Abstract
The development of effective malaria vaccines and immune biomarkers of malaria is a high priority for malaria control and elimination. Ags expressed by merozoites of Plasmodium falciparum are likely to be important targets of human immunity and are promising vaccine candidates, but very few Ags have been studied. We developed an approach to assess Ab responses to a comprehensive repertoire of merozoite proteins and investigate whether they are targets of protective Abs. We expressed 91 recombinant proteins, located on the merozoite surface or within invasion organelles, and screened them for quality and reactivity to human Abs. Subsequently, Abs to 46 proteins were studied in a longitudinal cohort of 206 Papua New Guinean children to define Ab acquisition and associations with protective immunity. Ab responses were higher among older children and those with active parasitemia. High-level Ab responses to rhoptry and microneme proteins that function in erythrocyte invasion were identified as being most strongly associated with protective immunity compared with other Ags. Additionally, Abs to new or understudied Ags were more strongly associated with protection than were Abs to current vaccine candidates that have progressed to phase 1 or 2 vaccine trials. Combinations of Ab responses were identified that were more strongly associated with protective immunity than responses to their single-Ag components. This study identifies Ags that are likely to be key targets of protective human immunity and facilitates the prioritization of Ags for further evaluation as vaccine candidates and/or for use as biomarkers of immunity in malaria surveillance and control.
Introduction
Malaria caused by Plasmodium falciparum remains a leading cause of global morbidity and mortality (1). The development of effective malaria vaccines is a high priority for malaria control and elimination, particularly in light of increasing drug resistance (2, 3), as well as the declining efficacy of vector control interventions in some populations that is compromising current control efforts (4). Effective immunity develops naturally in humans following exposure to P. falciparum infection, which has long provided a strong rationale that the development of malaria vaccines is achievable and highlights the importance of understanding the targets and mechanisms of immunity (5). Therefore, an important criterion for objectively identifying and prioritizing Ags for malaria vaccine development is the demonstration that a specific Ag is a target of acquired human immunity and that the immune response is associated with protection from symptomatic disease (6). Abs are important in protecting individuals from high parasitemias and clinical malaria, as demonstrated by passive-transfer studies in humans (7).
Acquired human immunity predominantly targets the blood stage of infection, and Ags expressed by the merozoite, the extracellular form of Plasmodium that infects erythrocytes, are especially important immune targets and vaccine candidates (5). Erythrocyte invasion occurs over several steps, with multiple interactions involving proteins on the merozoite surface and proteins contained within dedicated invasion organelles, the micronemes and rhoptries (8). These proteins are thought to represent the major protective Ab targets and most attractive merozoite vaccine candidates because of their exposure to host immune responses and their important roles in invasion. Abs to merozoite Ags may act by directly inhibiting parasite replication, through blocking binding of merozoite ligands to their receptor or binding partner, or by inhibiting processing that may be required for function (9–12). Abs can also act by Ab-dependent cellular inhibition involving monocytes and opsonization of merozoites for phagocytosis and killing by monocytes, macrophages, and neutrophils (13, 14).
Of the many known or predicted merozoite proteins, very few have been assessed as targets of human immunity (6), and few studies have examined Abs to multiple Ags concurrently in the same populations (15–21), as highlighted by the findings of a recent systematic review of longitudinal studies of human immunity (6). Detailed studies of human immune responses have only been performed for a small number of Ags (6), including MSP1, MSP2, MSP3, GLURP, AMA1, and EBA175. All of these progressed to phase 1 or 2 clinical trials, with mixed results; generally, phase 2 trials showed limited efficacy (5, 6). Additionally, there are no established correlates of protective human immunity or biomarkers of immunity that would be of great value in malaria surveillance and control. Recent advances in genomics and proteomics and methods for protein expression have facilitated the identification and expression of a greater number of Ags that are potential targets of immunity or vaccine candidates.
We sought to develop a comprehensive and rational approach to identify and prioritize an extensive list of known or predicted merozoite Ags as targets of human immunity to advance vaccine development and the identification of immune biomarkers for malaria surveillance. We expressed and screened 91 recombinant proteins corresponding to proteins located on the merozoite surface or the invasion organelles, which are likely to represent the major targets of protective Abs. Forty-six of these were assessed for Ab responses in a prospective study of 206 Papua New Guinean (PNG) children to identify proteins that are naturally immunogenic, to understand the acquisition of Ab responses, and to identify IgG responses most strongly associated with protection from malaria. The potential additive effects of combined responses to multiple Ags and the relationship between Ab levels and protective associations were also investigated.
Materials and Methods
Details of the study population and ethics approval
Plasma samples were obtained from a prospective treatment-reinfection cohort study of 206 school-aged children (5–14 y) conducted in Madang Province, PNG (22). The study commenced at the end of the high-transmission season in June 2004. An enrollment blood sample was obtained and was used for determining the Ab responses described in this article. All children were then treated with 7 d of artesunate (4 mg/kg/d) monotherapy to clear parasitemia, in accordance with the guidelines at that time. Treatment failures were differentiated from reinfection by msp2 genotyping. Participants were actively followed up with clinical assessment and finger-prick blood sampling every 2 wk at their school for 6 mo. Reinfection was determined using these finger-prick samples by light microscopy (LM) and post-PCR ligase detection reaction-florescent microsphere assay. PCR-based detection had a much higher sensitivity for detecting parasitemia compared with LM; 67.5% (n = 139) of children were PCR positive and 34.5% (n = 71) were LM positive for P. falciparum at enrollment (22). Consequently, PCR detection of parasitemia was used to classify children as infected or uninfected. A clinical episode of malaria was defined as a febrile episode (>37.5°C) with P. falciparum density > 5000/μl. Further details of the cohort are described elsewhere (22). Samples were also obtained from anonymous Australian residents (n = 9) as malaria-naive controls. Samples from malaria-exposed PNG adults were used as positive controls and to standardize interplate variation for each Ag.
Ethics approval for this study was obtained from the Medical Research Advisory Committee, Department of Health, PNG; the Alfred Hospital Ethics Committee (for Burnet Institute); Walter and Eliza Hall Institute of Medical Research (Melbourne); and the Veterans Affairs Medical Center (Cleveland, OH). Informed consent was obtained in writing from parents or guardians of all participants prior to enrollment.
Expression of rAgs
We aimed to include all merozoite proteins that have been established as important Ags, have a role in erythrocyte invasion, and/or have been localized on the merozoite surface or in the invasion organelles of the merozoite (Table I, Supplemental Table I). Ags were expressed or synthesized in different collaborating laboratories using a variety of expression methods, predominantly Escherichia coli and a wheat germ cell–free expression system (WGCF) (23, 24); a small number of Ags was expressed in yeast or generated as synthetic peptides (Table I). Purification tags included either 6-His tags or GST. All Ags were assessed for quality and purity by SDS-PAGE and Western blot (reduced and nonreduced). Ags were screened for immunoreactivity in ELISA assays using pools of serum from malaria-exposed PNG adults compared with malaria-naive Australian residents. Immobilization of Ags was assessed using anti-his Ab, where appropriate. Immunoreactivity by human serum Abs to the purification tags was assessed and found to be negligible. In addition to merozoite Ags, reactivity to a synthetic peptide (NANP6 repeat) of the circumsporozoite protein (CSP) was assessed as a marker of exposure to pre-erythrocytic Ags.
