Abstract
Background: Prior calculations of the burden of disease from toxic exposures have not included estimates of the burden from toxic waste sites due to the absence of exposure data.
Objective: We developed a disability-adjusted life year (DALY)-based estimate of the disease burden attributable to toxic waste sites. We focused on three low- and middle-income countries (LMICs): India, Indonesia, and the Philippines.
Methods: Sites were identified through the Blacksmith Institute’s Toxic Sites Identification Program, a global effort to identify waste sites in LMICs. At least one of eight toxic chemicals was sampled in environmental media at each site, and the population at risk estimated. By combining estimates of disease incidence from these exposures with population data, we calculated the DALYs attributable to exposures at each site.
Results: We estimated that in 2010, 8,629,750 persons were at risk of exposure to industrial pollutants at 373 toxic waste sites in the three countries, and that these exposures resulted in 828,722 DALYs, with a range of 814,934–1,557,121 DALYs, depending on the weighting factor used. This disease burden is comparable to estimated burdens for outdoor air pollution (1,448,612 DALYs) and malaria (725,000 DALYs) in these countries. Lead and hexavalent chromium collectively accounted for 99.2% of the total DALYs for the chemicals evaluated.
Conclusions: Toxic waste sites are responsible for a significant burden of disease in LMICs. Although some factors, such as unidentified and unscreened sites, may cause our estimate to be an underestimate of the actual burden of disease, other factors, such as extrapolation of environmental sampling to the entire exposed population, may result in an overestimate of the burden of disease attributable to these sites. Toxic waste sites are a major, and heretofore underrecognized, global health problem.
Keywords: Asia, burden of disease, chemical exposure, disability-adjusted life year, toxic waste sites
Toxic waste sites threaten the environment and human health in countries around the world. In developing countries these sites—and their risks to human health—have not been optimally assessed (Yáñez et al. 2002). Quantification of the burden of disease from toxic waste sites can assist public health planning and remediation efforts by complementing traditional waste site investigations and by framing these toxic exposures in the context of other exposures. Burden of disease estimates are typically expressed in disability-adjusted life years (DALYs). The DALY metric accounts for both the morbidity and mortality that result from a disease, injury, or health state (Prüss-Üstün et al. 2003).
Previous calculations of the burden of disease from toxic exposures have not included estimates from toxic waste sites because of an absence of data on exposures and health impacts. In 2004, Fewtrell et al. (2004) estimated that lead causes nearly 1% of the global burden of disease. Then in 2011, Prüss-Üstün et al. (2011) calculated that exposure to a variety of chemicals, including lead, secondhand smoke, and asbestos, accounts for 5.7% of total global DALYs and 8.3% of total global deaths. However, because of insufficient data, neither of these studies included estimates for disease and death attributable to exposures from toxic waste sites.
We aimed to develop a DALY-based estimate of the burden of disease and death attributable to toxic waste sites in India, Indonesia, and the Philippines. To our knowledge, no systematic evaluation of toxic waste sites in low- and middle-income countries (LMICs) had previously been performed. The paucity of data has precluded calculation of the burden of disease resulting from exposures at these sites. Through this effort we hope to ultimately calculate the contribution of toxic waste sites to the global burden of disease.
Methods
Site identification. In this study, we utilized data collected through Blacksmith Institute’s Toxic Sites Identification Program (TSIP), an effort to identify and screen contaminated sites in LMICs (Blacksmith Institute 2013). The TSIP, which is implemented jointly with the United Nations Industrial Development Organization, identifies point-source pollution from industrial sites that present a public health risk. A particular focus is placed on abandoned (legacy) sites, such as former tanneries, as well as small-scale artisanal sources, such as lead battery recycling and artisanal gold mining. Although other sources of contamination, such as large-scale mining, may also be included and screened, the majority of sites come from these two categories (i.e., legacy sites and artisanal sources). The TSIP excludes nonpoint sources, such as ambient urban air pollution, and non-chemical contamination, such as sewage-contaminated water. Ericson et al. (2012) described the types of sites identified in the TSIP.
The Blacksmith Institute developed an evaluation instrument, the Initial Site Screening (ISS), for rapid data collection and assessment of these sites (Blacksmith Institute 2013). The ISS is a modified and simplified version of the U.S. Environmental Protection Agency’s (EPA) Hazard Ranking System, used to prioritize and rank toxic waste sites in the U.S. EPA’s Superfund program (U.S. EPA 2012b). The ISS includes information on the concentration of the key toxic chemical, the primary environmental medium of the exposure pathway, and the size of the population at risk.
To undertake the TSIP, the Blacksmith Institute contracted and trained approximately 150 site investigators. These investigators identified and visited sites, collected environmental samples, took photographs and GPS coordinates, interviewed stakeholders, and categorized the potential contaminated environmental media. After being educated on the project and assured that participation in the interview process was voluntary, the stakeholders agreed to participate; written informed consent was not obtained. The investigators determined the dominant pollutant for each site based in part on prior testing or historical use of a site, then took samples to measure levels of the pollutant, typically in only one environmental medium. For sites where only total chromium was reported, the speciation coefficient of 0.6 was used to estimate hexavalent chromium (Avudainayagam et al. 2003; Kumar and Riyazuddin 2010). Between 2009 and 2012, investigators completed 1,510 such screenings in 49 countries. Since the majority of screenings occurred in 2010, we used 2010 as our baseline year for analysis. We have previously described the ISS protocol and TSIP in detail (Ericson et al. 2012).
