Abstract
Introduction
Prospective studies about the association between elevated circulating pregnancy-associated plasma protein A (PAPP-A) and adverse vascular events in patients with coronary heart diseases (CHD) are inconsistent. We performed a meta-analysis to clarify this issue.
Material and methods
We identified prospective studies by searching MEDLINE. The vascular outcomes included all-cause mortality, combination of all-cause mortality and non-fatal myocardial infarction (MI), and combined cardiovascular events. Prospective studies providing multivariable adjusted relative risks (RRs) and their 95% confidence intervals (CIs) of pre-mentioned outcomes were included. A random-effects model was used to calculate the pooled RRs. Subgroup and sensitivity analyses were used to explore the potential sources of heterogeneity or modifiable factors.
Results
Fourteen studies with a total of 12 830 participants were included. Elevated PAPP-A level was associated with all-cause mortality (pooled RR 1.74, 95% CI: 1.45 to 2.09, p < 0.001), combined all-cause mortality and non-fatal MI (RR 1.59, 95% CI: 1.37 to 1.85, p < 0.001) and combined cardiovascular events (RR 1.50, 95% CI: 1.22 to 1.85, p < 0.001). There was no significant heterogeneity. Subgroup and sensitivity analyses showed that the positive association was not affected by follow-up term, CHD type, different assay methods of PAPP-A, or studies with less than 5 adjusted variables.
Conclusions
Elevated serum PAPP-A level is associated with adverse vascular outcomes in patients with CHD.
Keywords: pregnancy-associated plasma protein A, coronary heart disease, mortality, myocardial infarction, revascularization, meta-analysis
Introduction
Coronary heart disease (CHD) is the leading cause of mortality worldwide, claiming 587 000 lives per year [1, 2]. The cardinal mechanism of CHD is progressive atherosclerosis. Various signaling molecules such as proinflammatory cytokines (e.g., high-sensitivity C-reactive protein, tumor necrosis factors, and soluble adhesion molecules), matrix metalloproteinase and growth factors have been implicated in the process of atherosclerosis [3, 4]. Recently, pregnancy-associated plasma protein A (PAPP-A), a newly discovered zinc-binding metalloprotein, has received considerable interest [5]. Clinical studies have reported that elevated plasma PAPP-A was a sensitive, specific and early biomarker for the diagnosis of acute coronary syndrome (ACS) [6–8]. Furthermore, prospective trials have reported that elevated PAPP-A was an independent risk factor for adverse vascular events [9–24]. Therefore, identifying this new biomarker could be helpful to improve diagnostic and therapeutic decision-making.
The PAPP-A primarily acts as a protease cleaving inhibitory binding proteins of insulin-like growth factors [25–27]. In vitro studies have found that PAPP-A is mainly secreted by coronary artery smooth muscle cells under the stimulation of proinflammatory cytokines. Activation of the nuclear factor-κB pathway seems to be involved [28, 29]. What is more, PAPP-A is not just a biomarker. Animal studies have shown that PAPP-A plays an important role in advanced atherosclerosis. An animal model with a PAPP-A knock-out gene could resist the progression of atherosclerosis, whereas an animal model with overexpression of PAPP-A had accelerated progression of atherosclerosis [26, 30–32]. Accumulating clinical evidence has suggested that PAPP-A is a prognostic indicator for adverse vascular events for CHD patients. However, these results are inconsistent. Some studies have reported that serum elevated PAPP-A is associated with adverse vascular outcomes, while others reported a null association [9–24]. So it remains uncertain whether elevated serum PAPP-A level is an independent risk factor for CHD. Therefore, we performed a meta-analysis to assess the association between elevated serum PAPP-A and relevant vascular events in patients with CHD.
Material and methods
Search strategy
We performed this meta-analysis according to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [33]. Two authors (Yuehua Li and Chenghui Zhou) identified articles through search of MEDLINE (PubMed) from 2000 to Feb 2013. The key word used in the search was “PAPP-A”. No language restriction was applied for searching and study inclusion.
Study selection
The inclusion criteria were determined as follows: (i) prospective study design; (ii) provide referent (lowest) and highest levels of serum PAPP-A; (iii) provide multivariable adjusted relative risks (RRs) and their corresponding 95% confidence intervals (CIs).
Outcomes
The primary outcome was all-cause mortality. The secondary outcomes included combination of all-cause mortality and non-fatal myocardial infarction (MI), and combined cardiovascular events (cardiac death, MI or revascularization).
