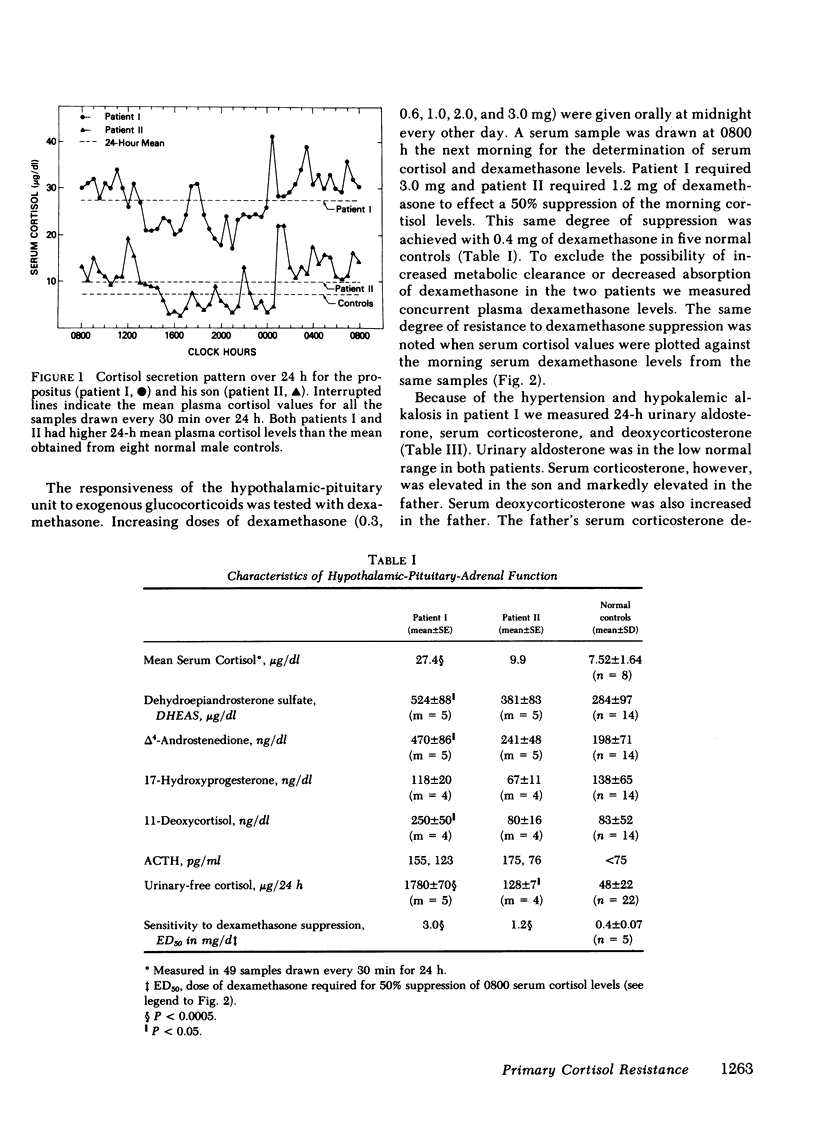
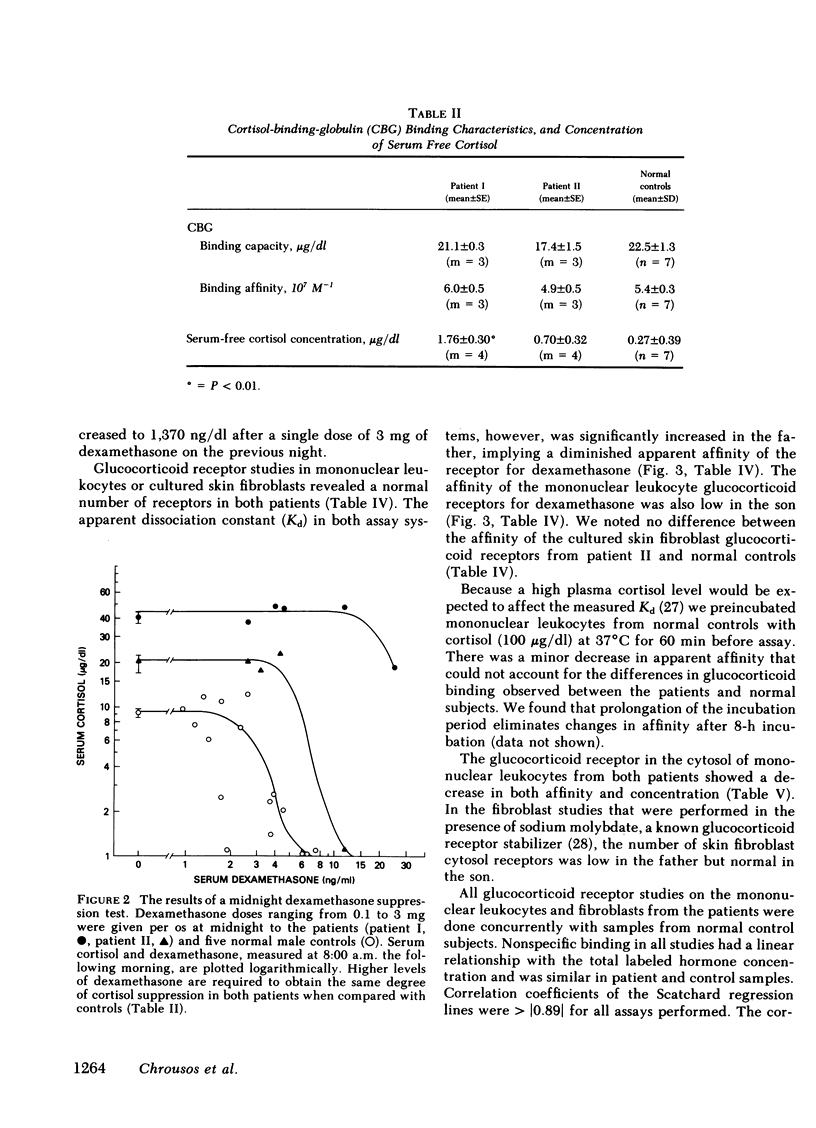
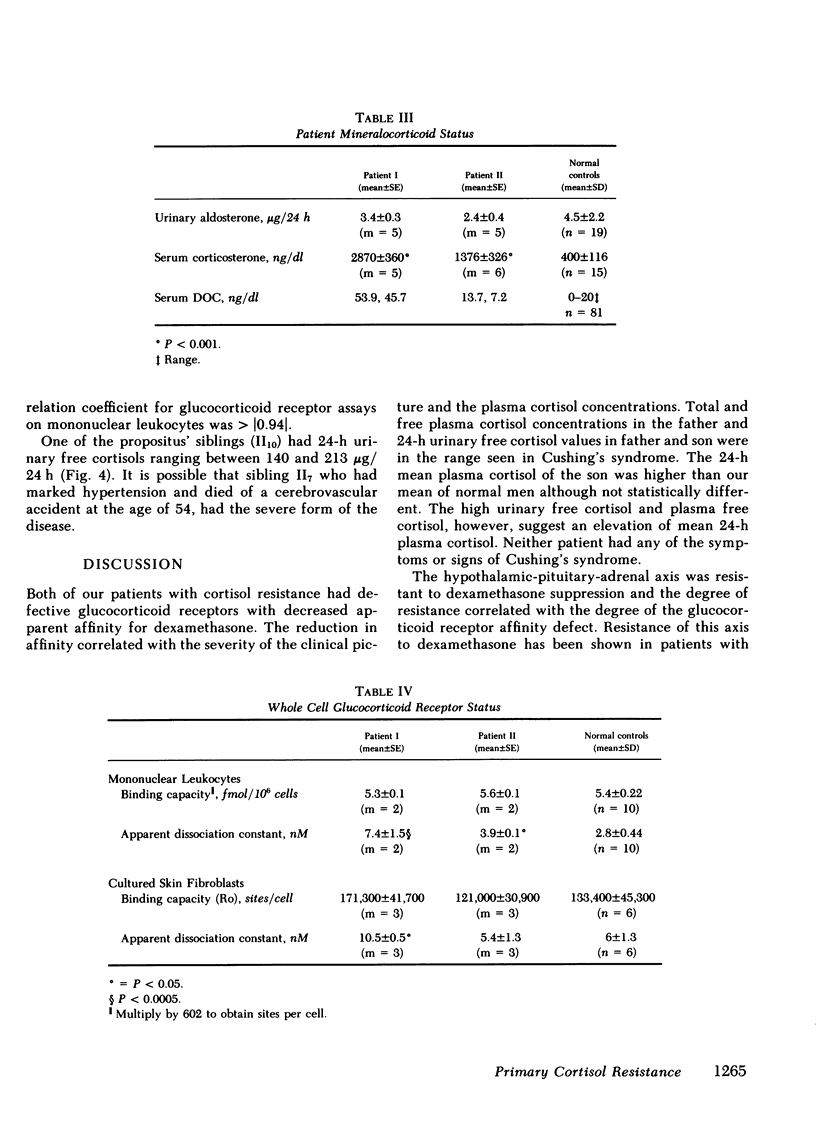
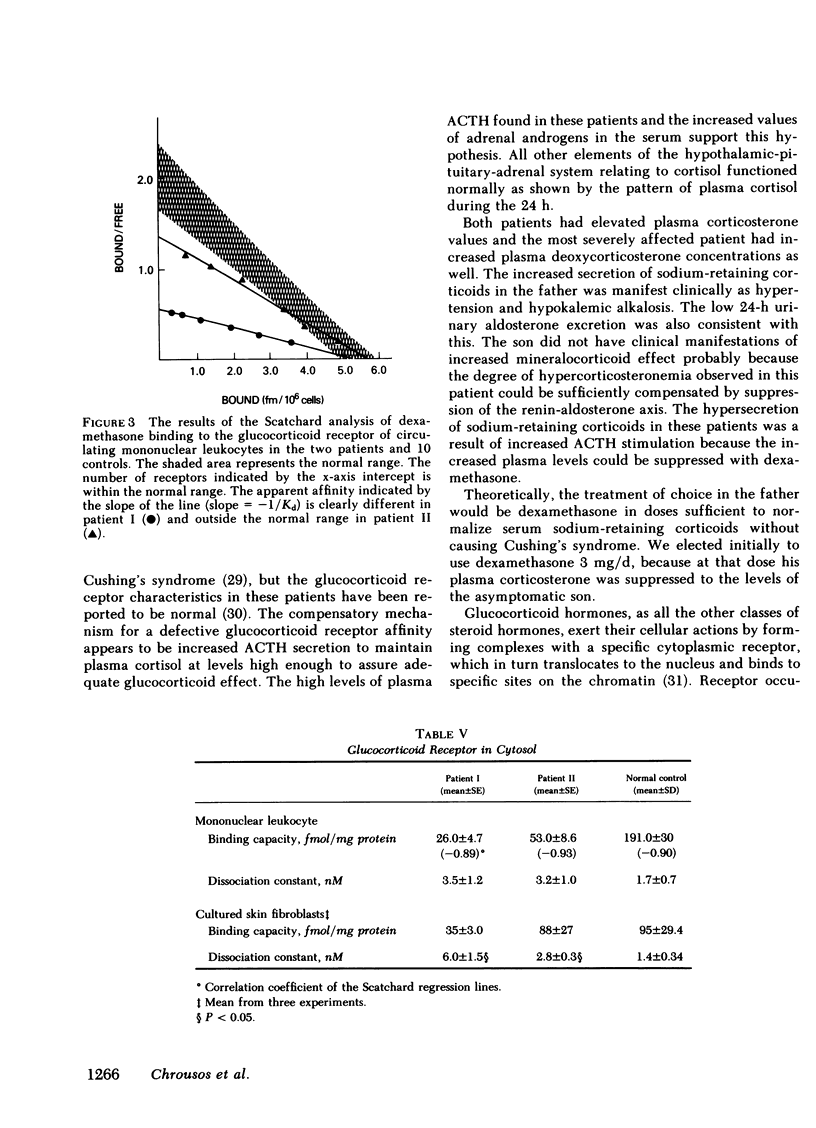
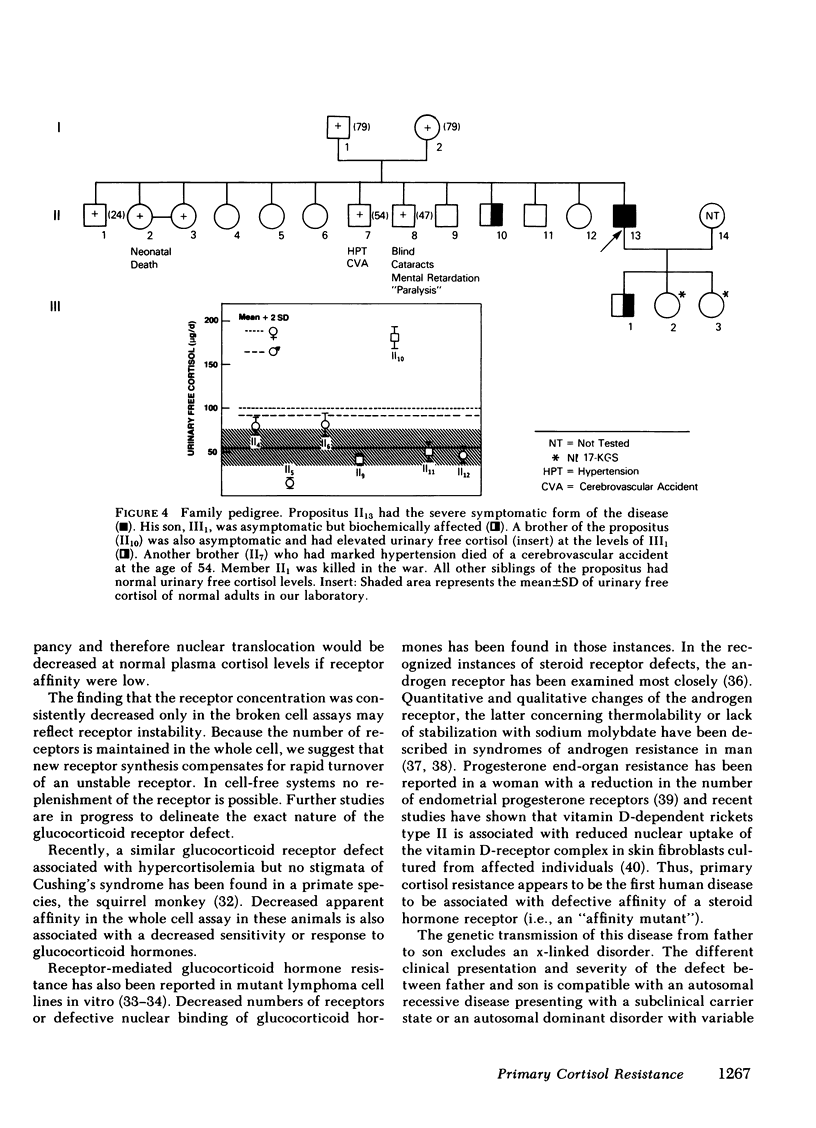
We have studied a man suspected of having primary cortisol resistance on the basis of high 24-h mean plasma cortisol levels (27.4 micrograms/dl) and no stigmata of Cushing's syndrome. His son had slightly elevated 24-h mean plasma cortisol levels (9.9 micrograms/dl; normal 7.52 micrograms/dl). Both had high plasma protein unbound cortisol and increased urinary free cortisol. Plasma ACTH concentration was high, and both were resistant to adrenal suppression by dexamethasone. The father appeared to have mineralocorticoid excess resulting in hypertension, hypokalemia, and metabolic alkalosis. This was found to be due to markedly elevated plasma levels of deoxycorticosterone and corticosterone. The son, who was normotensive, had mildly increased plasma corticosterone and normal deoxycorticosterone levels. To study the apparent end-organ resistance to cortisol, we examined the glucocorticoid receptor in the white cells and fibroblasts of these patients. In both tissues, using both whole cell and cytosol assays, the glucocorticoid receptor was found to have reduced affinity for dexamethasone. In the cytoxol assays, a reduced receptor number was found as well. We conclude that cortisol resistance is a rare familial syndrome owing to an abnormal glucocorticoid receptor with a decreased affinity for cortisol.