Population at risk of exposure. As part of the ISS, investigators estimated the population at risk of exposure for each site, indicating the number of persons regularly coming into contact with the contaminant in the relevant environmental medium. For example, if water contamination is documented, then the population at risk includes those individuals who use the water daily for drinking, food preparation, and other domestic purposes. Investigators used a range of approaches to obtain this information, including visual methods, satellite photographs, community census data, government interviews, and personal knowledge. The age distribution at sites was not recorded as part of the ISS. Therefore, we applied age distribution estimates from the U.S. Census Bureau (2012) for each country to the population around each site within that country. We divided each site’s estimated population into 17 age groups based on these distributions (e.g., 0–4, 5–9, 10–14 years).The World Health Organization (WHO) DALY calculator for cardiovascular disease resulting from adult lead exposure uses 5 age groups (i.e., 15–29, 30–44, 45–59, 60–69, 70–79 years). In this study, we condensed the 17 age groups into the appropriate 5 groups to enable our calculations.
Calculating risk per person. We divided human health effects into cancer and noncancer effects. For carcinogens, we used the U.S. EPA’s Regional Screening Level Calculator for Chemical Contaminants to calculate long-term cancer risk per unit toxicant (i.e., cancer probability per milligram per kilogram soil for agents found in soil or microgram per liter water for waterborne agents) (U.S. EPA 2012c). For noncancer health effects, reference doses (RfDs) and concentrations (RfCs) from the U.S. EPA’s Integrated Risk Information System (IRIS) database were applied to the exposure pathways and contamination levels at each site (U.S. EPA 2012a). The modeling assumed a linear dose response and used the health outcome associated with the RfD or RfC (e.g., liver toxicity, renal toxicity). A listing of the cancer and noncancer risks per unit of contaminant, with the exception of lead, is presented in Table 1. Given the availability of lead-specific modeling tools and dose–response relationships, we calculated disease incidence and DALYs from lead separately.
Table 1.
Per capita cancer and noncancer human health risks by chemical and media for chemicals other than lead.
| Chemical (media assessed) | Cancer risk | Noncancer risk | ||||
|---|---|---|---|---|---|---|
| Per µg/m3 in air | Permg/kg in soil | Per µg/L inwater | Per µg/m3 in air | Permg/kg in soil | Per µg/L inwater | |
| Aldrin (W) | NA | NA | 5.35 × 10–4 | NA | NA | 2.22 × 10–6 |
| Asbestos (A) | 2.30 × 10–1a | NA | NA | NA | NA | NA |
| Cadmium (A,S,W) | 1.80 × 10–3 | NA | NA | 5.00 × 10–5 | 2.67 × 10–8 | 1.33 × 10–7 |
| Chromium VI (A,S,W) | 8.40 × 10–2 | 9.71 × 10–8b | 2.09 × 10–5 | NA | NA | NA |
| DDT (W) | NA | NA | 1.07 × 10–5 | NA | NA | 1.33 × 10–7 |
| Lindane (S,W) | NA | 5.08 × 10–6 | 3.45 × 10–5 | NA | 8.85 × 10–8 | 2.22 × 10–7 |
| Mercury, inorganic (A,S,W) | NA | NA | NA | 5.68 × 10–8 | 8.85 × 10–8 | 2.22 × 10–7 |
| Abbreviations: A, air; DDT, dichlorodiphenyltrichloroethane; NA, not assessed; S, soil; W, water.aFibers/cubic centimeter. bInhaled airborne dust. | ||||||
Calculating incidence of disease. For each chemical, we considered up to three environmental media (soil, water, air) and corresponding routes of exposure (ingestion, dermal, and/or inhalation). To calculate disease incidence for all chemicals except lead, we multiplied the risk per person by the level of the contaminant in the relevant environmental medium. Because linear slope factors were utilized to calculate incidence, very high concentrations of contaminants resulted in correspondingly high estimates of disease incidence. To accommodate this limitation of the model, we arbitrarily capped incidence for all diseases at 5%.
For lead, we calculated the incidence of mild mental retardation and anemia in children and cardiovascular disease in adults resulting from lead-induced increases in blood pressure. We calculated the predicted mean blood lead levels (BLLs) that would result from lead exposures at each site by entering the soil and drinking-water lead levels measured at each site into the U.S. EPA’s Integrated Exposure, Uptake and Biokinetic (IEUBK) model for lead and Adult Lead Methodology (ALM) (Caravanos et al. 2012; U.S. EPA 1994; White et al. 1998). We calibrated default soil ingestion levels in the IEUBK model upward from 200 mg/day to 400 mg/day. This approach follows similar analyses done in Native American populations (400 mg/day), as well as in indigenous populations in Micronesia (500 mg/day), and is above the “upper bound” level (200 mg/day) used by the U.S. EPA (Harris and Harper 2004; Sun and Meinhold 1997; U.S. EPA 2011). Then we calculated the incidence of mild mental retardation and cardiovascular outcomes that would result from such BLLs, using spreadsheets developed by the WHO (2013). We also assumed that 20% of children with BLLs > 70 µg/dL develop anemia (Fewtrell et al. 2003).
Calculating years lived with disability (YLDs) and years of life lost (YLLs). The DALY metric is the sum of two components: YLD, which represents disease-related morbidity, and YLL, which represents the premature mortality from the disease. We calculated YLD and YLL for exposure to each contaminant through each relevant environmental medium. YLD is the product of the estimated years lived with a given disability multiplied by its specific disability weight (DW). The DW is a value from zero to one, depending on the severity of each disease, with zero representing ideal health and one representing death. For example, periodontal disease has a DW of 0.001, whereas a first-time stroke has a DW of 0.920 (WHO 2008).