Table I. rAgs of P. falciparum merozoites included in the study.
| Ag | Gene ID | Region | Amino Acid Residues | Expression System | Outcome or Plasma Dilution |
|---|---|---|---|---|---|
| MSPs (GPI anchored) | |||||
| MSP1-19a | PFI1475w | MSP1-19 | 1597–1704 | E. coli | 1/1000 |
| MSP1-42a | PFI1475w | MSP1-42 | 1362–1720 | E. coli | 1/1000 |
| MSP2a | PFB0300c | Full length | 19–249 | E. coli | 1/1000 |
| MSP4a | PFB0310c | Full length | 21–248 | E. coli | 1/1000 |
| MSP10a | PFF0995c | Full ectodomain | 29–506 | WGCF | 1/1000 |
| Pf12a | PFF0615c | Full ectodomain | 25–321 | E. coli | 1/1000 |
| Pf12p | PFF0620c | Full ectodomain | 21–352 | E. coli | Poor coating |
| Pf38a | PFE0395c | Full ectodomain | 22–327 | E. coli | 1/1000 |
| Pf92 | PF13-0338 | Full ectodomain | 28–772 | WGCF | Poor coating |
| MSPs (non-GPI anchored) | |||||
| MSP3a | PF10-0345 | Full ectodomain | 27–354 | WGCF | 1/1000 |
| MSP3a | PF10-0345 | Long synthetic peptide | 154–249 | Peptide | 1/250 |
| MSP6a | PF10-0346 | Full ectodomain | 23–371 | WGCF | 1/1000 |
| MSP7a | PF13-0197 | Full ectodomain | 24–351 | WGCF | 1/1000 |
| MSRP1a | PF13-0196 | Full ectodomain | 23–380 | WGCF | 1/500 |
| MSRP2 | MAL13P1.174 | Full ectodomain | 23–281 | WGCF | Poor immunoreactivity |
| ABRA/MSP9a | PFK1385c | Full ectodomain | 25–743 | WGCF | 1/250 |
| H101 | PF10-0347 | Full ectodomain | 25–424 | WGCF | Poor immunoreactivity |
| H103/MSP11a | PF10-0352 | Full ectodomain | 26–405 | WGCF | 1/250 |
| GLURPa | PF10-0344 | R2 peptide | 887–905 | Peptide | 1/1000 |
| S-Ag | PF10-0343 | Full ectodomain | 25–585 | WGCF | Poor coating |
| S-Ag | PF10-0343 | Repeat sequence | 136–151 | Peptide | Poor coating |
| SERA4 | PFB0345c | Papain domain | 376–962 | WGCF | Poor expression |
| SERA5a | PFB0340c | Central domain | 391–828 | E. coli | 1/500 |
| SERA5 | PFB0340c | Papain domain | 513–822 | WGCF | Poor expression |
| Pf41a | PFD0240c | Full ectodomain | 21–378 | E. coli | 1/1000 |
| MSPDBL1a,b | PF10-0348 | Full ectodomain | 26–697 | WGCF | 1/500 |
| MSPDBL1 (PfMSPDBL1)b | PF10-0348 | DBL domain | 243–443 | E. coli | Poor coating |
| MSPDBL2a,c | PF10-0355 | Full ectodomain | 30–762 | WGCF | 1/500 |
| MSP3.4 | PF10-0350 | Full ectodomain | 23–712 | WGCF | Poor immunoreactivity |
| ROM4 | PFE0340c | Full ectodomain | 17–759 | WGCF | Poor immunoreactivity |
| Microneme Ags | |||||
| AMA1a | PF11-0344 | Full ectodomain | 26–546 | WGCF | 1/1000 |
| EBA140RIIa | MAL13P1.60 | Region II | 146-713 | Pichia | 1/500 |
| EBA140RIII-Va | MAL13P1.60 | Region III–V | 746–1045 | E. coli | 1/500 |
| EBA175F2a | PF07-0128 | F2 | 447–795 | E. coli | 1/500 |
| EBA175RIIa | PF07-0128 | Region II | 145–760 | Pichia | 1/1000 |
| EBA175RIII-Va | PF07-0128 | Region III–V | 761–1271 | E. coli | 1/500 |
| EBA181RII | PFA0125c | Region II | 122–704 | WGCF | Poor expression |
| EBA181RIII-Va | PFA0125c | Region III–V | 755–1339 | E. coli | 1/500 |
| MTRAP | PF10-0281 | Full ectodomain | 24–432 | WGCF | Poor immunoreactivity |
| ASP | PFD0295c | Full ectodomain | 22–711 | WGCF | Poor coating |
| GAMAa | PF08-0008 | Full ectodomain | 25–714 | WGCF | 1/250 |
| GAMAa | PF08-0008 | N-terminal | 25–337 | WGCF | 1/500 |
| GAMAa | PF08-0008 | C-terminal | 500–714 | WGCF | 1/500 |
| Ripra | PFC1045c | EGF-like domain | 279–995 | WGCF | 1/500 |
| Ripr | PFC1045c | N-terminal | 238–368 | WGCF | Poor expression |
| Ripr | PFC1045c | C-terminal | 791–900 | WGCF | Poor expression |
| SUB2a | PF11-0381 | N-terminal | 19–681 | WGCF | 1/500 |
| SUB2 | PF11-0381 | C-terminal | 625–1136 | WGCF | Poor coating |
| Rhoptry proteins | |||||
| RAMAa | MAL7P1.208 | Full ectodomain | 18–786 | WGCF | 1/250 |
| PfRh1 | PFD0110w | N-terminal | 500–833 | WGCF | Poor expression |
| PfRh2-297a | PF13-0198 | PfRh2-297 | 297–726 | E. coli | 1/500 |
| PfRh2-2030a | PF13-0198 | PfRh2-2030 | 2030–2528 | E. coli | 1/500 |
| PfRh2aa | PF13-0198 | PfRh2a-2874 | 2874–3060 | E. coli | 1/500 |
| PfRh2ba | PF13-0198 | PfRh2b-2792 | 2792–3185 | E. coli | 1/500 |
| PfRh4.1 | PFD1150c | PfRh4.1 | 607–773 | E. coli | Poor expression |
| PfRh4.2a | PFD1150c | PfRh4.2 | 1277–1451 | E. coli | 1/1000 |
| PfRh4.4 | PFD1150c | PfRh4.4 | 1445–1619 | E. coli | Poor expression |
| PfRh4.9a | PFD1150c | PfRh4.9 | 28–340 | E. coli | 1/500 |
| PfRh5a | PFD1145c | Full ectodomain | 25–526 | WGCF | 1/500 |
| RALP-1a | MAL7P1.119 | Full ectodomain | 21–749 | WGCF | 1/500 |
| Rhop148 | PF13-0348 | N-terminal | 1–694 | WGCF | Poor expression |
| Rhop148 | PF13-0348 | C-terminal | 569–1262 | WGCF | Poor expression |
| RhopH1(9) | PFI1730w | N-terminal | 267–850 | WGCF | Poor expression |
| RhopH1(9) | PFI1730w | C-terminal | 752–1340 | WGCF | Poor expression |
| RhopH1(3.1)a | PFC0120w | N-terminal | 25–721 | WGCF | 1/500 |
| RhopH1(3.1) | PFC0120w | C-terminal | 722–1417 | WGCF | Poor coating |
| RhopH1(3.2) | PFC0110w | N-terminal | 25–722 | WGCF | Poor immunoreactivity |
| RhopH1(3.2) | PFC0110w | C-terminal | 723–1416 | WGCF | Poor expression |
| RhopH1(2)a | PFB0935w | N-terminal | 25–732 | WGCF | 1/500 |
| RhopH1(2) | PFB0935w | C-terminal | 733–1440 | WGCF | Poor coating |
| RhopH2 | PFI1445w | N-terminal | 20–693 | WGCF | Poor coating |
| RhopH2 | PFI1445w | C-terminal | 94–1378 | WGCF | Poor coating |
| RhopH3 | PFI0265c | C-terminal | 56–897 | WGCF | Poor coating |
| RON2a | PF14-0495 | N-terminal | 84–968 | WGCF | 1/500 |
| RON2 | PF14-0495 | C-terminal | 1786–2071 | WGCF | Poor coating |
| RON3 | PFL2505c | N-terminal | 574–1394 | WGCF | Poor expression |
| RON3 | PFL2505c | C-terminal | 1395–2215 | WGCF | Poor expression |
| RON4a | PF11-0168 | N-terminal | 25–709 | WGCF | 1/500 |
| RON4 | PF11-0168 | C-terminal | 710–1201 | WGCF | Poor expression |
| RON6a | PFB0680w | C-terminal | 124–950 | WGCF | 1/500 |
| SPATR | PFB0570w | Full ectodomain | 23–250 | WGCF | Poor coating |
| Pf34 | PFD0955w | Full ectodomain | 26–299 | WGCF | Poor immunoreactivity |
| Likely apical proteins without clearly defined localization | |||||
| Pf113a | PF14-0201 | C-terminal | 97–948 | WGCF | 1/500 |
| HYP | PF10-0119 | Full ectodomain | 22–267 | WGCF | Poor immunoreactivity |
| HYP | PFL0300c | Full ectodomain | 24–304 | WGCF | Poor immunoreactivity |
| HYP | Pf10-0166 | Full ectodomain | 26–310 | WGCF | Poor coating |
| HYP | PF14-0119 | Full ectodomain | 22–320 | WGCF | Poor immunoreactivity |
| PTRAMP | PFL0870w | Full ectodomain | 26–309 | WGCF | Poor immunoreactivity |
| HYP | PFD1130w | Full ectodomain | 27–362 | WGCF | Poor immunoreactivity |
| HYP | PFB0475c | Full ectodomain | 33–446 | WGCF | Poor immunoreactivity |
| Protease | PFD0230c | Papain domain | 116–939 | WGCF | Poor expression |
| Nonmerozoite Ags | |||||
| CSPa | PFC0210c | Repeat region | (NANP)6 | Peptide | 1/125 |
Bioinformatics approaches were used to identify merozoite Ags that were potential targets of protective Ab responses. All Ags listed demonstrated peak expression during late trophozoite/schizont stage, and all were predicted to have a signal peptide (except Rhop148). Selection was also made on the likely accessibility to Ab recognition (i.e., merozoite surface or invasion organelles). Ags predicted to have a GPI anchor (as listed) are likely to be localized to the merozoite surface. The localization of some Ags is still not well established. Known localization for all Ags was assessed using the ApiLoc database and used to categorize the Ags in the respective areas in the table. Homology to known merozoite Ags was also assessed using the PlasmoDB database. Ag expression was in E. coli, P. pastoris, or the WGCF system. Peptides were synthesized commercially.