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken S. C., Lippman M. E. A simple computer program for quantitation and Scatchard analysis of steroid receptor proteins. J Steroid Biochem. 1977 Jan;8(1):77–94. doi: 10.1016/0022-4731(77)90221-7. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Funder J. W. Hormone receptors. N Engl J Med. 1979 Nov 22;301(21):1149–1161. doi: 10.1056/NEJM197911223012104. [DOI] [PubMed] [Google Scholar]
- Baxter J. D., Tomkins G. M. Specific cytoplasmic glucocorticoid hormone receptors in hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1971 May;68(5):932–937. doi: 10.1073/pnas.68.5.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamness G. C., McGuire W. L. Scatchard plots: common errors in correction and interpretation. Steroids. 1975 Oct;26(4):538–542. doi: 10.1016/0039-128x(75)90073-2. [DOI] [PubMed] [Google Scholar]
- Cutler G. B., Jr, Glenn M., Bush M., Hodgen G. D., Graham C. E., Loriaux D. L. Adrenarche: a survey of rodents, domestic animals, and primates. Endocrinology. 1978 Dec;103(6):2112–2118. doi: 10.1210/endo-103-6-2112. [DOI] [PubMed] [Google Scholar]
- Eil C., Liberman U. A., Rosen J. F., Marx S. J. A cellular defect in hereditary vitamin-D-dependent rickets type II: defective nuclear uptake of 1,25-dihydroxyvitamin D in cultured skin fibroblasts. N Engl J Med. 1981 Jun 25;304(26):1588–1591. doi: 10.1056/NEJM198106253042608. [DOI] [PubMed] [Google Scholar]