For each chemical, we assigned the relevant type of cancer, noncancer health effect, and corresponding DW (Table 2) (U.S. EPA 2012a; WHO 2008). If the chemical’s health effect did not align with a disease in the WHO DW database, then we selected the most appropriate disease and DW on the basis of the target organ, duration of disease, and severity of disease. In the case of noncarcinogenic effects, the total number of years of life remaining at onset was multiplied by the appropriate DW to determine YLD (Prüss-Üstün et al. 2003). We chose to apply the exposure for the remainder of an individual’s life expectancy given that most LMICs do not have a systemic program to identify and remediate these sites. For carcinogens, we applied a DW and duration to each cancer stage: diagnosis (cancer-specific DW; 3 years); metastasis (DW 0.75; 1 year); and terminal (DW 0.81; 1 year) (WHO 2008). YLLs were calculated only for carcinogens. We used cancer incidence and survival data to calculate the resulting number of deaths (Ferlay et al. 2010; Sankaranarayanan et al. 2010). All cancers were assumed to last 5 years, before either going into remission or resulting in death.
Table 2.
Cancer and noncancer health effects and DWs of chemicals found at waste sites.
| Chemical | Cancer site (classification)a | Cancer-specific DWb | Health effect (noncancer) | DW (noncancer) |
|---|---|---|---|---|
| Aldrin (W) | Liver (probable) | 0.20 | Liver toxicity | 0.104c |
| Asbestos (A) | Lung (confirmed) | 0.15 | NA | NA |
| Cadmium (A) | Lung (probable) | 0.15 | NA | NA |
| Cadmium (W,S) | NA | NA | Renal toxicity | 0.091d |
| Chromium VI (A,W,S) | Lung (confirmed) | 0.15 | NA | NA |
| DDT (W) | Liver (probable) | 0.20 | Liver toxicity | 0.104c |
| Lead (A,W,S) | NA | NA | Mild mental retardation | 0.361 |
| Decrement in IQ | 0.024e | |||
| Cardiovascular disease | NAf | |||
| Anemia | 0.024 | |||
| Lindane (W,S) | Liver (possible) | 0.20 | Liver toxicity | 0.104c |
| Mercury, inorganic (W,S) | NA | NA | Renal toxicity | 0.091d |
| Abbreviations: A, air; DDT, dichlorodiphenyltrichloroethane; DW, disability weight; NA, not assessed; S, soil; W, water.aHuman carcinogenicity classification (U.S. EPA 2012a). bCancer-specific DW was applied for a duration of 3years, then a DW of 0.75 was applied for 1year (metastasis), followed by a DW of 0.81 for 1year (terminal stage). cAdvanced hepatic disease. dAcute glomerulonephritis. eDevelopmental disability associated with protein–energy malnutrition. fDALYs calculated with the environmental attributable fraction approach. | ||||
For lead, we utilized the environmentally attributable fraction approach in determining the contribution of lead exposure to the burden of cardiovascular disease (ischemic heart disease, cerebrovascular disease, hypertensive disease, and other cardiac disease) (Fewtrell et al. 2003). The WHO has calculated the fraction of cardiovascular disease attributable to lead exposure based on BLL. By entering the predicted BLL and total cardiovascular disease DALYs for each country into a WHO spreadsheet, we calculated the DALYs attributable to cardiovascular disease from lead exposure at toxic waste sites in each of the three countries. In addition, for children with BLLs > 10 µg/dL who did not have mental retardation, we applied the DW for developmental disability from protein–energy malnutrition (0.024) as a proxy DW for lifelong disability from IQ loss in the absence of mental retardation. Prior research suggests that the loss of IQ points may impact cardiovascular and all-cause mortality, resulting in increased morbidity and mortality (Batty et al. 2010; Lager et al. 2009).
We then applied weighting factors to the resulting YLD and YLL for each chemical, including a discount rate to account for inherent inaccuracies when predicting future events, and age weights to reflect the relative societal value of different age groups (Mathers et al. 2006). The notation DALYs(r,K) signifies the discount rate (r) and age weight (K) used. Our primary results are expressed as DALYs(3,1), which include a 3% discount rate and the full age weight. We also calculated DALYs(3,0) with the 3% discount rate only, and DALYs(0,0) without any weighting to provide a range of estimates (Mathers et al. 2006).
For example, the drinking water at one site in India had an aldrin level of 0.063 ppb. The oral RfD for aldrin for liver toxicity is 3.0 × 10–5 mg/kg/day, which converts to a risk of 8.57 × 10–10 per microgram per liter of drinking water (U.S. EPA 2012a, 2012c). The DW for advanced hepatic disease is 0.104. Assuming the 4,000 persons potentially exposed consume 2 L of drinking water each day, we calculated 1.62 DALYs(3,0), 1.64 DALYs(3,1), and 2.02 DALYs(0,0) resulting from exposure to aldrin in drinking water at this site.
Sensitivity analysis. In addition to calculating DALYs with varying rates and weights, we also altered inputs into our model to conduct a sensitivity analysis. We varied the total population at risk by 25%, changed the disease incidence cap from the default value of 5% to 2.5% or 7.5%, and removed the additional DW for lead-induced IQ (intelligence quotient) losses that did not result in mild mental retardation. For a remediation scenario, we also assumed that remediation had reduced all pollutants to concentrations below international standards (Blacksmith Institute 2011). By subtracting the resulting DALYs from our primary estimate, we quantified the potential impact of remediating these sites.
