Abstract
There have been considerable advances in the past few years in our understanding of how chronic kidney disease (CKD) predisposes to acute kidney injury (AKI) and vice versa. This review shows, however, that few studies have focused on the elderly or conducted stratified analysis by age. It does appear that elderly patients with estimated glomerular filtration rate (eGFR) 45–59 ml/min/1.73 m2 are at higher risk for AKI compared with their counterparts with eGFR >60 ml/min/1.73 m2. This is a similar relationship to that seen in younger patients, although effect size appears smaller. As the incidence of AKI has been increasing over the past several years, the proportion of elderly patients surviving after AKI has also been increasing. Since AKI heightens the risk for the development and acceleration of CKD, this implies significant public health concerns with regard to the absolute number of elderly persons developing incident CKD.
Key Words: Acute kidney injury, Aging, Chronic kidney disease, Glomerular filtration rate
Introduction
Acute kidney injury (AKI) is particularly common among the elderly [1]. Pre-existing chronic kidney disease (CKD) is arguably the strongest risk factor for AKI [2]. Here, we review the recent literature on the relationship between AKI and CKD in elderly patients.
CKD and Predisposition to AKI
There has been considerable debate recently about the definition of ‘chronic kidney disease’ in elderly patients [see Winearls and Glassock, pp. c2–c4 this issue]. Doubts have been raised whether an estimated glomerular filtration rate (eGFR) cutoff of 60 ml/min/1.73 m2 is appropriate to define CKD in all segments of the population, especially among the elderly, since there is a natural age-associated decline in renal function [3]. To inform this debate, one major thrust of active research in the CKD field is to better define the association between levels of eGFR and clinical outcomes [4]. To address the issue of CKD classification in the elderly, numerous papers have examined outcomes such as cardiovascular disease events (e.g. myocardial infarction or congestive heart failure) and mortality in age-stratified analyses [5,6,7].
However, until recently, no published study examined AKI as an outcome, even though a strong argument can be made that AKI compared to cardiovascular disease is more directly linked pathophysiologically to CKD. Therefore, any observed association between CKD and AKI is less likely to be due to confounding than analogous associations between CKD and cardiovascular disease [8].
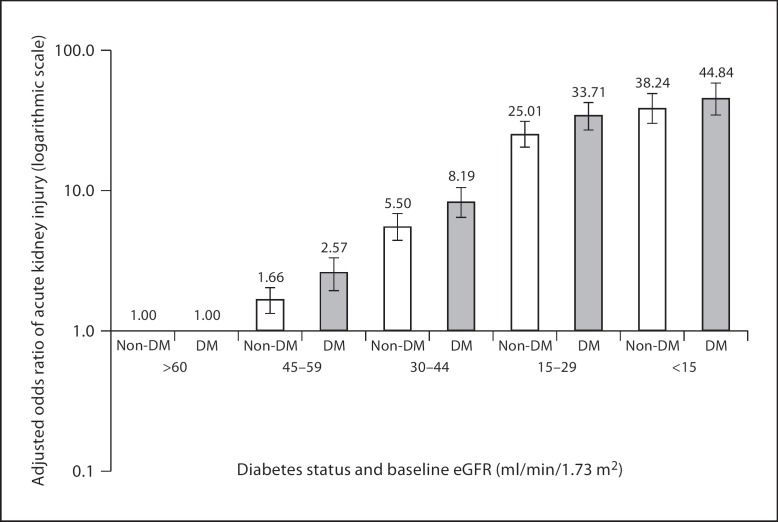
A 2008 paper by Hsu et al. [2] quantified how risk of dialysis-requiring AKI varied by severity of preexisting CKD among a large cohort of patients receiving usual medical care in a Northern California integrated health care delivery system. That study found that an increase in risk of AKI becomes apparent starting below an eGFR of 60 ml/min/1.73 m2. Even subjects with an eGFR 45–59 ml/min/1.73 m2 have on average a two-fold increase in adjusted odds ratio of AKI compared with subjects with an eGFR of 60 ml/min/1.73 m2 or above – with risk being higher among subjects with diabetes than among those without (fig. 1). These data support the National Kidney Foundation Chronic Kidney Disease Guidelines in which persons with eGFRs chronically below 60 ml/min/1.73 m2 are classified as having CKD, regardless of other factors. Further age stratification analyses showed that the adjusted odds ratio was 2.73 (95% CI 2.12–3.51; p < 0.0001) for those aged ≤65 years, comparing patients with eGFR 45–59 ml/min/1.73 m2 with their counterparts with eGFR ≥60 ml/min/1.73 m2; for patients aged >65 years, the corresponding adjusted odds ratio was 1.33 (95% CI 1.08–1.64; p = 0.008) [unpubl. data].