- Eil C., Lippman M. E., Loriaux D. L. A dispersed-whole cell method for the determination of androgen receptors in human skin fibroblasts. Steroids. 1980 Apr;35(4):389–404. doi: 10.1016/0039-128x(80)90140-3. [DOI] [PubMed] [Google Scholar]
- Gorden P., Sherman B. M., Simopoulos A. P. Glucose intolerance with hypokalemia: an increased proportion of circulating proinsulin-like component. J Clin Endocrinol Metab. 1972 Jan;34(1):235–240. doi: 10.1210/jcem-34-1-235. [DOI] [PubMed] [Google Scholar]
- Griffin J. E. Testicular feminization associated with a thermolabile androgen receptor in culutred human fibroblasts. J Clin Invest. 1979 Dec;64(6):1624–1631. doi: 10.1172/JCI109624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. E., Wilson J. D. The syndromes of androgen resistance. N Engl J Med. 1980 Jan 24;302(4):198–209. doi: 10.1056/NEJM198001243020404. [DOI] [PubMed] [Google Scholar]
- Gross H. A., Ruder H. J., Brown K. S., Lipsett M. B. A radioimmunoassay for plasma corticosterone. Steroids. 1972 Dec;20(6):681–695. doi: 10.1016/0039-128x(72)90051-7. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Kao M., Voina S., Nichols A., Horton R. Parallel radioimmunoassay for plasma cortisol and 11-deoxycortisol. Clin Chem. 1975 Oct;21(11):1644–1647. [PubMed] [Google Scholar]