We also estimated that an additional 5,000 unscreened sites exist in these countries, and that these sites present similar conditions as the screened sites. The TSIP prioritized screenings in part by the scale of the problem, measured in population at risk. Thus, these 5,000 sites are unlikely to have comparably large populations. We therefore assumed that the population at risk for each of these additional sites was the median of the population at risk of screened sites, which is lower than the mean population for screened sites. By contrast, the DALY per person estimates for the 5,000 unscreened sites are unlikely to be lower than those identified at the screened sites. Sites were not prioritized for screening based on the level of the contaminant in the pathway. Therefore, we applied the average DALY per person for the screened sites to the population at the unscreened sites.
Results
Sites evaluated. Blacksmith Institute–trained investigators screened 498 sites in India, Indonesia, and the Philippines, with an estimated population at risk of exposure of approximately 12 million. Of the 23 separate chemicals documented at these sites, 8 occurred at more than one site and had established dose–response relationships correlating exposure with specific outcomes. We included in the analysis only the 373 sites containing 1 of these 8 chemicals. Figure 1 displays the geographical distribution of the sites in India (n = 221), Indonesia (n = 73), and the Philippines (n = 79). The estimated population at risk of exposure at these 373 sites was 8,629,750 (mean, 23,136, median, 7,000), which is 0.61% of the total population of the three countries. Of the exposed population, 3,449,592 were < 18 years of age and 2,184,220 were women of childbearing age (15–49 years of age). We estimated that an additional 5,000 unscreened sites exist in the three countries, with a population of 7,000 persons per site. This additional population equals 35,000,000, resulting in a total population of 43,629,750 for the screened and unscreened sites.
Figure 1.
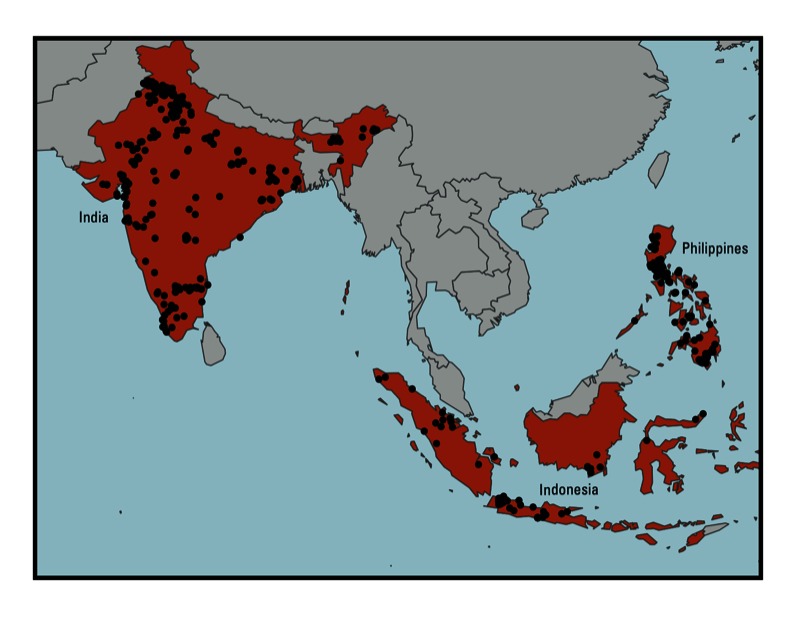
Locations of 373 toxic waste sites in India, Indonesia, and the Philippines in 2010.
YLD and YLL at screened sites. We estimated 588,112 person-years lived with disease and 240,610 person-years lost as a result of chemical exposures in 2010 at the 373 toxic waste sites (Table 3). According to our estimates, lead was the largest contributor of the eight chemicals to YLD (523,630 YLD, 89% of total YLD), and hexavalent chromium was the largest contributor to YLL (235,483 YLL, 97.9% of total YLL). In Table 3, inhalation of soil and dust is incorporated into the soil results.
Table 3.
YLDs, YLLs, and DALYs by chemical.
| Chemical | No. of sites | Estimated population at risk | YLDs | YLLs | DALYs |
|---|---|---|---|---|---|
| Aldrin | 5 | 133,000 | 212 | 812 | 1,024 |
| Asbestos | 3 | 25,000 | 974 | 4,218 | 5,192 |
| Cadmium | 53 | 976,600 | 15 (S=1, W=14) | 0 | 15 |
| Chromium VI | 128 | 3,231,750 | 63,174 (S=3,582, W=59,592) | 235,483 (S=14,467, W=221,016) | 298,657 |
| DDT | 4 | 180,000 | 4 | 18 | 22 |
| Lead | 79 | 1,829,900 | 523,630 | 0 | 523,630 |
| Lindane | 9 | 131,300 | 20 (S=2, W=18) | 79 (S=6, W=73) | 99 |
| Mercury, inorganic | 92 | 2,122,200 | 83 (S=32, W=51) | 0 | 83 |
| Total | 373 | 8,629,750 | 588,112 | 240,610 | 828,722 |
| Abbreviations: DDT, dichlorodiphenyltrichloroethane; S, soil; W, water. | |||||
Premature deaths and DALYs at screened and unscreened sites.We estimated that 828,722 DALYs(3,1) resulted from chemical exposures at the 373 sites in 2010. By applying the value of 0.10 DALYs(3,1) per person from the screened sites to the population at the unscreened sites, we estimated that 3,500,000 DALYs(3,1) resulted from exposure at the unscreened sites. The total estimated DALYs(3,1) for the screened and unscreened sites was 4,328,722. We also calculated that 66,747 persons would die prematurely from cancer, specifically liver and lung cancer, from exposures at these sites.