These Ags were tested in the prospective cohort of PNG children at the plasma dilution indicated. Ags were not tested in the cohort if they were poorly expressed or yielded poor results in screening ELISAs (poor Ag coating or poor immunoreactivity when tested with malaria-exposed adult controls).
This protein has recently been renamed DBLMSP.
This protein has recently been renamed MSP3.8.
S-Ag repeat sequence peptide: KVSNGGEDEVSNGRED.
HYP, Hypothetical protein.
The quality of protein expression and folding had been validated previously for some of the protein constructs used by demonstrating that vaccine-induced Abs raised against the recombinant protein recognize native protein (25–37); vaccine-induced Abs have functional antiparasite activity in growth-inhibition assays (GIAs) or Ab-dependent cellular-inhibition (ADCI) assays (12, 28, 30, 33–43); or the recombinant protein has appropriate biological function by binding its receptor (25, 28, 34, 35, 38, 39) (Supplemental Table I). Validation of WGCF as an appropriate system for expression of P. falciparum merozoite proteins has also been established in several ways. For example, recombinant proteins representing PfRh5, GAMA, and EBA175 bound the surface of the erythrocyte, consistent with their proposed function in invasion and suggesting that they were folded correctly (44–46). Abs generated by immunization to several Ags showed relevant functional activity in GIAs (e.g., AMA1, EBA175, and GAMA) (33, 44–46). As described below, many of the WGCF-expressed proteins showed strong reactivity with Abs from malaria-exposed donors, but not with malaria-naive donors, and Abs to many Ags were strongly associated with protection from malaria, further suggesting that the proteins were correctly folded or representative of native proteins.
Measurement of IgG responses by ELISA
Each Ag was individually optimized for coating concentration, and the plasma concentration was selected on the basis that it reflected reactivity on the linear aspect of the Ab-response gradient (47). It was necessary to vary the plasma dilutions used to test each Ag to ensure a good spread of data points, and these ranged from 1/250 to 1/1000; AMA1 was the only exception and tested at 1/4000 (Table I). All Ags were optimally coated, diluted in PBS, onto Maxisorp microtiter plates (Nunc) at saturating concentrations, which were typically 0.5–1.0 μg/ml. Diluted serum samples were incubated for 2 h. Secondary Ab was polyclonal sheep anti-human IgG HRP Ab used at 1/2500 (Chemicon). Color was developed with an ABTS liquid substrate system (Sigma) and stopped after 15 min with 1% NaDodSO4 (SDS). OD was measured at 405 nm using a GENios microplate reader (Tecan). Sera from nine Australian blood donors were used as negative controls, and sera from three malaria-exposed PNG adults were used as positive controls. All wash steps were done in PBS/0.05% Tween 20. All blocking and Ab dilutions were in 5% skim milk/PBS/0.05% Tween 20, with incubations at room temperature. All samples were tested in duplicate and were repeated if there was a discrepancy >25% between duplicates. Background absorbance was determined using PBS controls on each plate and was deducted from all other values. Variability across plates was standardized using a titration of the positive controls. Poor plate coating of Ags was defined by lack of reactivity using serum Abs of malaria-exposed donors and Abs to the recombinant protein tag (e.g., His-tag).
Statistical analysis
Ab responses for the cohort were not distributed normally, and it was not possible to transform all of the data; therefore, nonparametric methods of analysis were used. The association between categorical variables was assessed using χ2 tests, and differences in median Ab responses between groups were assessed using Wilcoxon rank-sum or Kruskal–Wallis tests, as appropriate. Seropositivity was calculated as the mean of malaria-naive controls plus 3 SD for each response.
Survival analysis used Ab responses categorized into three equal groups (low, medium, and high tertiles). Tertile responses were calculated using the “xtile” command within STATA to create new tertile variables, based on OD values after adjustment for plate-to-plate variation. This method of categorization overcame the finding of high seroprevalence for most responses, and it also allowed a dose-response relationship to be examined while maintaining sufficient numbers of participants in each group for statistical power. The Cox proportional-hazards model was used to calculate hazard ratios (HRs) for time to first symptomatic malaria episode (defined as fever and P. falciparum parasitemia > 5000/μl) between Ab-responder groups. Treatment failures (n = 12) were excluded from the survival analysis (22). Assumptions of proportional hazards were assessed using the Schoenfeld residuals test and visual inspection of data using log-log plots. In most cases, Ab variables showed nonproportional hazards over the follow-up time; therefore, an interaction term between the Ab variable and time (three categories: t = 0–100, t = 101–150, and t >150 d) was included in the analysis. Although some children had multiple episodes of parasitemia or symptomatic malaria, the analysis presented examined the time to first symptomatic episode only. This is consistent with previous analytic approaches used in this cohort (48–51). A range of demographic, clinical, and biological variables was assessed as potential confounders of associations between Abs and malaria outcomes. Only host age (≤9 y or >9 y) and location of residence (defined as distance from the sea: <1 km or >1 km) were identified as being significantly associated with Abs and malaria outcomes (22, 50). Therefore, all Cox-regression analyses were performed unadjusted and with adjustments for these previously defined covariates in this study cohort (22, 50). Previous analyses in this cohort showed that concurrent parasitemia and erythrocyte polymorphisms are not significant confounders (48, 52). Regression analysis investigating multiple Ab responses was limited by their highly correlated nature. Interaction terms were also investigated between Ab tertile categories but, again, the highly correlated nature of the responses meant that some cells had so few children as to make them uninterpretable. Reported p values were not adjusted for multiple comparisons (53); rather, the interpretation of associations is based on the level of significance and the direction and magnitude of the association between Ab responses and prospective risk for malaria.