Fig. 1.
Multivariable association of baseline eGFR and dialysis-requiring acute kidney injury stratified by the presence or absence of diabetes mellitus (DM) [modified from ref. [2]].
A recent publication by Grams et al. [9] using data from the Atherosclerosis Risk in Communities (ARIC) Study obtained similar results. For example, the adjusted risk of AKI approximately doubled going from an eGFR of 60 to 45 ml/min/1.73 m2. Interestingly, Grams et al., who used the CKD-EPI equation [10] to explore higher eGFR levels, reported that compared with those with eGFR 75 ml/min/1.73 m2, relative hazards for AKI was nearly doubled in ARIC participants with eGFR of 60 ml/min/1.73 m2. This is a stronger and earlier signal than that seen in numerous studies of eGFR and death or cardiovascular disease – where in some instances risk may not rise appreciably until eGFR is below 45 ml/min/1.73 m2[11,12,13]. This argues that the NKF threshold of an eGFR of 60 ml/min/1.73 m2 may actually be too conservative in some clinical settings such as AKI. No age-stratified eGFR analysis was presented by Grams et al. [9].
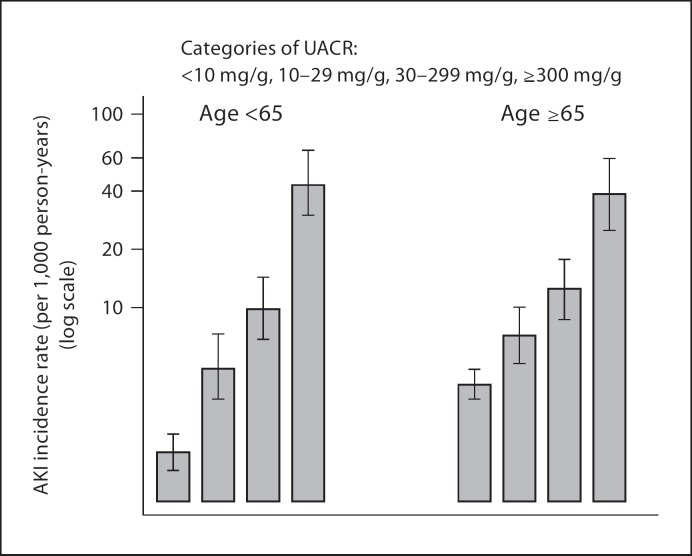
In addition to a low eGFR, the other main manifestation of CKD is proteinuria and the importance of proteinuria in the classification of CKD has received much attention recently. Hsu et al. [2] first reported that proteinuria is an important independent risk factor for AKI. Patients with documented dipstick proteinuria appear to be 2–3 times as likely as patients without it to develop AKI, independent of eGFR. Grams et al. [9] further quantified a graded relationship between severity of proteinuria (albuminuria) and risk of AKI. With participants who had urine albumin-to-creatinine ratios <10 mg/g as a reference, the adjusted hazards ratios of AKI were 1.9, 2.2, and 4.8 for urine albumin-to-creatinine ratio groups of 11–29, 30–299 and ≥300 mg/g, respectively. Age-stratified analysis showed that the associations between crude rates of AKI and severity of baseline albuminuria were similar in those above or below age 65 years (fig. 2).
Fig. 2.
Incidence rates of acute kidney injury by level of baseline albuminuria, stratified by age above or below 65 years [modified from ref. [9]]. UACR = Urine albumin-to-creatinine ratio.
Several other recent studies have also concluded that proteinuria is a risk factor for AKI in the setting of cardiac catheterization [14], cardiac surgery [15] and in the general population [16]. But no comparisons of older and younger patients were reported.
AKI and Predisposition to CKD
A plethora of data over the past several years, both from experimental studies in animals and from human studies, indicates that AKI not infrequently leads to CKD.
In experimental animals, a robust fibrotic response in the kidney is apparent several days to weeks after a single episode of AKI induced by ischemia-reperfusion injury [17,18]. This fibrotic response is enhanced by older age [19]. The primary mediator of this relationship is multifactorial, implicating microvascular damage [17], increased sensitivity to angiotensin II [20] and upregulation of genes associated with inflammation, remodeling and fibrosis [21,22]. In aged mice, expression of zinc-α2-glycoprotein may influence the exaggerated fibrotic response [19]. A significant manifestation of the post-AKI injury phenotype is salt-sensitive hypertension [23,24].