Sensitivity analysis. Removal of age weights yielded 814,934 DALYs(3,0), whereas removal of age weights and the discount rate yielded 1,557,121 DALYs(0,0) (Table 4). If the actual exposed population around these sites is 25% less or 25% greater than our estimate, the resulting DALYs(3,1) would be 621,541 and 1,035,902, respectively. If the additional DW for lead-induced IQ loss not resulting in mental retardation is removed, our overall estimate would be 483,201 DALYs(3,1). In addition, if disease incidence is capped at 2.5% or 7.5%, the resulting DALYs(3,1) would be 730,627 and 922,479, respectively. The remediation scenario yielded 30,317 DALYs(3,1), in contrast with our primary estimate of 828,722 DALYs(3,1). Thus, our estimates suggest that 798,405 DALYs(3,1) could be eliminated by remediation of these sites to achieve international standards.
Table 4.
Sensitivity analysis estimates.
| Scenario | Total DALYs |
|---|---|
| Primary estimate of screened sites | 828,722 DALYs(3,1) |
| Estimate without age weights | 814,934 DALYs(3,0) |
| Estimate without age weights or discount rate | 1,557,121 DALYs(0,0) |
| Remediation scenario | 30,317 DALYs(3,1) |
| If actual exposed population is 25% less | 621,541 DALYs(3,1) |
| If actual exposed population is 25% greater | 1,035,902 DALYs(3,1) |
| If additional DW for lead-induced IQ loss not resulting in MMR is removed | 483,201 DALYs(3,1) |
| If incidence is capped at 2.5% | 730,627 DALYs(3,1) |
| If incidence is capped at 7.5% | 922,479 DALYs(3,1) |
| Estimate of unscreened sites | 3,500,000 DALYs(3,1) |
| Estimate of screened and unscreened sites | 4,328,722 DALYs(3,1) |
Discussion
We estimated that 8,629,750 persons were at risk of exposure to one of eight industrial pollutants at 373 toxic waste sites in three countries in 2010, resulting in 828,722 DALYs(3,1). This estimate represents a burden of disease equal to 0.22% of the total estimated DALYs(3,1) from all causes in India, Indonesia, and the Philippines (WHO 2008). Alteration of the discount rate and age weight leads to a range of estimates, from 814,934 DALYs(3,0) to 828,722 DALYs(3,1) to 1,557,121 DALYs(0,0). Lead and hexavalent chromium account for 99.2% of the total DALYs estimated for the 8 waste site chemical exposures evaluated. The additional DW for lead-induced IQ loss not resulting in mental retardation accounts for 483,201 DALYs(3,1), which represents approximately 58% of total DALYs(3,1). Inclusion of an estimated number of unscreened sites increased the estimated population at risk of exposure to 43,629,750, and the total DALYs(3,1) to 4,328,722. As part of a larger project attempting to calculate the burden of disease of toxic waste sites in LMICs, the present analysis indicates that the burden of disease associated with these sites is substantial and comparable to well-described diseases and environmental risk factors. For example, the WHO (2009) estimated that outdoor air pollution causes 1,448,612 DALYs(3,1) and malaria causes 725,000 DALYs(3,1) in these three countries. Overall, the present analysis begins to address the paucity of knowledge regarding health effects from toxic waste sites in LMICs and helps frame this issue in the context of other public health problems.
Given the limited scope of this project and the understanding that the screened sites represent only a portion of the total existing sites, we estimated that 5,000 unscreened sites exist in these three countries. The U.S. EPA (2004) estimates that there are approximately 294,000 contaminated sites in the United States alone that require some form of remediation. India’s population is nearly four times that of the United States, with nearly one third of Indian urban residents living in informal housing settlements, where unregulated cottage industries can proliferate without zoning or emissions controls (UN-HABITAT 2007).
Pollutants at toxic waste sites in LMICs can potentially have profound health effects. Lead and cadmium adversely affect neurodevelopment in children, with the in utero period being the life stage of greatest vulnerability (Ciesielski et al. 2012; Hu et al. 2006). Children and women of childbearing age constitute 65.3% of the total exposed population in this analysis, highlighting the potential impact on these vulnerable populations. The majority of the chemicals are nephrotoxic or hepatotoxic, and kidney and liver toxicity accounted for the majority of noncancer health effects. Several are known carcinogens, including asbestos, cadmium, and chromium.
Previous work has described the difficulty in identifying which toxic chemicals are being generated in India via industrial processes, as well as which ones are being imported for recycling or disposal (Dutta et al. 2006). The actual amount being produced and imported, and the ultimate fate of many of these chemicals, is unclear. Misra and Pandey (2005) discussed the complex requirements for proper handling of toxic waste to prevent human exposures and highlight the barriers to achieving this goal in countries such as India. Waste is often handled without adequate control mechanisms, such as proper infrastructure and personal protective equipment, in dense, highly populated areas, exposing not only workers but also residents in the surrounding communities.