To assess the effect of Ab combinations, a summation of the quartile responses from each Ag-specific response was used. The quartile responses for each Ag were designated as 0, 1, 2, or 3, representing low to high IgG responses. These quartile categories were then added together for each combination (i.e., a combination of two different responses yields a score between 0 and 6). These additive combination scores were then used to create three equal groups reflecting low, intermediate, and high combination responses. These tertile combination responses were used in the Cox-regression analysis, as described above. All statistical analyses were performed using STATA 9.2 (StataCorp) or Prism 5.01 (GraphPad) software. Ab responses to MSP1–19, MSP2, AMA1, EBAs, PfRh2, and PfRh4 in this population were reported previously (48–51), and the data were reanalyzed for comparison with other Ags and in combination analyses.
Results
Selection and screening of merozoite Ags
The aim of this study was to assess a comprehensive repertoire of merozoite proteins that may be plausible targets of protective Ab responses in humans, focusing on Ags that are known or proposed to be located on the merozoite surface or located within the invasion organelles. We compiled a list of all established merozoite Ags (Table I, Supplemental Table I), and bioinformatics approaches were used to identify other potential merozoite Ags from the P. falciparum genome on the basis of expression profile, signal peptides, putative GPI anchor, and homology to other known merozoite Ags. The likely localization of these Ags was checked in the ApiLoc database (54) to determine whether they were located on the merozoite surface or in the apical organelles. The rationale was that these Ags might be accessible to Abs and, therefore, could be targets for naturally acquired or vaccine-induced protective Abs that inhibit merozoite invasion or by promoting opsonization of merozoites for phagocytosis and killing by monocytes, macrophages, and neutrophils. All known and candidate proteins that have been localized to the merozoite surface, rhoptries, or micronemes were expressed as recombinant proteins (n = 91; Fig. 1A). For some Ags, protein expression and purification in E. coli or yeast had already been developed (Supplemental Table I), and these proteins were used if available (E. coli, n = 21; Pichia pastoris, n = 2). For the remainder, expression was attempted using WGCF (n = 65). Recombinant expression of GLURP and S-Ag was not successful, and synthetic peptides were generated. The long synthetic peptide of MSP3 was also included, because it was previously included in multiple cohort studies and has progressed to clinical trials (55). Abs to CSP peptide (NANP6) were also assessed as a marker of responses to sporozoite Ags as a comparison with merozoite Ags.
FIGURE 1.
Flow chart of screening and evaluation of merozoite Ags. (A) Merozoite Ags were identified by late schizont–stage transcription, appropriate signal sequences, putative GPI anchor, or homology to other known merozoite Ags. These were expressed as recombinant proteins if known or reported to have localization to the merozoite surface or apical organelles. Expressed proteins were then purified and screened for quality by SDS-PAGE and Western blots. Coating efficiency and immunoreactivity to naturally acquired human responses were assessed by ELISA. Ags of sufficient quality, coating efficiency, and immunoreactivity were then tested in a cohort of 206 PNG children. (B) The localization of merozoite Ags tested in the cohort. Forty-six recombinant proteins were tested, and these represented 36 distinct merozoite proteins (i.e., some were subregions of the same merozoite protein). Details on the recombinant proteins that were tested in the study are provided in Table I.
The quality of these 91 proteins was assessed by SDS-PAGE and Western blotting, which excluded 16 proteins of poor quality (Fig. 1A). The remaining 75 Ags were screened for Ab reactivity against a panel of sera from malaria-exposed residents of PNG and nonexposed residents of Australia. An additional 16 proteins were excluded because of poor ELISA plate coating, and 13 Ags were excluded because of poor immunoreactivity (protein detected in plate wells but very little or no reactivity with Abs from malaria-exposed donors). The remaining 46 proteins were used to study Ab responses in a longitudinal cohort of PNG children (Fig. 1A). The expression system for these 46 proteins included E. coli (n = 18), WGCF (n = 24), yeast (n = 2), and peptides (n = 2; not including CSP peptide). These 46 recombinant proteins represented 36 merozoite proteins (i.e., some recombinant proteins represented multiple regions of the same merozoite Ag; Fig. 1B). For some Ags it was possible to assess protein quality by additional methods (see Materials and Methods). Expression of proteins in E. coli or P. pastoris was validated previously for several Ags, and validation of WGCF as an appropriate system for expression of P. falciparum merozoite proteins was also established previously (Supplemental Table I). Additionally, we compared the reactivity of human Abs between WGCF-expressed proteins and well-characterized proteins that were expressed in established bacterial- or yeast-expression systems; this demonstrated a high degree of correlation in the reactivity among individuals between proteins expressed in different systems (e.g., AMA1: r = +0.921, p < 0.0001; MSP2: r = +0.944, p < 0.0001).
Acquisition of Abs to merozoite Ags and their association with age and concurrent parasitemia
To identify Ags that are targets of naturally acquired Abs, we determined whether Abs to each of the 46 proteins were specific to individuals exposed to P. falciparum infection and examined the association of Abs with age and active P. falciparum infection, which may reflect boosting of responses. Median IgG responses for the cohort were significantly higher than for malaria-naive individuals for all Ags when tested at the same dilution, reflecting the specificity of P. falciparum Ab responses (data not shown). IgG seropositivity to most Ags was high: almost all were >75% (Table II). Notable exceptions included Ripr (44.8%) and PfRh5 (53.2%). The median seroprevalence of responses appeared to differ according to the localization of the Ags; merozoite surface proteins (MSPs) typically had higher seropositivity than did micronemal and rhoptry Ags (89.8, 87.2, and 84.1%, respectively; p = 0.057). There was no significant difference between the median seroprevalence of GPI- and non–GPI-anchored MSPs (90.6 and 89.8%, respectively; p = 0.801).
Table II. Associations among Ab prevalence, age, and parasitemia.
| Ag | Region | No. Positive (%) | Age ≤9 y (no. [%]) | Age >9 y (no. [%]) | p Value | PCR−(no. [%]) | PCR+ (no. [%]) | p Value |
|---|---|---|---|---|---|---|---|---|
| Surface proteins (GPI anchored) | ||||||||