The preponderance of evidence from epidemiologic studies supports the notion that AKI leads to CKD in elderly persons. First, older age is associated with a greater chance of nonrecovery of renal function back to baseline after AKI by the time of hospital discharge [25]. Second, several studies have demonstrated that even after adjustment for several important covariates, AKI is independently associated with an increased risk for both CKD and end-stage renal disease (ESRD) (table 1). In elderly patients, the risk for ESRD after a single episode of AKI is elevated 2-fold in those with mild AKI [26], and elevated by 3- to 13-fold in those with more severe AKI [16,26,27,28]. The annual absolute risk for developing ESRD is approximately 0.6–1.2% after mild AKI [14,26], but 1.7–2.9% after severe AKI [14,26,27,28]. The annualized risk of ESRD increases to 7–9% if the AKI occurs in an individual with a preexisting history of CKD [16,27,28]. The relative risk for ESRD may [28] or may not [27] be higher in those of older age (vs. younger age) after AKI. If the latter is true (lower relative risk of ESRD with older age vs. younger age), the effect is likely confounded by the competing risk of death.
Table 1.
Adjusted rate ratios for ESRD or doubling of serum creatinine by baseline kidney function and proteinuria
| Study | Setting | Patients n | AKI definition | ESRD |
Comments | |
|---|---|---|---|---|---|---|
| incidence rate person-years | adjusted HR | |||||
| Amdur et al. [29] | hospitalized veterans | 113,272 | ICD-9 codes | mean ages by group, years | ||
| ATN | 20.0%a | 6.64a | 63.8 | |||
| ARF | 13.2%a | 4.03a | 66.5 | |||
| controls (no AKI) | 3.3%a | 1.0a | 68.7 | |||
| CKD without AKI | 24.7%a | 6.5a | 74.4 | |||
| No age-specific analyses reported | ||||||
| Hsu et al. [34] | hospitalized with preexisting CKD | 39,805 | acute dialysis | 12.7% at 6 months | 1.47 (0.95–2.28) | mean age 66.6 years in those with AKI no age-specific analyses reported |
| Ishani et al. [30] | Medicare | 233,803 | ICD-9 based: | incidence rate; adjusted HR for ESRD by age strata | ||
| AKI | 27.5/1,000 | 13.0 (11.0–16.0) | (total population; not by AKI/no AKI) | |||
| AKI on CKD | 101.5/1,000 | 41.2 (34.6–49.1) | age, years: | |||
| 67–70: 8/1,000; 1.0 | ||||||
| 71–75: 6.9/1,000; 0.87 (0.74–1.02) | ||||||
| 76–80: 5.7/1,000; 0.72 (0.61−0.85) | ||||||
| 81–85: 4.3/1,000; 0.63 (0.52−0.76) | ||||||
| ≥86: 1.9/1,000; 0.36 (0.28−0.46) | ||||||
| Lo et al. [30] | population-based cohort | 3,773 | in-hospital dialysis vs. matched non-AKI | 479/1,000b 17/1,000b | 28.1 (21.1–37.6)b | mean age of AKI 63.5 |
| no age-specific analyses reported | ||||||
| Newsome et al. [26] | Medicare-Acute MI | 87,094 | change in SCr: | entire cohort was aged ≥67 | ||
| None | 2.3/1,000 | 1.0 | no age-specific analyses reported | |||
| Cr ↑ 0.1 | 2.3/1,000 | 1.45 | ||||
| Cr ↑ 0.2 | 3.6/1000 | 1.97 | ||||
| Cr ↑ 0.3–0.5 | 6.3/1,000 | 2.36 | ||||
| Cr ↑ 0.6–3.0 | 20.0/1,000 | 3.26 | ||||
| Wald et al. [28] | population-based cohort | 17,367 | in-hospital dialysis vs. matched non-AKI | 26/1,000 | 3.23 (2.7–3.86) | absolute risk and excess risk for ESRD after AKI higher in patients aged ≥65 (AKI 9.5% vs. non-AKI 2.8%) compared to those aged <65 (7.4 vs. 3.2%) |
| 9/1,000 | ||||||
| James et al. [16] | coronary angiography | 11,249 | no AKI | 3.7%c | 1.0 | mean age 67 in those with AKI |
| mild AKI | 9.4%c | 1.60 (1.19–2.14) | no age-specific analyses reported | |||
| moderate/severe AKI | 21.8%c | 3.12 (1.95–4.99) | ||||
| James et al. [14] | population-based cohort | 920,985 | AKI (ICD-9 and −10) vs. no AKI | 3.5/1,000d | 21–230, depending on baseline GFR and degree of proteinuria | no age-specific analyses reported |
| 0.78/1,000d | ||||||
Figures shown in parentheses are 95% CI. ATN = Acute tubular necrosis; ARF = acute renal failure.