- Keller D. W., Wiest W. G., Askin F. B., Johnson L. W., Strickler R. C. Pseudocorpus luteum insufficiency: a local defect of progesterone action on endometrial stroma. J Clin Endocrinol Metab. 1979 Jan;48(1):127–132. doi: 10.1210/jcem-48-1-127. [DOI] [PubMed] [Google Scholar]
- Kontula K., Pelkonen R., Andersson L., Sivula A. Glucocorticoid receptors in adrenocorticoid disorders. J Clin Endocrinol Metab. 1980 Sep;51(3):654–657. doi: 10.1210/jcem-51-3-654. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan J., Jackson R., Adlin E. V., Channick B. J. A simple radioimmunoassay for urinary aldosterone. J Clin Endocrinol Metab. 1974 Feb;38(2):189–193. doi: 10.1210/jcem-38-2-189. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Dahmer M. K., Hammond N. D., Sando J. J., Pratt W. B. Molybdate inhibition of glucocorticoid receptor inactivation and transformation. J Biol Chem. 1979 Dec 10;254(23):11884–11890. [PubMed] [Google Scholar]
- Liotta A., Krieger D. T. A sensitive bioassay for the determination of human plasma ACTH levels. J Clin Endocrinol Metab. 1975 Feb;40(2):268–267. doi: 10.1210/jcem-40-2-268. [DOI] [PubMed] [Google Scholar]
- Meikle A. W., Lagerquist L. G., Tyler F. H. A plasma dexamethasone radioimmunoassay. Steroids. 1973 Aug;22(2):193–202. doi: 10.1016/0039-128x(73)90085-8. [DOI] [PubMed] [Google Scholar]