| MSP1 | MSP1–19 | 199 (96.6) | 85 (93.4) | 114 (99.1) | 0.024 | 62 (92.5) | 137 (98.6) | 0.025 |
| MSP1 | MSP1–42 | 191 (92.7) | 80 (87.9) | 111 (96.5) | 0.018 | 57 (85.1) | 134 (96.4) | 0.003 |
| MSP2 | Full length | 182 (88.4) | 74 (81.3) | 108 (93.9) | 0.005 | 51 (76.1) | 131 (94.2) | <0.001 |
| MSP4 | Full length | 194 (94.2) | 80 (87.9) | 114 (99.1) | 0.001 | 57 (85.1) | 137 (98.6) | <0.001 |
| MSP10 | Full ectodomain | 178 (86.4) | 71 (78.0) | 107 (93.0) | 0.002 | 50 (74.6) | 128 (92.1) | 0.001 |
| Pf12 | Full ectodomain | 196 (95.2) | 81 (89.0) | 115 (100.0) | <0.001 | 59 (88.1) | 137 (98.6) | 0.001 |
| Pf38 | Full ectodomain | 182 (88.4) | 77 (84.6) | 105 (91.3) | 0.137 | 53 (79.1) | 129 (92.8) | 0.004 |
| Surface proteins (non-GPI anchored) | ||||||||
| MSP3 | Full ectodomain | 180 (87.4) | 70 (76.9) | 110 (95.7) | <0.001 | 50 (74.6) | 130 (93.5) | <0.001 |
| MSP3 | LSP | 135 (65.5) | 48 (52.8) | 87 (75.7) | 0.001 | 34 (50.8) | 101 (72.7) | 0.002 |
| MSP6 | Full ectodomain | 182 (88.4) | 76 (83.5) | 106 (92.2) | 0.054 | 50 (74.6) | 132 (95.0) | <0.001 |
| MSP7 | Full ectodomain | 199 (96.6) | 85 (93.4) | 114 (99.1) | 0.024 | 63 (94.0) | 136 (97.8) | 0.157 |
| MSRP1 | Full ectodomain | 175 (85.0) | 68 (74.7) | 107 (93.0) | <0.001 | 50 (74.6) | 125 (89.9) | 0.004 |
| ABRA/MSP9 | Full ectodomain | 185 (89.8) | 75 (82.4) | 110 (95.7) | 0.002 | 53 (79.1) | 132 (95.0) | <0.001 |
| H103/MSP11 | Full ectodomain | 195 (94.7) | 82 (90.1) | 113 (98.3) | 0.010 | 58 (86.6) | 137 (98.6) | <0.001 |
| GLURP | R2 peptide | 185 (89.8) | 74 (81.3) | 111 (96.5) | <0.001 | 55 (82.1) | 130 (93.5) | 0.011 |
| SERA5 | Full ectodomain | 199 (96.6) | 85 (93.4) | 114 (99.1) | 0.024 | 61 (91.0) | 138 (99.8) | 0.002 |
| Pf41 | Full ectodomain | 183 (88.8) | 73 (80.2) | 110 (95.7) | <0.001 | 51 (76.1) | 132 (95.0) | <0.001 |
| MSPDBL1 | DBL domain | 193 (93.7) | 81 (89.0) | 112 (97.4) | 0.014 | 58 (86.6) | 135 (97.1) | 0.004 |
| MSPDBL2 | Full ectodomain | 186 (90.3) | 75 (82.4) | 111 (96.5) | 0.001 | 53 (79.1) | 133 (95.7) | <0.001 |
| Micronemal proteins | ||||||||
| AMA1 | Full ectodomain | 195 (94.7) | 81 (89.0) | 114 (99.1) | 0.001 | 58 (86.6) | 137 (98.6) | <0.001 |
| EBA140 | Region II | 176 (85.4) | 74 (81.3) | 102 (88.7) | 0.136 | 51 (76.1) | 125 (89.9) | 0.009 |
| EBA140 | Region III–V | 165 (80.1) | 66 (72.5) | 99 (86.1) | 0.016 | 47 (70.2) | 118 (84.9) | 0.013 |
| EBA175 | F2 | 186 (90.3) | 75 (82.4) | 111 (96.5) | 0.001 | 56 (83.6) | 130 (93.5) | 0.024 |
| EBA175 | Region II | 161a (79.3) | 64 (70.3) | 97 (86.6) | 0.004 | 46 (69.7) | 115 (83.9) | 0.019 |
| EBA175 | Region III–V | 184 (89.3) | 75 (82.4) | 109 (94.8) | 0.004 | 50 (74.6) | 134 (96.4) | <0.001 |
| EBA181 | Region III–V | 183 (88.8) | 73 (80.2) | 110 (95.7) | <0.001 | 56 (83.6) | 127 (91.4) | 0.097 |
| GAMA | Full ectodomain | 180 (87.4) | 74 (81.3) | 106 (92.2) | 0.020 | 51 (76.1) | 129 (92.8) | 0.001 |
| GAMA | N-terminal | 169a (83.3) | 71 (78.0) | 98 (87.5) | 0.072 | 48 (72.7) | 121 (88.3) | 0.005 |
| GAMA | C-terminal | 56a (27.6) | 26 (28.6) | 30 (26.8) | 0.777 | 11 (16.7) | 45 (32.9) | 0.016 |
| Ripr | EGF-like domain | 91a (44.8) | 35 (38.5) | 56 (50.0) | 0.100 | 26 (39.4) | 65 (47.5) | 0.280 |
| SUB2 | N-terminal | 177a (87.2) | 71 (78.02) | 106 (94.6) | <0.001 | 50 (75.8) | 127 (92.7) | 0.001 |
| Rhoptry proteins | ||||||||
| RAMA | Full ectodomain | 177 (85.9) | 72 (79.1) | 105 (91.3) | 0.013 | 48 (71.6) | 129 (92.8) | <0.001 |
| PfRh2 | PfRh2-297 | 161 (78.2) | 67 (73.6) | 94 (81.7) | 0.162 | 45 (67.2) | 116 (83.5) | 0.008 |
| PfRh2 | PfRh2-2030 | 194 (94.2) | 80 (87.9) | 114 (99.1) | 0.001 | 59 (88.1) | 135 (97.2) | 0.009 |
| PfRh2 | PfRh2a | 191 (94.1) | 82 (91.1) | 109 (96.5) | 0.108 | 58 (87.9) | 133 (97.1) | 0.009 |
| PfRh2 | PfRh2b | 175 (85.0) | 72 (79.1) | 103 (89.6) | 0.037 | 47 (70.1) | 128 (92.1) | <0.001 |
| PfRh4 | PfRh4.2 | 195 (94.7) | 82 (90.1) | 113 (98.3) | 0.010 | 60 (89.6) | 135 (97.1) | 0.024 |
| PfRh4 | PfRh4.9 | 193 (93.7) | 81 (89.0) | 112 (97.4) | 0.014 | 57 (85.1) | 136 (97.8) | <0.001 |
| PfRh5 | Full ectodomain | 108a (53.2) | 35 (38.5) | 73 (65.2) | <0.001 | 28 (42.4) | 80 (58.4) | 0.033 |
| RALP-1 | Full ectodomain | 189a (93.1) | 81 (89.0) | 108 (96.4) | 0.038 | 56 (84.9) | 133 (97.1) | 0.001 |
| RhopH1(3.1) | N-terminal | 120a (59.1) | 38 (41.8) | 82 (73.2) | <0.001 | 32 (48.5) | 88 (64.2) | 0.033 |
| RhopH1(2) | N-terminal | 154a (75.9) | 60 (65.9) | 94 (83.9) | 0.003 | 45 (68.2) | 109 (79.6) | 0.076 |
| RON2 | N-terminal | 167a (82.3) | 62 (68.1) | 105 (93.8) | <0.001 | 41 (62.1) | 126 (92.0) | <0.001 |
| RON4 | N-terminal | 191a (94.1) | 81 (89.0) | 110 (98.2) | 0.006 | 56 (84.9) | 135 (98.5) | <0.001 |
| RON6 | C-terminal | 164a (79.6) | 67 (73.6) | 97 (86.6) | 0.020 | 41 (62.1) | 123 (89.8) | <0.001 |
| Likely apical proteins | ||||||||
| Pf113 | C-terminal | 178a (87.7) | 74 (81.3) | 104 (92.9) | 0.013 | 51 (77.3) | 127 (92.7) | 0.002 |
| CSP | NANP repeat | 106 (51.5) | 46 (50.6) | 60 (52.2) | 0.817 | 31 (46.3) | 75 (54.0) | 0.301 |
The total prevalence of IgG responses to each Ag tested in a cohort of PNG children. The effects of age (≤9 or >9 y of age, n = 91 and n = 115, respectively) and concurrent parasitemia (determined by PCR, n = 67 and n = 139, respectively) on IgG prevalence are also examined. The total number of study participants was n = 206, unless indicated otherwise.
χ2 tests were used to determine statistical significance (p ≤ 0.05 shown in bold type).
n = 203.
The acquisition of Ab responses in malaria-endemic regions generally increases with age, largely reflecting a cumulative increase in exposure to blood-stage parasitemia over time. Consistent with this, the seroprevalence of Abs was higher in older children compared with younger children for the majority of Ags (statistically significant for 38 of 46 proteins [82.6%]) (Table II); for those Ag-specific responses that did not reach statistical significance, there was a clear trend toward a difference between age groups. The same was found for Ab levels, with significantly higher median levels among older children compared with younger children for most Ags (examples shown in Fig. 2A).
FIGURE 2.
Examples of the associations between Ab levels and age or concurrent parasitemia. Selected examples of the relationship between Ab levels (relative OD) to specific Ags and age (children ≤9 y or >9 y of age) (A) or concurrent parasitemia (PCR negative, PCR positive) (B). Bar graphs show the relative OD and interquartile range for IgG responses against merozoite Ags MSPDBL1, ABRA/MSP9, Ripr, PfRh5, RON2 (left to right). Relative OD was calculated as proportion of absolute OD compared with the median OD of the cohort. Mann–Whitney U test was used to assess the difference in median values (p < 0.05 in all cases comparing younger versus older children or PCR-negative versus PCR-positive children). The associations between Ab prevalence and age or concurrent parasitemia are shown for all Ags in Table II.