Data extraction
Data extraction was conducted by two independent authors (Yuehua Li and Chenghui Zhou). Discrepancies were resolved by group discussion. We did not contact the authors of the original studies for missing data. The extracted data included first author's name, publication year, sample size, number of events, male proportion, mean age, duration of follow-up, assay methods for measuring PAPP-A, cut-off value of PAPP-A, adjusted covariates and RRs and their corresponding 95% CIs. We extracted RRs from the most fully adjusted model for the highest levels of PAPP-A compared with the lowest ones.
Statistical analysis
We considered the hazard ratio or odds ratio as RRs in the prospective studies. Compared with the lowest category of PAPP-A, the pooled RRs and their 95% CIs were estimated by a random-effects model to incorporate the inter-study variability [34]. The heterogeneity was assessed by the Q statistic, I 2 and p value. We tried to explore the potential sources of heterogeneity by subgroup analysis according to follow-up term, assay methods of measuring PAPP-A, and different types of CHD patients (patients with stable CHD, suspected or established ACS). The point-of-care (POC) time-resolved immunofluorometric assay could also be a kit which is based on a comparable strategy like an enzyme-linked immunosorbent assay (ELISA) kit. Therefore, assay methods were classified by ELISA or POC assay and other methods. We also performed sensitivity analysis by excluding studies which provided the RRs with less than 5 adjusted variables.
We assessed publication bias by Begg's funnel plot and Egger's regression test [35]. Two-sided p value < 0.05 was considered to be significant. All of these analyses were completed by using STATA software (10.0 version, Stata Corporation, TX, USA).
Results
Search results
We identified 1337 articles in the initial search. Of those studies, 1291 citations were excluded based on titles and abstracts due to experimental studies, reviews, or non-relevant. Forty-six potential articles were selected for the detailed assessment. We further excluded 32 ones for the following reasons: cross-sectional design (n = 18), not providing the needed endpoints (n = 10), comment or review (n = 2), not providing the multivariable adjusted RRs (n = 2). The study of Iversion et al. [14] was divided into two cohorts because they provided separated data for patients with ST-segment elevated myocardial infarction and non-ST-segment elevated ACS, respectively. Finally, 14 prospective studies (15 independent cohorts) concerning 12 830 participants and 1813 cases were included in our meta-analysis [9–17, 19–23]. Of the 15 cohorts, there were 7 endpoints of all-cause mortality [11, 12, 14–17, 23], 7 combinations of all-cause mortality and non-fatal MI [12–16, 20, 22], and 4 combinations of cardiac death, MI or revascularization [9, 10, 19, 21].
Study characteristics
The characteristics of 14 included studies are presented in Table I. Ten were conducted in Europe [10, 11, 13–16, 19, 20, 22, 23], two in the United States [9, 12], one in Canada [17], and one in Asia [21]. The sample size ranged from 129 to 3782, the average age from 52 years to 70.5 years, and the follow-up term from 6 months to 8.8 years. Serum PAPP-A was measured by ELISA or POC assay in 11 studies [9–12, 14–21], others in 3 studies [13, 22, 23]. The cut-off value of serum PAPP-A was derived from the median in 2 studies [17, 23], others in 12 studies [9–16, 19–22].