Our estimates highlight the need for remediation of these sites, with a focus on addressing the key pollutant and dominant environmental medium. High-dose, mass poisonings periodically come to worldwide attention, such as recent events in Nigeria and Senegal, prompting immediate focus and remediation (Dooyema et al. 2012; Haefliger et al. 2009). However, exposures from most toxic waste sites continue unabated. Research has documented that waste site remediation can be cost-effective while reducing toxic exposures (Guerriero et al. 2011; Jones et al. 2011).
We must note several limitations of this analysis. We examined only eight chemicals and restricted the analysis to only one chemical per site. Persons living near toxic waste sites are often exposed to multiple chemicals simultaneously (DeRosa et al. 1996; Hu et al. 2007; Vrijheid 2000). Therefore, health effects may be increased or decreased due to the existence of coexposures and the potential for synergistic or antagonistic effects. For example, Claus Henn et al. (2011) documented a synergistic effect between lead and manganese in a Mexico City pregnancy cohort, with the impact of lead on child neurodevelopment increasing in the group with higher levels of manganese.
For most of the chemicals, we assigned only one cancer and one noncancer health effect. In addition, only a limited number of diseases have an associated DW, which prevented the inclusion of some health effects. For example, exposure to hexavalent chromium can cause nasal perforation. However, there is no DW for nasal perforation, so this health effect was not included in the analysis. In several cases there were no specific DWs that aligned properly with the projected health effect. Because there is no DW for liver toxicity, for example, we applied the DW for advanced hepatic disease to those chemicals known to cause liver toxicity. Although the major health effect of mercury is the impact of in utero methylmercury exposure on neurodevelopment, we were unable to capture this health effect for various reasons (e.g., limited methylmercury samples, no methylmercury biomonitoring). An additional source of uncertainty is the calculation of YLD for cancer, in which each cancer stage was assigned a different duration and DW.
Limited environmental sampling occurred at most sites, forcing us to extrapolate results of several samples to the entire population at risk. Biomarkers of exposure were not obtained, so we were unable to confirm completed pathways of exposure. In the case of lead, we attempted to offset this limitation by utilizing the U.S. EPA’s IEUBK model and ALM, which predict BLLs expected as a consequence of environmental lead exposure. However, these models may overestimate BLLs when predicted BLLs are > 30 µg/dL given the uncertainty in the relationship between environmental lead levels and BLLs at this level (Hogan et al. 1998). It is also likely that the actual exposures to the pollutants vary, with some individuals being exposed to lower levels. Despite evidence of prenatal exposure to environmental toxicants causing adverse health effects (Wigle et al. 2008), our analysis did not account for effects of prenatal exposures other than lead.
In addition, we assumed that exposures continued for a lifetime because there are no established waste site remediation programs in most LMICs. Although complete elimination of the toxic exposure may not be feasible for each site, a reduction in high-level exposure would decrease our disease burden estimates. Remediation of all sites such that pollutant concentrations are below international standards could save 798,405 DALYs(3,1). Finally, a key limitation of this analysis is its reliance on slope factors, reference doses, and reference concentrations, largely based on animal testing. These regulatory values may overestimate the disease burden given the limitations of animal testing and the assumptions required to extrapolate toxicity data from animals to humans (e.g., applying uncertainty factors). Acknowledging these limitations, we believe our analysis presents the best possible estimate of the burden of disease from these sites given current data.
Further research should better define the specific exposures occurring at toxic waste sites in LMICs by linking environmental sampling levels, biomarkers of disease, and health outcomes and focusing on uniquely vulnerable populations such as women who are pregnant, children, and the elderly. Such enhanced surveillance data will help provide context when comparing toxic waste sites with more recognized public health threats. This research should not preclude the immediate remediation of existing sites given the disease and resulting costs to society that result from such exposures. Given that the majority of the DALYs estimated for the eight chemicals evaluated were due to lead and chromium exposures, remediation could be facilitated by selectively targeting lead- and chromium-contaminated sites.
Conclusions
This study documents that chemical pollutants from toxic waste sites are a large and heretofore insufficiently studied public health problem in the three low- and middle-income Asian countries that we examined (India, Indonesia, and the Philippines). Disease and death caused by toxic chemicals contribute to the total burden of disease in these countries. We estimate that > 8 million persons in these countries suffered disease, disability, or death from exposures to industrial contaminants in 2010, resulting in 828,722 DALYs(3,1). These findings underscore the urgent need for toxic waste sites around the world to be characterized and remediated and for the health of affected populations to be monitored.
Acknowledgments
We thank A. Prüss-Üstün and P. Haefliger for reviewing early drafts of this manuscript.
Footnotes
This work was funded in part by the Asian Development Bank, the European Commission, the Green Cross Switzerland, the United Nations Industrial Development Organization, and the World Bank.
J.C., B.E., J.S.A., B.S., P.S., and R.F. are employed either part-time or full-time by the Blacksmith Institute, which is an international nonprofit organization dedicated to solving pollution problems in low- and middle-income countries. The Icahn School of Medicine at Mount Sinai receives funding from the Blacksmith Institute, some of which helps support K.C-S. P.J.L. is a member of the Board of Directors for the Blacksmith Institute.