Cumulative incidence at 20% percentile follow-up of 10.9 months in ATN and 57 months in ARF.
Endpoint was CKD stage 4 or higher.
Cumulative incidence with median follow-up of 21 months for composite endpoint of decline in eGFR >4 ml/min/1.73 m2 or ESRD.
Endpoint was ESRD or doubling of serum creatinine (SCr).
ESRD only represents the most severe manifestation of CKD. Less severe stages of CKD are still associated with a markedly increased risk of cardiovascular disease, poorer quality of life and increased health care costs [11]. The incidence rate of CKD (stage 4 or worse) is approximately 120 per 1,000 person-years after non-dialysis-requiring AKI [29] and 479 per 1,000 person-years in those who required dialysis for AKI [30]. These absolute incidence rates are commensurate with adjusted hazard ratios of at least 4 for non-dialysis-requiring AKI and 28 for dialysis-requiring AKI (compared to no AKI, respectively) [29,30].
In a recent study examining the rate of eGFR decline in patients undergoing cardiac catheterization, James et al. [14] demonstrated that the rate of decline in eGFR was 1.0 ml/min/1.73 m2 per year after mild AKI and 2.8 ml/min/1.73 m2 per year after moderate or severe AKI (compared with 0.1 ml/min/1.73 m2 per year in those without AKI). Although these rates were adjusted for age (along with proteinuria and comorbidities), it is unclear whether older age was associated with a more rapid decline of eGFR, as would be hypothesized based on data from experimental animals. Factors that clearly modify the effects of the relationship between AKI and progressive CKD are level of baseline renal function and degree of proteinuria [16]. The risk for progressive CKD attributable to AKI attenuates with lower levels of baseline eGFR and higher levels of proteinuria.
With regard to public health, approximately 40 million people in the US are aged ≥65 in 2010. Since the incidence of AKI in this elderly population is approximately 3,000 per 100,000 person-years [1] and 75% will survive to discharge after AKI, and the incidence of stage 4 or worse CKD after AKI is approximately 120 per 1,000 person-years [estimated from Kaplan-Meier curve in figure 3 of reference 29] then approximately 100,000 elderly persons per year in the United States are developing new CKD after an episode of AKI. Clearly, the challenges for the nephrology community are to find strategies to either prevent AKI or prevent the transition from AKI to CKD. Until these strategies are developed and proven to be effective, CKD and ESRD after AKI in elderly patients represent a substantial public health burden.
Conclusion
In summary, elderly patients with eGFR 45–59 ml/min/1.73 m2 are at higher risk for AKI compared with their counterparts with eGFR >60 ml/min/1.73 m2. This is a similar relationship to that seen in younger patients, although effect size appears smaller. As the incidence of AKI has been increasing over the past several years [1], the proportion of elderly patients surviving after AKI has also been increasing [31,32,33]. Since AKI heightens the risk for the development and acceleration of CKD, this implies significant public health concerns with regard to the absolute number of elderly persons developing incident CKD.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgements
S.C. and C.-y.H. are members of the ASsess, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) consortium and have been supported by the NIH (U01 DK82223, K23DK080132). S.C. has also been supported by the Hartford Foundation Center of Excellence in Aging at Yale Subspecialty Scholar Award, and by the American Society of Nephrology-ASP Junior Development Award in Geriatric Nephrology.