Table I.
Characteristics of 15 included studies of PAPP-A in predicting adverse outcomes in patients with CHD
| Source (author, year, country) | No. of subjects | No. of events | Type of CHD | Age [years] | Male [%] | Follow-up [years] | PAPP-A assay | Cut-off of PAPP-A [mIU/l] | Outcomes | Adjustment for covariates |
|---|---|---|---|---|---|---|---|---|---|---|
| Lund et al. [19], 2003, Finland | 136 | 26 | ACS | 66.0 | 51.0 | 0.5 | POC assay | 2.9 | Combination of cardiac death, nonfatal MI, revascularization | Age, gender, DM, smoking, hypertension, previous MI, congestive heart failure, CRP |
| Heesch et al. [13], 2005, Netherlands | 547 | 64 | ACS | 61.0 | 70.9 | 0.5 | Roche Elecsys | 12.6 | Combination of all-cause mortality and nonfatal MI | Sex, age, DM, smoking, hypertension, hyperlipidemia, CHD history, CRP, TnT, ST-segment depression |
| Elesber et al. [12], 2006, USA | 103 | 24 | Stable CHD | 65.0 | 71.0 | 4.9 | BTA ELISA | 4.8 | All-cause mortality, combination of all-cause mortality and ACS | Age, EF, BMI, DM, coronary atherosclerotic burden, CRP, history of MI, CABG and revascularization, medication |
| Brugger-Andersen et al. [10], 2008, Norway | 300 | 83 | MI | 64.0 | 79.3 | 3.75 | DSL ELISA | 0.72 | TnT-positive coronary event or cardiac death | Age, sex, hypertension, smoking, DM, HF, EF, BMI, creatinine, MI location and characteristics, thrombolytics |
| Consuegra-Sanche et al. [11], 2008, Britain | 663 | 106 | Stable CHD | 62.9 | 74.5 | 8.8 | BTA ELISA | 4.6 | All-cause mortality | Age, sex, hypertension, hyperlipidemia, creatinine, vessel score, EF, BMI, DM, CRP, history of CABG and revascularization |
| Iversion-1 et al. [14], 2009, Denmark | 123 | 94 | NSTE-ACS | 70.5 | 60.0 | 2.95 | Sandwich ELISA | 15.5 | All-cause mortality, combination of all-cause mortality and nonfatal MI | Age, family history of CHD, DM, previous MIor revascularization, TnT, CRP, CKMB, potassium, hyperlipidemia, hypertension, hemoglobin |
| Iversion-2 et al. [14], 2009, Denmark | 314 | STEMI | 62.8 | 65.0 | 2.95 | |||||
| Kavsak et al. [17], 2009, Canada | 320 | 50 | Suspected ACS | 64.0 | 60.0 | 2 | DSL ELISA | 1.62 | All-cause mortality | Age, sex, presentation of TnT > 0.02 µg/l |
| Lund et al. [20], 2010, Finland | 267 | 57 | NSTE-ACS | 69.0 | 45.5 | 1 | POC assay | 1.74 | Combination of all-cause mortality and nonfatal MI | Sex, age, DM, previous MI, ischemic electrocardiogram findings |
| Iversion et al. [15], 2010, Denmark | 415 | 147 | Suspected ACS | 67.0 | 57.0 | 3.4 | Sandwich ELISA | 12.4 | All-cause mortality, combination of all-cause mortality and nonfatal MI | Age, sex, BMI, DM, creatinine, hypertension, hyperlipidemia, stroke, EF, vessel score, CRP, CKMB, hemoglobin, history of MI, CABG and revascularization |
| Mei et al. [21], 2011, China | 129 | 25 | ACS | 52 | 73.0 | 1.7 | DSL ELISA | 11.33 | Combination of cardiac death, nonfatal MI, revascularization, rehospitalization | Sex, hyperlipidemia, TIMI grade, EF |