References
- Avudainayagam S, Megharaj M, Owens G, Kookana RS, Chittleborough D, Naidu R. Chemistry of chromium in soils with emphasis on tannery waste sites. Rev Environ Contam Toxicol. 2003;178:53–91. doi: 10.1007/0-387-21728-2_3. [DOI] [PubMed] [Google Scholar]
- Batty GD, Deary IJ, Benzeval M, Der G. Does IQ predict cardiovascular disease mortality as strongly as established risk factors? Comparison of effect estimates using the West of Scotland Twenty-07 cohort study. Eur J Cardiovasc Prev Rehabil. 2010;17(1):24–7. doi: 10.1097/HJR.0b013e328321311b. [DOI] [PubMed] [Google Scholar]
- Blacksmith Institute. Maximum Recommended Levels. 2011. Available: http://www.blacksmithinstitute.org/files/FileUpload/files/Maximum%20Recommended%20Levels.pdf [accessed 17 December 2012]
- Blacksmith Institute. Investigator Handbook. 2013. Available: http://blacksmithinstitute.org/coordinator-resources.html [accessed 25 April 2013]
- Caravanos J, Chatham-Stephens K, Ericson B, Landrigan P, Fuller R. The burden of disease from pediatric lead exposure at hazardous waste sites in 7 Asian countries. Environ Res. 2012;120:119–125. doi: 10.1016/j.envres.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Ciesielski T, Weuve J, Bellinger DC, Schwartz J, Lanphear B, Wright RO. Cadmium exposure and neurodevelopmental outcomes in U.S. children. Environ Health Perspect. 2012;120:758–763. doi: 10.1289/ehp.1104152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernández-Avila M, et al. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ Health Perspect. 2011;120:126–131. doi: 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRosa CT, Johnson BL, Fay M, Hansen H, Mumtaz MM. Public health implications of hazardous waste sites: Findings, assessment and research. Food Chem Toxicol. 1996;34:1131–1138. doi: 10.1016/s0278-6915(97)00084-7. [DOI] [PubMed] [Google Scholar]
- Dooyema CA, Neri A, Lo Y-C, Durant J, Dargan PI, Swarthout T, et al. Outbreak of fatal childhood lead poisoning related to artisanal gold mining in northwestern Nigeria, 2010. Environ Health Perspect. 2012;120:601–607. doi: 10.1289/ehp.1103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta SK, Upadhyay VP, Sridharan U. Environmental management of industrial hazardous wastes in India. J Environ Sci Eng. 2006;48(2):143–150. [PubMed] [Google Scholar]
- Ericson B, Caravanos J, Chatham-Stephens K, Landrigan P, Fuller R.2012Approaches to systematic assessment of environmental exposures posed at hazardous waste sites in the developing world: the Toxic Sites Identification Program. Environ Monit Assess 18521755–1766.; 10.1007/s10661-012-2665-2[Online 17 May 2012] [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008: Estimated Cancer Incidence, Mortality, Prevalence and Disability-Adjusted Life Years (DALYs) Worldwide in 2008. 2010. Available: http://globocan.iarc.fr [accessed 7 June 2012]
- Fewtrell L, Kaufmann R, Prüss-Üstün A. Lead: Assessing the Environmental Burden of Disease at National and Local Levels. WHO Environmental Burden of Disease Series, No. 2. Geneva:World Health Organization. 2003. Available: http://www.who.int/quantifying_ehimpacts/publications/en/leadebd2.pdf [accessed 22 May 2013]
- Fewtrell LJ, Prüss-Üstün A, Landrigan P, Ayuso-Mateos JL. Estimating the global burden of disease of mild mental retardation and cardiovascular diseases from environmental lead exposure. Environ Res. 2004;94(2):120–133. doi: 10.1016/s0013-9351(03)00132-4. [DOI] [PubMed] [Google Scholar]
- Guerriero C, Bianchi F, Cairns J, Cori L.2011Policies to clean up toxic industrial contaminated sites of Gela and Priolo: a cost-benefit analysis. Environ Health 1068; 10.1186/1476-069X-10-68[Online 28 July 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger P, Mathieu-Nolf M, Lociciro S, Ndiaye C, Coly M, Diouf A, et al. Mass lead intoxication from informal used lead-acid battery recycling in Dakar, Senegal. Environ Health Perspect. 2009;117:1535–1540. doi: 10.1289/ehp.0900696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SG, Harper BL. Exposure Scenario For CTUIR Traditional Subsistence Lifeways. Pendleton, OR:Confederated Tribes of the Umatilla Indian Reservation. 2004. Available: http://health.oregonstate.edu/sites/default/files/research/pdf/tribal-grant/CTUIR-SCENARIO.pdf [accessed 22 May 2013]
- Hogan K, Marcus A, Smith R, White P. Integrated exposure uptake biokinetic model for lead in children: empirical comparisons with epidemiologic data. Environ Health Perspect. 1998;106(suppl 6):1557–1567. doi: 10.1289/ehp.98106s61557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shine J, Wright RO. The challenge posed to children’s health by mixtures of toxic waste: the Tar Creek superfund site as a case-study source. Pediatr Clin North Am. 2007;54(1):155–175. doi: 10.1016/j.pcl.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Téllez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, et al. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006;114:1730–1735. doi: 10.1289/ehp.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Diop A, Block M, Smith-Jones A, Smith-Jones A. Assessment and remediation of lead contamination in Senegal. J Health Pollution. 2011;1:16–25. [Google Scholar]