- Meikle A. W., Lagerquist L. G., Tyler F. H. Apparently normal pituitary-adrenal suppressibility in Cushing's syndrome: dexamethasone metabolism and plasma levels. J Lab Clin Med. 1975 Sep;86(3):472–478. [PubMed] [Google Scholar]
- Murakami T., Brandon D. D., Loriaux D. L., Lipsett M. B. Effect of cortisol, T3 and T4, on the glucocorticoid receptor concentration in leukocytes. J Steroid Biochem. 1980 Sep;13(9):1125–1127. doi: 10.1016/0022-4731(80)90147-8. [DOI] [PubMed] [Google Scholar]
- Murakami T., Brandon D., Rodbard D., Loriaux D. L., Lipsett M. B. Glucocorticoid receptor in circulating mononuclear leukocytes. Endocrinology. 1979 Feb;104(2):500–505. doi: 10.1210/endo-104-2-500. [DOI] [PubMed] [Google Scholar]
- Perrin F. M., Forest M. G. Time course of the effect of adrenalectomy on transcortin binding characteristics: appraisal of different methods of calculation. Endocrinology. 1975 Apr;96(4):869–878. doi: 10.1210/endo-96-4-869. [DOI] [PubMed] [Google Scholar]
- Rifka S. M., Pita J. C., Jr, Loriaux D. L. Mechanism of interaction of digitalis with estradiol binding sites in rat uteri. Endocrinology. 1976 Oct;99(4):1091–1096. doi: 10.1210/endo-99-4-1091. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Lewald J. E. Computer analysis of radioligand assay and radioimmunoassay data. Acta Endocrinol Suppl (Copenh) 1970;147:79–103. doi: 10.1530/acta.0.065s079. [DOI] [PubMed] [Google Scholar]