| Iversion et al. [16], 2011, Denmark | 4243 | 531 | Stable CHD | 64.8 | 69.0 | 2.8 | Sandwich ELISA | 4.0 | All-cause mortality, combination of all-cause mortality and nonfatal MI | Age, sex, hypertension, smoking, DM, previous MI, medication |
| Oemrawsingh et al. [22], 2011, Netherlands | 1090 | 167 | NSTE-ACS | 63 | 73.0 | 4 | Roche Elecsys | 12.6 | Combination of all-cause mortality and nonfatal MI | Age, sex, hypertension, smoking, DM, TnT, hyperlipidemia, previous MI, heart failure, revascularization, inflammatory markers, vascular disease, electrocardiogram findings |
| Schaub et al. [23], 2012, Swizerland | 398 | 39 | Suspected ACS | 64 | 66.0 | 2.3 | Roche Elecsys | 4.9 | All-cause mortality | Age, sex, hypertension, smoking, DM, history of CHD, MI, renal failure, inflammatory markers |
| Bonaca et al. [9], 2012, USA | 3782 | NR | NSTE-ACS | NR | 65.0 | 1 | Beckman ELISA | 6.0 | Combination of cardiovascular death and MI | Age, sex, hypertension, smoking, DM, history of CHD, TnI, inflammatory markers |
ACS – acute coronary syndrome, BMI – body mass index, BTA – biotin-tyramide-amplified, CABG – coronary artery bypass graft, CHD – coronary artery disease, CKMB – creatine kinase-MB, CRP – C-reactive protein, DM – diabetes mellitus, DSL – Diagnostic Systems Laboratory, EF – ejective fraction, ELISA – enzyme-linked immunosorbent assay, MI – myocardial infarction, NSTE – non-ST-segment elevated, PAPP-A – pregnancy-associated plasma protein A, POC assay – Point-of-care time-resolved immunofluorometric assay, Roche Elecsys – Elecsys 2010 assay system, Roche Diagnostics, STEMI – ST-segment elevated myocardial infarction, TIMI – thrombolysis in myocardial infarction, TnT – troponin T. 1 – data of NSTE-ACS group, 2 – data of STEMI group
Main analysis
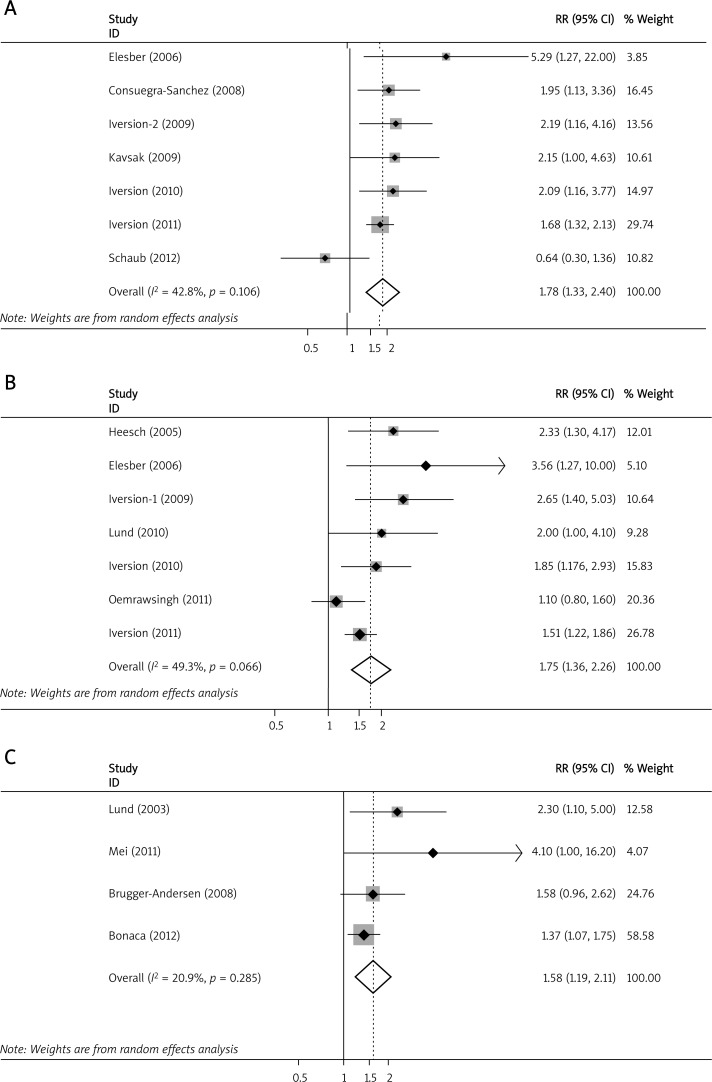
Compared with the lowest levels, the pooled RR for all-cause mortality was 1.78 (95% CI: 1.33 to 2.40, p < 0.001; I 2 = 42.8%, p for heterogeneity = 0.106) (Figure 1A); for combined all-cause mortality and non-fatal MI it was 1.75 (95% CI: 1.36 to 2.26, p < 0.001; I 2 = 49.3%, p for heterogeneity = 0.066) (Figure 1B); for combined cardiovascular events it was 1.58 (95% CI: 1.19 to 2.11, p < 0.00; I 2 = 20.9%, p for heterogeneity = 0.285) (Figure 1C) for high elevated PAPP-A.
Figure 1.
Meta-analysis of elevated PAPP-A and risk of adverse events. Meta-analysis of elevated PAPP-A in predicting (A) all-cause mortality; B – combined all-cause mortality and non-fatal myocardial infarction; C – combined cardiovascular events
CI – confidence interval, PAPP-A – pregnancy-associated plasma protein A, RR – relative risk
Table II shows the subgroup analyses. For the endpoint of all-cause mortality, duration of follow-up and different type of CHD (stable CHD or ACS) did not modify the positive association of elevated PAPP-A with all-cause mortality. Six studies [11, 12, 14–17] detected serum PAPP-A in ELISA and one study [23] used one-step enzyme immunoassay, so we did not perform the subgroup analysis by different assay methods. For the endpoint of combined all-cause mortality and non-fatal MI, duration of follow-up, different assay methods of measuring PAPP-A and different type of CHD were not modifiable factors.
Table II.