- Kumar AR, Riyazuddin P. Chromium speciation in groundwater of a tannery polluted area of Chennai City, India. Environ Monit Assess. 2010;160(1–4):579–591. doi: 10.1007/s10661-008-0720-9. [DOI] [PubMed] [Google Scholar]
- Lager A, Bremberg S, Vågerö D.2009The association of early IQ and education with mortality: 65 year longitudinal study in Malmö, Sweden. BMJ 10339b5282; doi.org/10.1136/bmj.b5282[Online 11 December 2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Salomon JA, Ezzati M, Begg S, Vander Hoorn S, Lopez AD. Sensitivity and uncertainty analyses for burden of disease and risk factors estimates. In: Global Burden of Disease and Risk Factors (Lopez AD, Mathers CD, Ezzati M, et al., eds). Washington, DC:World Bank, 399–426. 2006. Available: http://files.dcp2.org/pdf/GBD/GBD05.pdf [accessed 22 May 2013] [PubMed]
- Misra V, Pandey SD. Hazardous waste, impact on health and environment for development of better waste management strategies in future in India. Environ Int. 2005;31(3):417–431. doi: 10.1016/j.envint.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Prüss-Üstün A, Mathers C, Corvalán C, Woodward A. Introduction and Methods: Assessing the Environmental Burden of Disease at National and Local Levels. Geneva:World Health Organization (Prüss-Üstün A, Campbell-Lendrum D, Corvalán C, Woodward A, eds). WHO Environmental Burden of Disease Series, No. 1. Geneva:World Health Organization. 2003. Available: http://www.who.int/quantifying_ehimpacts/publications/en/9241546204.pdf [accessed 22 May 2013]
- Prüss-Üstün A, Vickers C, Haefliger P, Bertollini R.2011Knowns and unknowns on burden of disease due to chemicals: a systematic review. Environ Health 109; 10.1186/1476-069X-10-9[Online 21 January 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan R, Swaminathan R, Brenner H, Chen K, Chia KS, Chen JG, et al. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncol. 2010;11:165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- Sun LC, Meinhold CB. Gastrointestinal absorption of plutonium by the Marshall Islanders. Health Phys. 1997;73(1):167–175. doi: 10.1097/00004032-199707000-00013. [DOI] [PubMed] [Google Scholar]
- UN-HABITAT, Global Urban Observatory. Urban Info. 2007. Available: http://www.devinfo.info/urbaninfo/ [accessed 27 June 2012]
- U.S. Census Bureau. International Data Base. 2012. Available: http://www.census.gov/population/international/data/idb/informationGateway.php [accessed 31 May 2012]
- U.S. EPA (U.S. Environmental Protection Agency). Washington, DC: U.S. EPA; 1994. Guidance Manual for the IEUBK Model for Lead in Children. PB93-963510, OSWER 9285.7-15-1. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). New Report Projects Number, Cost and Nature of Contaminated Site Cleanups in the U.S. over Next 30 years. 2004. Available: http://www.epa.gov/superfund/accomp/news/30years.htm [accessed 31 May 2012]
- U.S. EPA (U.S. Environmental Protection Agency). Exposure Factors Handbook: 2011 Edition. Washington, DC:U.S. EPA. 2011. Available: http://www.epa.gov/ncea/efh/pdfs/efh-complete.pdf [accessed 21 May 2013]
- U.S. EPA (U.S. Environmental Protection Agency). Integrated Risk Information System (IRIS). 2012a. Available: http://www.epa.gov/ncea/iris/index.html [accessed 9 August 2012] [PubMed]
- U.S. EPA (U.S. Environmental Protection Agency). Introduction to the Hazard Ranking System (HRS). 2012b. Available: http://www.epa.gov/superfund/programs/npl_hrs/hrsint.htm [accessed 25 April 2013]
- U.S. EPA (U.S. Environmental Protection Agency). Regional Screening Levels for Chemical Contaminants at Superfund Sites. 2012c. Available: http://epa-prgs.ornl.gov/cgi-bin/chemicals/csl_search [accessed 9 August 2012]
- Vrijheid M. Health effects of residence near hazardous waste landfill sites: a review of epidemiologic literature. Environ Health Perspect. 2000;108(suppl 1):101–112. doi: 10.1289/ehp.00108s1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PD, Van Leeuwen P, Davis BD, Maddaloni M, Hogan KA, Marcus AH, et al. The conceptual structure of the integrated exposure uptake biokinetic model for lead in children. Environ Health Perspect. 1998;106(suppl 6):1513–1530. doi: 10.1289/ehp.98106s61513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). The Global Burden of Disease: 2004 Update. Geneva:WHO. 2008. Available: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf [accessed 21 May 2013]
- WHO (World Health Organization). Environmental Burden of Disease: Country Profiles. 2009. Available: http://www.who.int/quantifying_ehimpacts/national/countryprofile/intro/en/index.html [accessed 27 November 2012]
- WHO (World Health Organization). Global Burden of Disease (GBD). 2013. Available: http://www.who.int/healthinfo/global_burden_disease/en/ [accessed 25 April 2013]
- Wigle DT, Arbuckle TE, Turner MC, Bérubé A, Yang Q, Liu S, et al. Epidemiologic evidence of relationships between reproductive and child health outcomes and environmental chemical contaminants. J Toxicol Environ Health B Crit Rev. 2008;11(5–6):373–517. doi: 10.1080/10937400801921320. [DOI] [PubMed] [Google Scholar]
- Yáñez L, Ortiz D, Calderón J, Batres L, Carrizales L, Mejía J, et al. Overview of human health and chemical mixtures: problems facing developing countries. Environ Health Perspect. 2002;110:901–909. doi: 10.1289/ehp.110-1241270. [DOI] [PMC free article] [PubMed] [Google Scholar]