- Rosenau W., Baxter J. D., Rousseau G. G., Tomkins G. M. Mechanism of resistance to steroids: glucocorticoid receptor defect in lymphoma cells. Nat New Biol. 1972 May 3;237(70):20–24. doi: 10.1038/newbio237020a0. [DOI] [PubMed] [Google Scholar]
- Ruder H. J., Guy R. L., Lipsett M. B. A radioimmunoassay for cortisol in plasma and urine. J Clin Endocrinol Metab. 1972 Aug;35(2):219–224. doi: 10.1210/jcem-35-2-219. [DOI] [PubMed] [Google Scholar]
- Schiebinger R. J., Albertson B. D., Barnes K. M., Cutler G. B., Jr, Loriaux D. L. Developmental changes in rabbit and dog adrenal function: a possible homologue of adrenarche in the dog. Am J Physiol. 1981 Jun;240(6):E694–E699. doi: 10.1152/ajpendo.1981.240.6.E694. [DOI] [PubMed] [Google Scholar]
- Schmidt T. J., Harmon J. M., Thompson E. B. 'Activation-labile' glucocorticoid-receptor complexes of a steroid-resistant variant of CEM-C7 human lymphoid cells. Nature. 1980 Jul 31;286(5772):507–510. doi: 10.1038/286507a0. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Mechanisms of steroid resistance. Cell. 1974 Aug;2(4):221–227. doi: 10.1016/0092-8674(74)90014-2. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Jubiz W. A source of error in equilibrium dialysis. Steroids. 1980 Oct;36(4):393–403. doi: 10.1016/0039-128x(80)90028-8. [DOI] [PubMed] [Google Scholar]
- Vingerhoeds A. C., Thijssen J. H., Schwarz F. Spontaneous hypercortisolism without Cushing's syndrome. J Clin Endocrinol Metab. 1976 Nov;43(5):1128–1133. doi: 10.1210/jcem-43-5-1128. [DOI] [PubMed] [Google Scholar]