Subgroup analysis
| Characteristic | All-cause mortality | Combination of all-cause mortality and non-fatal MI | ||||||
|---|---|---|---|---|---|---|---|---|
| Data points, No. | Pooled RR (95% CI) | Value of p for heterogeneity | Value of p for subgroup difference | Data points, No. | Pooled RR (95% CI) | Value of p for heterogeneity | Value of p for subgroup difference | |
| All studies | 7 | 1.90 (1.45-2.50) | 0.11 | 7 | 1.75 (1.36-2.26) | 0.051 | ||
| Follow-up term [years] | ||||||||
| < 3 | 5 | 1.62 (0.93-2.81) | 0.05 | 0.81 | 4 | 1.85 (1.39-2.47) | 0.22 | 0.31 |
| ≥ 3 | 4 | 1.85 (1.34-2.53) | 0.28 | 3 | 1.67 (0.96-2.91) | 0.04 | ||
| Type of CHD | ||||||||
| Stable CHD | 3 | 1.85 (1.34-2.53) | 0.28 | 0.81 | 2 | 1.99 (0.91-4.36) | 0.11 | 0.79 |
| ACS | 4 | 1.62 (0.93-2.81) | 0.05 | 5 | 1.80 (1.26-2.57) | 0.06 | ||
| Assay for PAPP-A | ||||||||
| ELISA or POC assay | 6 | 5 | 1.83 (1.43-2.36) | 0.25 | 0.18 | |||
| Other | 1 | 2 | 1.54 (0.74-3.20) | 0.03 | ||||
ACS – acute coronary syndrome, CHD – coronary heart disease, ELISA – enzyme-linked immunosorbent assay, MI – myocardial infarction, PAPP-A – pregnancy-associated plasma protein A, POC assay – point-of-care time-resolved immunofluorometric assay
Two studies provided RR with less than five adjusted variables [17, 21]. Sensitivity analyses showed that, when excluding the study of Kavsak et al. [17], the pooled RR for mortality is 1.75 (95% CI 1.25 to 2.44; p = 0.001); when excluding the study of Mei et al. [21], the pooled RR for cardiovascular events is 1.46 (95% CI 1.18 to 1.81; p = 0.000).
Publication bias
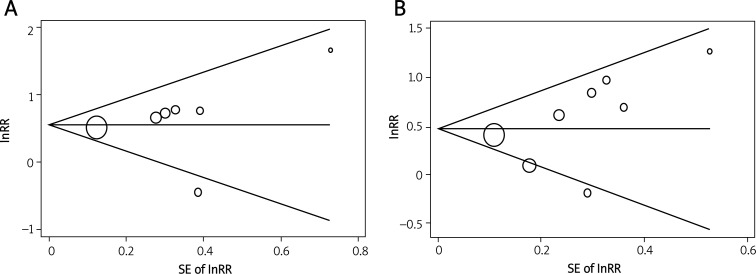
No evidence of publication bias was observed in Begg's funnel plot. The results were further confirmed in Begg's adjusted rank correlation test (p = 0.18, p = 0.10,) and Egger's regression test (p = 0.16, p = 0.56) for the association of serum elevated PAPP-A with all-cause mortality, and combined all-cause mortality and non-fatal MI (Figures 2A and 2B).
Figure 2.
Begg's funnel plot of individual studies on the association of circulating PAPP-A levels and risk of all-cause mortality, and combined all-cause mortality and non-fatal myocardial infarction. The studies that evaluated higher PAPP-A levels and risk of all-cause mortality (A), and combined all-cause mortality and non-fatal myocardial infarction (B) were plotted with lnRRs on the vertical axis and the SEs of the lnRRs along the horizontal axis. The graph symbols are sized by weights
PAPP-A – pregnancy-associated plasma protein A, RR – relative risk, SE – standard error
Discussion
In our meta-analysis of 14 prospective studies, we observed that circulating elevated PAPP-A predicts adverse vascular events in patients with CHD. Patients with elevated PAPP-A were associated with a 1.78-fold higher risk for all-cause mortality, 1.75-fold for combined all-cause mortality and MI, and 1.58-fold for combined cardiovascular events (including cardiac death, myocardial infarction or revascularization). The positive association between elevated PAPP-A and adverse vascular events was not modified by duration of follow-up, different methods for measuring PAPP-A and different type of CHD.
Whether PAPP-A is an independent predictor for adverse vascular events is controversial. Several clinical trials have reported a positive relationship while others did not. Almost all the included studies have controlled for established cardiovascular risk factors or prognostic indicators, such as age, gender, hyperlipidemia, hypertension, diabetes, smoking, history of CHD, left ventricular ejection fraction, troponin, inflammatory markers, and so on [9–17, 19–23]. We combined all these studies and found that serum PAPP-A is an independent risk factor for patients with CHD. Furthermore, our sensitivity analyses showed consistent results when we excluded the studies with less than 5 adjusted variables [17, 21]. So the results of our meta-analysis indicated that elevated PAPP-A was an independent risk factor for adverse vascular events for CHD patients.
Heterogeneity is often a concern of a meta-analysis. Different studies have been performed in different types of patients (e.g., patients with stable CHD, ACS, or suspected ACS), with different duration of follow-up, so the included trials were varied with different cut-off values of PAPP-A even under the same assay methods [9–22]. However, our subgroup analyses found that patient type, follow-up term and assay methods of serum PAPP-A were not factors modifying the results. Nevertheless, the cut-off value of PAPP-A in CHD patients and the potential modifying factors require further exploration in larger prospective studies.