The potential boosting effect of concurrent parasitemia at the time of sample collection on IgG responses was explored. Seroprevalence was higher in PCR-positive individuals compared with PCR-negative individuals for 42 of 46 (91.3%) merozoite Ags (Table II). Median Ab levels were also higher among children with concurrent parasitemia compared with aparasitemic children for the majority of Ags (examples shown in Fig. 2B). In contrast, responses to CSP peptide did not show any statistically significant differences with regard to age (p = 0.817) or P. falciparum infection status (p = 0.301).
Association between Abs to merozoite Ags and reduced risk for malaria
The relationship between Ag-specific IgG responses and prospective risk for episodes of symptomatic malaria was examined in a longitudinal cohort of 206 PNG children for all 46 Ags. Following the initial clearance of parasitemia at enrollment among children in the cohort, high P. falciparum reinfection rates were observed in the subsequent 6 mo of follow-up by active and passive case detection; 95.3% had reinfection detected by PCR, whereas 87.6% had it detected by LM (22). In 6 mo of follow-up, 38.8% of the cohort had a symptomatic episode of malaria (defined as fever and P. falciparum parasitemia > 5000/μl). Individuals were classified into three equal groups (tertiles), reflecting high, intermediate, or low IgG responses for each Ag. The risk for malaria was compared between children with high IgG responses and children with low IgG responses for each Ag; analysis examining the intermediate tertile response group is described later in relation to Ab dose-response associations with protection. For 36 of 46 merozoite Ags tested (78.3%), children with high Ab levels had a reduced risk for malaria (unadjusted HR [uHR] < 1 by Cox-regression analysis) that was statistically significant compared with those with low levels (Table III). Adjusting the Cox-regression models for the predefined confounders age and location (22) had little impact on the magnitude of protective associations (Table III), and protective associations calculated from unadjusted and adjusted models were highly correlated (data not shown).
Table III. Association between Abs and protection from symptomatic malaria.
| Ag | Region | Tertile | uHR (95% CI) | p Value | Adjusted HR (95% CI) | p Value |
|---|---|---|---|---|---|---|
| Surface proteins (GPI-anchored) | ||||||
| MSP1 | MSP1–19 | MvL | 0.53 (0.23–1.21) | 0.132 | 0.66 (0.28–1.54) | 0.334 |
| HvL | 0.42 (0.17–1.03) | 0.059 | 0.57 (0.22–1.44) | 0.235 | ||
| MSP1 | MSP1–42 | MvL | 0.39 (0.15–1.01) | 0.052 | 0.47 (0.18–1.21) | 0.116 |
| HvL | 0.59 (0.26–1.35) | 0.211 | 0.80 (0.34–1.85) | 0.596 | ||
| MSP2 | Full length | MvL | 0.69 (0.31–1.55) | 0.369 | 0.87 (0.38–2.01) | 0.747 |
| HvL | 0.42 (0.16–1.09) | 0.073 | 0.56 (0.21–1.49) | 0.246 | ||
| MSP4 | Full length | MvL | 0.29 (0.11–0.82) | 0.019 | 0.36 (0.13–1.01) | 0.053 |
| HvL | 0.68 (0.31–1.49) | 0.330 | 0.92 (0.40–2.08) | 0.833 | ||
| MSP10 | Full ectodomain | MvL | 0.26 (0.10–0.69) | 0.007 | 0.31 (0.11–0.83) | 0.020 |
| HvL | 0.38 (0.16–0.92) | 0.031 | 0.42 (0.17–1.02) | 0.055 | ||
| Pf12 | Full ectodomain | MvL | 0.53 (0.22–1.27) | 0.155 | 0.60 (0.25–1.44) | 0.251 |
| HvL | 0.58 (0.24–1.39) | 0.223 | 0.70 (0.29–1.68) | 0.422 | ||
| Pf38 | Full ectodomain | MvL | 0.34 (0.14–0.82) | 0.016 | 0.36 (0.15–0.88) | 0.024 |
| HvL | 0.31 (0.12–0.78) | 0.013 | 0.34 (0.13–0.87) | 0.024 | ||
| Surface proteins (not-GPI anchored) | ||||||
| MSP3 | Full ectodomain | MvL | 0.20 (0.07–0.59) | 0.004 | 0.26 (0.09–0.77) | 0.015 |
| HvL | 0.44 (0.19–1.01) | 0.052 | 0.58 (0.24–1.36) | 0.209 | ||
| MSP3 | Long synthetic peptide | MvL | 0.38 (0.16–0.90) | 0.028 | 0.44 (0.18–1.06) | 0.066 |
| HvL | 0.27 (0.10–0.72) | 0.009 | 0.36 (0.13–1.00) | 0.049 | ||
| MSP6 | Full ectodomain | MvL | 0.62 (0.28–1.36) | 0.230 | 0.75 (0.34–1.69) | 0.491 |
| HvL | 0.23 (0.08–0.70) | 0.009 | 0.32 (0.10–0.98) | 0.046 | ||
| MSP7 | Full ectodomain | MvL | 0.32 (0.12–0.88) | 0.027 | 0.39 (0.14–1.08) | 0.071 |
| HvL | 0.66 (0.30–1.46) | 0.304 | 0.82 (0.36–1.85) | 0.635 | ||
| MSRP1 | Full ectodomain | MvL | 0.19 (0.07–0.57) | 0.003 | 0.23 (0.08–0.69) | 0.009 |
| HvL | 0.35 (0.15–0.83) | 0.017 | 0.44 (0.18–1.06) | 0.066 | ||
| ABRA/MSP9 | Full ectodomain | MvL | 0.36 (0.15–0.86) | 0.022 | 0.41 (0.17–1.00) | 0.049 |
| HvL | 0.32 (0.13–0.81) | 0.016 | 0.42 (0.16–1.10) | 0.077 | ||
| H103MSP11 | Full ectodomain | MvL | 0.49 (0.21–1.14) | 0.098 | 0.55 (0.23–1.29) | 0.167 |
| HvL | 0.36 (0.14–0.92) | 0.032 | 0.46 (0.18–1.18) | 0.107 | ||
| GLURP | R2 peptide | MvL | 0.65 (0.28–1.51) | 0.320 | 0.81 (0.35–1.89) | 0.625 |
| HvL | 0.49 (0.20–1.22) | 0.127 | 0.65 (0.26–1.64) | 0.363 | ||
| SERA5 | Full ectodomain | MvL | 0.25 (0.10–0.63) | 0.003 | 0.31 (0.12–0.78) | 0.013 |
| HvL | 0.27 (0.11–0.68) | 0.006 | 0.33 (0.13–0.83) | 0.018 | ||
| Pf41 | Full ectodomain | MvL | 0.27 (0.11–0.69) | 0.006 | 0.31 (0.12–0.80) | 0.016 |
| HvL | 0.35 (0.14–0.83) | 0.018 | 0.39 (0.16–0.95) | 0.039 | ||
| MSPDBL1 | DBL domain | MvL | 0.67 (0.31–1.45) | 0.314 | 0.78 (0.36–1.7) | 0.532 |