References
- 1.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM, Go AS. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winearls CG, Glassock RJ. Dissecting and refining the staging of chronic kidney disease. Kidney Int. 2009;75:1009–1014. doi: 10.1038/ki.2009.49. [DOI] [PubMed] [Google Scholar]
- 4.K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 5.O’Hare AM, Bertenthal D, Walter LC, Garg AX, Covinsky K, Kaufman JS, Rodriguez RA, Allon M. When to refer patients with chronic kidney disease for vascular access surgery: should age be a consideration? Kidney Int. 2007;71:555–561. doi: 10.1038/sj.ki.5002078. [DOI] [PubMed] [Google Scholar]
- 6.O’Hare AM, Choi AI, Bertenthal D, Bacchetti P, Garg AX, Kaufman JS, Walter LC, Mehta KM, Steinman MA, Allon M, McClellan WM, Landefeld CS. Age affects outcomes in chronic kidney disease. J Am Soc Nephrol. 2007;18:2758–2765. doi: 10.1681/ASN.2007040422. [DOI] [PubMed] [Google Scholar]
- 7.Chronic Kidney Disease Prognosis Consortium Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho K, Hsu CY. Quantifying severity of chronic kidney disease as a risk factor for acute kidney injury. J Am Soc Nephrol. 2010;21:1602–1604. doi: 10.1681/ASN.2010080816. [DOI] [PubMed] [Google Scholar]
- 9.Grams ME, Astor BC, Bash LD, Matsushita K, Wang Y, Coresh J. Albuminuria and estimated glomerular filtration rate independently associate with acute kidney injury. J Am Soc Nephrol. 2010;21:1757–1764. doi: 10.1681/ASN.2010010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 12.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462293 adults in Taiwan. Lancet. 2008;371:2173–2182. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 13.Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M for the Alberta Kidney Disease N Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 14.James MT, Ghali WA, Tonelli M, Faris P, Knudtson ML, Pannu N, Klarenbach SW, Manns BJ, Hemmelgarn BR. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78:803–809. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
- 15.Huang TM, Wu VC, Young GH, Lin YF, Shiao CC, Wu PC, Li WY, Yu HY, Hu FC, Lin JW, Chen YS, Lin YH, Wang SS, Hsu RB, Chang FC, Chou NK, Chu TS, Yeh YC, Tsai PR, Huang JW, Lin SL, Chen YM, Ko WJ, Wu KD. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol 2010. [DOI] [PMC free article] [PubMed]
- 16.James MT, Hemmelgarn BR, Wiebe N, Pannu N, Manns BJ, Klarenbach SW, Tonelli M. Glomerular filtration rate, proteinuria, and the incidence and consequences of acute kidney injury: a cohort study. Lancet. 2010;376:2096–2103. doi: 10.1016/S0140-6736(10)61271-8. [DOI] [PubMed] [Google Scholar]
- 17.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–F899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 18.Ko GJ, Grigoryev DN, Linfert D, Jang HR, Watkins T, Cheadle C, Racusen L, Rabb H. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol. 2010;298:F1472–F1483. doi: 10.1152/ajprenal.00619.2009. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt R, Marlier A, Cantley LG. Zag expression during aging suppresses proliferation after kidney injury. J Am Soc Nephrol. 2008;19:2375–2383. doi: 10.1681/ASN.2008010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips SA, Pechman KR, Leonard EC, Friedrich JL, Bian JT, Beal AG, Basile DP. Increased ANG II sensitivity following recovery from acute kidney injury: role of oxidant stress in skeletal muscle resistance arteries. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1682–R1691. doi: 10.1152/ajpregu.00448.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basile DP, Fredrich K, Alausa M, Vio CP, Liang M, Rieder MR, Greene AS, Cowley AW., Jr Identification of persistently altered gene expression in the kidney after functional recovery from ischemic acute renal failure. Am J Physiol Renal Physiol. 2005;288:F953–F963. doi: 10.1152/ajprenal.00329.2004. [DOI] [PubMed] [Google Scholar]
- 22.Grigoryev DN, Liu M, Hassoun HT, Cheadle C, Barnes KC, Rabb H. The local and systemic inflammatory transcriptome after acute kidney injury. J Am Soc Nephrol. 2008;19:547–558. doi: 10.1681/ASN.2007040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pechman KR, De Miguel C, Lund H, Leonard EC, Basile DP, Mattson DL. Recovery from renal ischemia-reperfusion injury is associated with altered renal hemodynamics, blunted pressure natriuresis, and sodium-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1358–R1363. doi: 10.1152/ajpregu.91022.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP. Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol. 2007;293:F269–F278. doi: 10.1152/ajprenal.00279.2006. [DOI] [PubMed] [Google Scholar]
- 25.Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis. 2008;52:262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, Allison JJ. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med. 2008;168:609–616. doi: 10.1001/archinte.168.6.609. [DOI] [PubMed] [Google Scholar]
- 27.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 29.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in US veterans: focus on acute tubular necrosis. Kidney Int. 2009;76:1089–1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 30.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 32.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 33.Bagshaw SM, George C, Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11:R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS. Non-recovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4:891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]