Several mechanisms may be involved in the probability of elevated PAPP-A in predicting adverse vascular events in CHD patients. First, PAPP-A is not only a biomarker of ACS but also plays an important role in atherosclerosis [31, 36]. Animal studies have demonstrated that PAPP-A knock-out mice could resist progression of atherosclerosis [26]. Vice versa, over-expression of the PAPP-A gene in a mouse model of atherosclerosis could accelerate the development of atherosclerosis [32]. In addition, PAPP-A knock-out mice could resist proliferation and migration of vascular smooth muscle cells. Vascular smooth muscle cells are the major cells which lead to restenosis and fatal events after the first ACS [37]. So it seemed plausible that elevated PAPP-A levels could predict restenosis or subsequent vascular events. Second, circulating PAPP-A is related to the extent of atherosclerosis. Clinical studies have reported that patients with complex stenosis have higher levels of PAPP-A than ones with smooth stenosis [38, 39]. Therefore, patients with higher PAPP-A levels may be at higher risk for vascular events. Third, various studies have demonstrated that PAPP-A is positively correlated with local inflammation, which was a well-established risk factor for prognosis of CHD patients [29, 36, 40]. So PAPP-A may also be useful in improving risk stratification for CHD patients [41–43]. Finally, in our meta-analysis, we found that an elevated PAPP-A level seemed to be more associated with all-cause mortality or combined all-cause mortality and non-fatal MI than combined cardiovascular events. The possible explanation was that PAPP-A is not only a cardiac biomarker, but also a non-cardiac biomarker [44]. Recent studies have found that PAPP-A is also an biomarker associated with malignant cancer and end-stage renal disease [45, 46]. So patients with an elevated PAPP-A level not only have a high risk of cardiovascular events, but also have a higher risk of death.
Our study has several strengths. The major strength is that all of the included studies are of prospective design, which eliminates revision bias and minimizes selection bias. Furthermore, we performed a series of subgroup and sensitivity analyses to explore potential sources of heterogeneity. The consistent results from subgroup and sensitivity analyses have strengthened the statistical power.
Our study also has a few limitations. First, the residual confounders may be potential limitations. Most included studies have adjusted a wide range of potential confounders. However, we cannot exclude the potential influence of unadjusted variables on the results. Second, the predictive value of circulating PAPP-A in CHD patients may be affected by study design. However, we did not find that the predictive value of PAPP-A varied by short or long follow-up term, stable CHD or ACS, or different assay measurements. Third, all of the included studies are varied with different cut-off values of PAPP-A. Given the nature of a meta-analysis, we could not provide the cut-off value of serum PAPP-A. Finally, potential publication bias might influence the results; however, we found no evidence of publication bias.
In conclusion, elevated serum PAPP-A level is associated with adverse vascular outcomes in patients with CHD. The independent predictive value of PAPP-A was not affected by follow-up term, assay methods, or types of CHD. Further studies should test the newly defined cut-off value of elevated PAPP-A and put PAPP-A in use from bench to bedside.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Mercando AD, Lai HM, Aronow WS, et al. Reduction in atherosclerotic events: a retrospective study in an outpatient cardiology practice. Arch Med Sci. 2012;8:57–62. doi: 10.5114/aoms.2012.27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Glass CK, Witztum JL. Atherosclerosis. The Road Ahead. Cell. 2001;104:503–16. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 5.Dudziak J, Majewski M, Jerzykowska O, et al. Diagnostic value of circulating pregnancy-associated plasma protein a in atherosclerotic plaque rupture complications. Post Kardiol Interw. 2011;7:302–10. [Google Scholar]