| HvL | 0.18 (0.05–0.61) | 0.006 | 0.23 (0.07–0.80) | 0.021 | ||
| MSPDBL2 | Full ectodomain | MvL | 0.34 (0.13–0.86) | 0.023 | 0.41 (0.16–1.06) | 0.067 |
| HvL | 0.39 (0.16–0.94) | 0.036 | 0.55 (0.22–1.37) | 0.200 | ||
| Microneme proteins | ||||||
| AMA1 | Full ectodomain | MvL | 0.31 (0.12–0.77) | 0.012 | 0.40 (0.16–1.02) | 0.055 |
| HvL | 0.32 (0.13–0.80) | 0.015 | 0.36 (0.14–0.91) | 0.031 | ||
| EBA140 | Region II | MvL | 0.59 (0.27–1.31) | 0.196 | 0.61 (0.28–1.35) | 0.225 |
| HvL | 0.24 (0.08–0.71) | 0.010 | 0.28 (0.09–0.83) | 0.022 | ||
| EBA140 | Region III–V | MvL | 0.35 (0.15–0.83) | 0.017 | 0.40 (0.17–0.95) | 0.039 |
| HvL | 0.20 (0.07–0.58) | 0.003 | 0.25 (0.09–0.76) | 0.014 | ||
| EBA175 | Region F2 | MvL | 0.59 (0.26–1.29) | 0.182 | 0.64 (0.28–1.41) | 0.268 |
| HvL | 0.24 (0.08–0.73) | 0.011 | 0.51 (0.11–0.98) | 0.046 | ||
| EBA175 | Region II | MvL | 0.27 (0.10–0.72) | 0.009 | 0.30 (0.11–0.81) | 0.018 |
| HvL | 0.46 (0.20–1.06) | 0.069 | 0.61 (0.26–1.44) | 0.263 | ||
| EBA175 | Region III–V | MvL | 0.39 (0.17–0.90) | 0.028 | 0.50 (0.21–1.16) | 0.106 |
| HvL | 0.21 (0.07–0.61) | 0.004 | 0.27 (0.09–0.81) | 0.019 | ||
| EBA181 | Region III–V | MvL | 0.37 (0.15–0.88) | 0.025 | 0.42 (0.17–1.01) | 0.052 |
| HvL | 0.32 (0.13–0.82) | 0.017 | 0.40 (0.16–1.04) | 0.060 | ||
| GAMA | Full ectodomain | MvL | 0.23 (0.08–0.61) | 0.003 | 0.27 (0.10–0.73) | 0.010 |
| HvL | 0.28 (0.11–0.71) | 0.007 | 0.35 (0.14–0.89) | 0.027 | ||
| GAMA | N-terminal | MvL | 0.12 (0.03–0.39) | <0.001 | 0.13 (0.04–0.45) | 0.001 |
| HvL | 0.25 (0.10–0.63) | 0.003 | 0.31 (0.13–0.80) | 0.015 | ||
| GAMA | C-terminal | MvL | 0.60 (0.27–1.33) | 0.210 | 0.69 (0.31–1.53) | 0.360 |
| HvL | 0.25 (0.08–0.75) | 0.013 | 0.27 (0.09–0.82) | 0.021 | ||
| Ripr | EGF-like domain | MvL | 0.41 (0.18–0.94) | 0.035 | 0.51 (0.22–1.21) | 0.127 |
| HvL | 0.20 (0.07–0.60) | 0.004 | 0.24 (0.08–0.73) | 0.012 | ||
| SUB2 | N-terminal | MvL | 0.32 (0.13–0.76) | 0.010 | 0.41 (0.17–0.99) | 0.046 |
| HvL | 0.19 (0.07–0.57) | 0.003 | 0.27 (0.09–0.81) | 0.020 | ||
| Rhoptry proteins | ||||||
| RAMA | Full ectodomain | MvL | 0.33 (0.14–0.78) | 0.012 | 0.40 (0.17–0.98) | 0.044 |
| HvL | 0.18 (0.06–0.54) | 0.002 | 0.23 (0.08–0.70) | 0.010 | ||
| PfRh2 | PfRh2-297 | MvL | 0.46 (0.20–1.07) | 0.073 | 0.52 (0.22–1.23) | 0.136 |
| HvL | 0.37 (0.15–0.95) | 0.039 | 0.42 (0.16–1.08) | 0.070 | ||
| PfRh2 | PfRh2-2030 | MvL | 0.68 (0.32–1.47) | 0.327 | 0.68 (0.31–1.48) | 0.334 |
| HvL | 0.19 (0.05–0.64) | 0.008 | 0.20 (0.06–0.70) | 0.012 | ||
| PfRh2 | PfRh2a | MvL | 0.28 (0.11–0.70) | 0.006 | 0.30 (0.12–0.77) | 0.012 |
| HvL | 0.25 (0.09–0.68) | 0.006 | 0.27 (0.10–0.73) | 0.010 | ||
| PfRh2 | PfRh2b | MvL | 0.44 (0.2–0.98) | 0.045 | 0.49 (0.22–1.08) | 0.076 |
| HvL | 0.09 (0.02–0.40) | 0.001 | 0.12 (0.03–0.52) | 0.005 | ||
| PfRh4 | PfRh4.2 | MvL | 0.58 (0.27–1.24) | 0.162 | 0.71 (0.33–1.55) | 0.393 |
| HvL | 0.11 (0.02–0.46) | 0.003 | 0.13 (0.03–0.58) | 0.007 | ||
| PfRh4 | PfRh4.9 | MvL | 0.63 (0.26–1.52) | 0.303 | 0.74 (0.31–1.79) | 0.504 |
| HvL | 0.70 (0.30–1.63) | 0.402 | 0.75 (0.32–1.77) | 0.516 | ||
| PfRh5 | Full ectodomain | MvL | 0.46 (0.20–1.06) | 0.068 | 0.53 (0.23–1.25) | 0.150 |
| HvL | 0.27 (0.10–0.72) | 0.010 | 0.35 (0.12–0.96) | 0.042 | ||
| RALP-1 | Full ectodomain | MvL | 0.36 (0.16–0.82) | 0.014 | 0.41 (0.18–0.93) | 0.033 |
| HvL | 0.09 (0.02–0.36) | 0.001 | 0.11 (0.03–0.48) | 0.003 | ||
| RhopH1(3.1) | N-terminal | MvL | 0.40 (0.18–0.92) | 0.030 | 0.47 (0.20–1.08) | 0.075 |
| HvL | 0.14 (0.04–0.48) | 0.002 | 0.19 (0.05–0.66) | 0.009 | ||
| RhopH1(2) | N-terminal | MvL | 0.49 (0.22–1.08) | 0.078 | 0.57 (0.25–1.28) | 0.173 |
| HvL | 0.15 (0.04–0.49) | 0.002 | 0.18 (0.05–0.64) | 0.008 | ||
| RON2 | N-terminal | MvL | 0.57 (0.26–1.25) | 0.163 | 0.72 (0.32–1.59) | 0.415 |
| HvL | 0.17 (0.05–0.58) | 0.005 | 0.21 (0.06–0.72) | 0.013 | ||
| RON4 | N-terminal | MvL | 0.54 (0.25–1.18) | 0.124 | 0.64 (0.29–1.41) | 0.272 |
| HvL | 0.10 (0.02–0.43) | 0.002 | 0.13 (0.03-0.59) | 0.008 | ||
| RON6 | C-terminal | MvL | 0.57 (0.26–1.24) | 0.153 | 0.69 (0.31–1.53) | 0.363 |
| HvL | 0.16 (0.05–0.54) | 0.003 | 0.21 (0.06–0.73) | 0.014 | ||
| Miscellaneous Ags | ||||||
| Pf113 | C-terminal | MvL | 0.27 (0.11–0.68) | 0.006 | 0.33 (0.13–0.83) | 0.018 |
| HvL | 0.22 (0.08–0.59) | 0.003 | 0.30 (0.11–0.84) | 0.021 | ||
| CSP | NANP repeat | MvL | 1.28 (0.50–3.24) | 0.606 | 1.39 (0.55–3.55) | 0.486 |
| HvL | 1.58 (0.65–3.87) | 0.316 | 1.86 (0.75–4.57) | 0.178 |
IgG responses were stratified into three equal groups (tertiles, n = 69 for each group) to examine the dose-response effect of Ag-specific Ab responses on subsequent episodes of symptomatic P. falciparum malaria. HRs were calculated using the Cox proportional-hazards model comparing those with medium-versus-low (MvL) and high-versus-low (HvL) levels of Abs for the risk for symptomatic malaria over a 6-mo follow-up (first symptomatic episode only). Covariates included in the adjusted model include age and the location of residence.
The p values < 0.05 are in bold type.
CI, Confidence interval.