- 6.Iversen KK, Teisner AS, Teisner B, et al. Pregnancy-associated plasma protein A in non-cardiac conditions. Clin Biochem. 2008;41:548–53. doi: 10.1016/j.clinbiochem.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Iversen KK, Teisner AS, Teisner B, et al. Pregnancy associated plasma protein A, a novel, quick, and sensitive marker in ST-elevation myocardial infarction. Am J Cardiol. 2008;101:1389–94. doi: 10.1016/j.amjcard.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Hajek P, Macek M, Sr, Lashkevich A, et al. Influence of concomitant heparin administration on pregnancy-associated plasma protein-A levels in acute coronary syndrome with ST segment elevation. Arch Med Sci. 2011;7:977–83. doi: 10.5114/aoms.2011.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaca MP, Scirica BM, Sabatine MS, et al. Prospective evaluation of pregnancy-associated plasma protein-A and outcomes in patients with acute coronary syndromes. J Am Coll Cardiol. 2012;60:332–8. doi: 10.1016/j.jacc.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Brugger-Andersen T, Aarsetoy H, Grundt H, et al. The long-term prognostic value of multiple biomarkers following a myocardial infarction. Thromb Res. 2008;123:60–6. doi: 10.1016/j.thromres.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Consuegra-Sanchez L, Petrovic I, Cosin-Sales J, et al. Prognostic value of circulating pregnancy-associated plasma protein-A (PAPP-A) and proform of eosinophil major basic protein (Pro-Mbp) levels in patients with chronic stable angina pectoris. Clin Chim Acta. 2008;391:18–23. doi: 10.1016/j.cca.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Elesber AA, Conover CA, Denktas AE, et al. Prognostic value of circulating pregnancy-associated plasma protein levels in patients with chronic stable angina. Eur Heart J. 2006;27:1678–84. doi: 10.1093/eurheartj/ehl042. [DOI] [PubMed] [Google Scholar]
- 13.Heeschen C, Dimmeler S, Hamm CW, et al. Pregnancy-associated plasma protein-a levels in patients with acute coronary syndromes: comparison with markers of systemic inflammation, platelet activation, and myocardial necrosis. J Am Coll Cardiol. 2005;45:229–37. doi: 10.1016/j.jacc.2004.09.060. [DOI] [PubMed] [Google Scholar]
- 14.Iversen KK, Dalsgaard M, Teisner AS, et al. Usefulness of pregnancy-associated plasma protein A in patients with acute coronary syndrome. Am J Cardiol. 2009;104:1465–71. doi: 10.1016/j.amjcard.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Iversen KK, Dalsgaard M, Teisner AS, et al. Pregnancy-associated plasma protein-a, a marker for outcome in patients suspected for acute coronary syndrome. Clin Biochem. 2010;43:851–7. doi: 10.1016/j.clinbiochem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Iversen KK, Teisner B, Winkel P, et al. Pregnancy associated plasma protein-A as a marker for myocardial infarction and death in patients with stable coronary artery disease: a prognostic study within the Claricor trial. Atherosclerosis. 2011;214:203–8. doi: 10.1016/j.atherosclerosis.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Kavsak PA, Wang X, Henderson M, et al. PAPP-A as a marker of increased long-term risk in patients with chest pain. Clin Biochem. 2009;42:1012–8. doi: 10.1016/j.clinbiochem.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laterza OF, Cameron SJ, Chappell D, et al. Evaluation of pregnancy-associated plasma protein A as a prognostic indicator in acute coronary syndrome patients. Clin Chim Acta. 2004;348:163–9. doi: 10.1016/j.cccn.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Lund J, Qin QP, Ilva T, et al. Circulating pregnancy-associated plasma protein A predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation. 2003;108:1924–6. doi: 10.1161/01.CIR.0000096054.18485.07. [DOI] [PubMed] [Google Scholar]
- 20.Lund J, Wittfooth S, Qin QP, et al. Free vs. total pregnancy-associated plasma protein A (PAPP-A) as a predictor of 1-year outcome in patients presenting with non-ST-elevation acute coronary syndrome. Clin Chem. 2010;56:1158–65. doi: 10.1373/clinchem.2009.136960. [DOI] [PubMed] [Google Scholar]
- 21.Mei WY, Du ZM, Zhao Q, et al. Pregnancy-associated plasma protein predicts outcomes of percutaneous coronary intervention in patients with non-ST-elevation acute coronary syndrome. Heart Lung. 2011;40:e78–83. doi: 10.1016/j.hrtlng.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Oemrawsingh RM, Lenderink T, Akkerhuis KM, et al. Multimarker risk model containing troponin-T, interleukin 10, myeloperoxidase and placental growth factor predicts long-term cardiovascular risk after non-ST-segment elevation acute coronary syndrome. Heart. 2011;97:1061–6. doi: 10.1136/hrt.2010.197392. [DOI] [PubMed] [Google Scholar]
- 23.Schaub N, Reichlin T, Meune C, et al. Markers of plaque instability in the early diagnosis and risk stratification of acute myocardial infarction. Clin Chem. 2012;58:246–56. doi: 10.1373/clinchem.2011.172940. [DOI] [PubMed] [Google Scholar]
- 24.Schulz O, Reinicke M, Kramer J, et al. Pregnancy-associated plasma protein a values in patients with stable cardiovascular disease: use of a new monoclonal antibody-based assay. Clin Chim Acta. 2011;412:880–6. doi: 10.1016/j.cca.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFbps. Growth Horm IGF Res. 2007;17:10–8. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Harrington SC, Simari RD, Conover CA. Genetic deletion of pregnancy-associated plasma protein-A is associated with resistance to atherosclerotic lesion development in apolipoprotein E-deficient mice challenged with a high-fat diet. Circ Res. 2007;100:1696–702. doi: 10.1161/CIRCRESAHA.106.146183. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence JB, Oxvig C, Overgaard MT, et al. The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc Natl Acad Sci U S A. 1999;96:3149–53. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conover CA, Harrington SC, Bale LK. Differential regulation of pregnancy associated plasma protein-A in human coronary artery endothelial cells and smooth muscle cells. Growth Horm IGF Res. 2008;18:213–20. doi: 10.1016/j.ghir.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conover CA, Bale LK, Harrington SC, et al. Cytokine stimulation of pregnancy-associated plasma protein A expression in human coronary artery smooth muscle cells: inhibition by Resveratrol. Am J Physiol Cell Physiol. 2006;290:C183–8. doi: 10.1152/ajpcell.00199.2005. [DOI] [PubMed] [Google Scholar]
- 30.Bayes-Genis A, Conover CA, Schwartz RS. The insulin-like growth factor axis: a review of atherosclerosis and restenosis. Circ Res. 2000;86:125–30. doi: 10.1161/01.res.86.2.125. [DOI] [PubMed] [Google Scholar]
- 31.Bayes-Genis A, Conover CA, Overgaard MT, et al. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345:1022–9. doi: 10.1056/NEJMoa003147. [DOI] [PubMed] [Google Scholar]
- 32.Conover CA, Mason MA, Bale LK, et al. Transgenic overexpression of pregnancy-associated plasma protein-A in murine arterial smooth muscle accelerates atherosclerotic lesion development. Am J Physiol Heart Circ Physiol. 2010;299:H284–91. doi: 10.1152/ajpheart.00904.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (Moose) Group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 34.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sangiorgi G, Mauriello A, Bonanno E, et al. Pregnancy-associated plasma protein-A is markedly expressed by monocyte-macrophage cells in vulnerable and ruptured carotid atherosclerotic plaques: a link between inflammation and cerebrovascular events. J Am Coll Cardiol. 2006;47:2201–11. doi: 10.1016/j.jacc.2005.11.086. [DOI] [PubMed] [Google Scholar]
- 37.Resch ZT, Simari RD, Conover CA. Targeted disruption of the pregnancy-associated plasma protein-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia after vascular injury. Endocrinology. 2006;147:5634–40. doi: 10.1210/en.2006-0493. [DOI] [PubMed] [Google Scholar]
- 38.Cosin-Sales J, Christiansen M, Kaminski P, et al. Pregnancy-associated plasma protein A and its endogenous inhibitor, the proform of eosinophil major basic protein (Prombp), are related to complex stenosis morphology in patients with stable angina pectoris. Circulation. 2004;109:1724–8. doi: 10.1161/01.CIR.0000124716.67921.D2. [DOI] [PubMed] [Google Scholar]
- 39.Cosin-Sales J, Kaski JC, Christiansen M, et al. Relationship among pregnancy associated plasma protein-A levels, clinical characteristics, and coronary artery disease extent in patients with chronic stable angina pectoris. Eur Heart J. 2005;26:2093–8. doi: 10.1093/eurheartj/ehi433. [DOI] [PubMed] [Google Scholar]
- 40.Resch ZT, Chen BK, Bale LK, et al. Pregnancy-associated plasma protein A gene expression as a target of inflammatory cytokines. Endocrinology. 2004;145:1124–9. doi: 10.1210/en.2003-1313. [DOI] [PubMed] [Google Scholar]
- 41.Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002;105:1760–3. doi: 10.1161/01.cir.0000015464.18023.0a. [DOI] [PubMed] [Google Scholar]
- 42.Biasillo G, Leo M, Della Bona R, et al. Inflammatory biomarkers and coronary heart disease: from Bench to bedside and back. Intern Emerg Med. 2010;5:225–33. doi: 10.1007/s11739-010-0361-1. [DOI] [PubMed] [Google Scholar]
- 43.Morrow DA, Cannon CP, Jesse RL, et al. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–75. doi: 10.1161/CIRCULATIONAHA.107.182882. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Zhou C, Zhou X, et al. PAPP-A in cardiac and non-cardiac conditions. Clin Chim Acta. 2013;417:67–72. doi: 10.1016/j.cca.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Bulut I, Coskun A, Ciftci A, et al. Relationship between pregnancy-associated plasma protein-A and lung cancer. Am J Med Sci. 2009;337:241–4. doi: 10.1097/MAJ.0b013e31818967a3. [DOI] [PubMed] [Google Scholar]
- 46.Etter C, Straub Y, Hersberger M, et al. Pregnancy-associated plasma protein-A is an independent short-time predictor of mortality in patients on maintenance haemodialysis. Eur Heart J. 2010;31:354–9. doi: 10.1093/eurheartj/ehp429. [DOI] [PubMed] [Google Scholar]