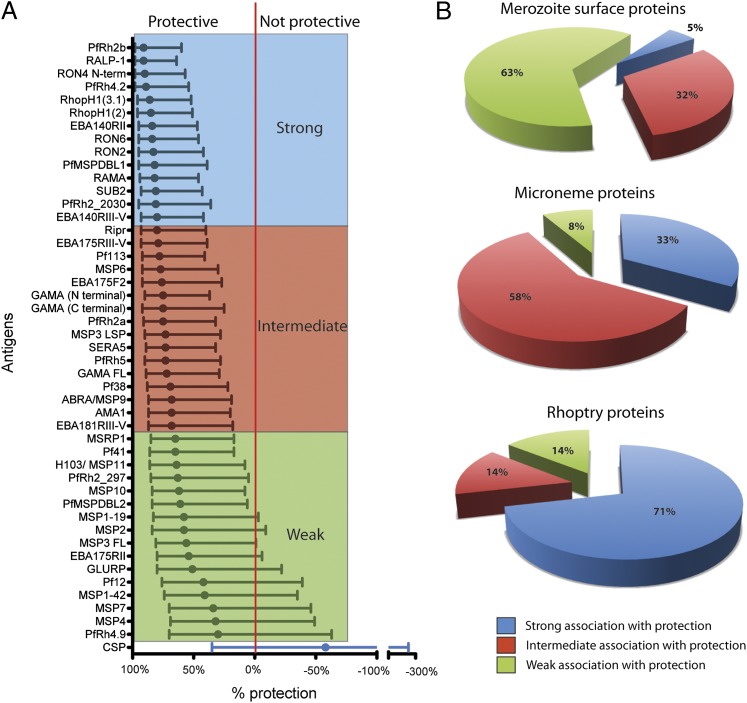
When Ag-specific responses were ranked according to the strength of their protective associations, many of the Ags that were most strongly associated with protection were relatively understudied rhoptry and micronemal proteins, particularly proteins with a demonstrated role in erythrocyte invasion, rather than the more extensively studied MSPs (Fig. 3A). To assess this further, Ag-specific responses were classified into three equal groups, defined as strong, intermediate, and weak categories (Fig. 3A); however, it should be noted that, even in the intermediate group, there were Ab responses that were strongly associated with protective outcomes (HRs ranging from 0.20 to 0.32). The majority of responses to rhoptry and microneme Ags were in the strong or intermediate protective-association groups, whereas the majority of responses against merozoite surface Ags were in the weak association group (p < 0.001, Fig. 3B).
FIGURE 3.
Associations between Abs to merozoite Ags and protection from symptomatic malaria. (A) Association between Abs and protection from symptomatic malaria for each Ag tested in the longitudinal cohort of 206 children. Ags are ranked by the strength of their protective association from most to least protective (top to bottom). The protective association (percentage) for each Ag is derived from the HR calculated by the unadjusted Cox-regression analysis (comparing children with high versus low IgG responses). Circles indicate the percentage protection, and the error bars indicate the 95% confidence interval. The red vertical line indicates percentage protection = 0% (HR = 1). Responses are colored according to the strength of the protective association: strong, intermediate, and weak. These groups were defined as three equal groups, but it should be noted that the intermediate group contains many responses strongly associated with protection. (B) Pie charts showing the number of responses with strong, intermediate, and weak associations with protection according to their location within the merozoite. A higher proportion of microneme and rhoptry proteins had strong-intermediate protective associations for Abs compared with merozoite surface Ags (p < 0.001, χ2 test). The data for HRs (and 95% confidence interval) are provided in Table III.
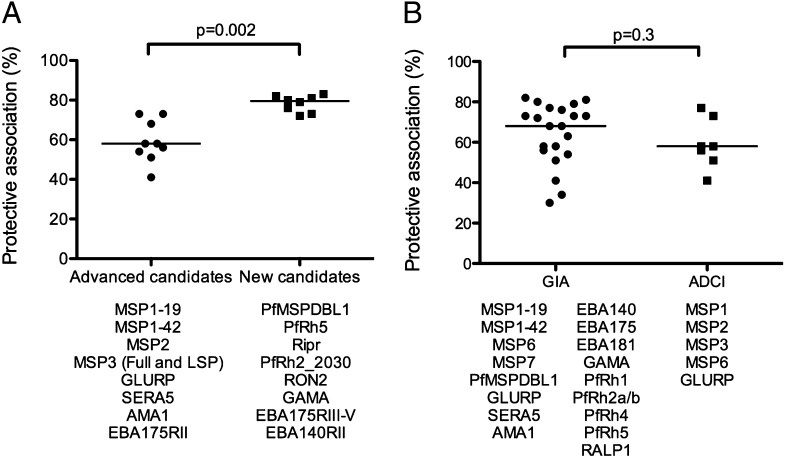
Interestingly, IgG responses against a number of recently described erythrocyte invasion ligands, such as PfRh5, Ripr, PfRh2, RON2, GAMA, EBA175RIII-V, EBA140RII, and MSPDBL1, were strongly associated with protective immunity, highlighting these Ags as potentially important vaccine targets or biomarkers of immunity (Fig. 3A, Table III). For each of these Ags, Abs generated in experimental animals have reported functional activity and have protein-binding activity relevant to invasion (Supplemental Table I), which further supports a protective role of Abs to these proteins. The median protective association for these “new” vaccine candidates was significantly stronger than was that of established Ags that had advanced to phase 1 or phase 2 vaccine trials (median risk reduction for new versus established candidates was 79.5% [interquartile range: 73.8–81.8%] and 58.0% [interquartile range: 52.5–70.5%], respectively; p = 0.002, Fig. 4A). Knowledge of the function of Abs to merozoite Ags is also important for determining whether an Ag is an important target of immunity and for prioritizing and validating candidates for vaccine development (8). The two most widely used assays of Ab function are GIAs, which measure the direct inhibitory activity of Abs, and ADCI assays, which measure Ab activity in the presence of monocytes. We compared protective associations for Ag-specific Abs that showed GIA and/or ADCI activity in previous studies (Fig. 4B). We found no clear difference in the protective association for Ag-specific Abs that are active in GIAs versus ADCI assays, and there was a broad range in the levels of protective association for Ags in each group.
FIGURE 4.
Protective associations for Ag-specific responses comparing advanced versus new vaccine candidates, as well as activity in different functional assays. (A) Protective associations for IgG responses against “advanced” vaccine candidates and “new” vaccine candidates are compared. Ags included in the comparison are listed below the graph. Advanced vaccine candidates were defined as those that had reached phase 1 or 2 vaccine trials. New candidates were selected on the basis that they had confirmed localization to the merozoite surface or apical organelles, the protein has a reported binding function relevant to invasion, and preclinical vaccine studies reported and demonstrated functional activity of Abs. The protective association for new candidates was significantly stronger than for advanced candidates. (B) Protective associations are shown for IgG responses against Ags that have known functional in vitro activity. Ags were grouped based on whether Abs to them have previously demonstrated functional activity in GIAs and ADCI assays, which are the two most widely used functional assays in merozoite Ag–vaccine development. There was no significant difference in the protective association between the two groups. Protective associations were calculated from the uHRs (Table III). Protective associations for each Ag are indicated (dots), as is the median for each group (horizontal line).
Different patterns of Ab levels associated with protective immunity
Although it was not possible to give an absolute quantification of Ag-specific Ab levels in this study, there was evidence of different Ag-specific dose-response patterns in the survival analysis examining the relationship between Ab levels and risk for malaria (Table III, Supplemental Fig. 1). These patterns may provide insights into how Abs function in vivo or how Ab levels can be used as biomarkers to predict immunity or susceptibility in populations. To illustrate this, each Ag-specific IgG response was classified into one of three categories (Supplemental Table II): 1) gradient responses, 2) High level Ab responses required for protection, 3) Moderate-high level Ab responses required for protection. Category 1 responses were those for which low-level Abs had little or no protective association, moderate levels had intermediate protective association, and high levels were associated with the highest degree of protection, suggesting that increasing Ab levels correlate with increasing levels of protective immunity (e.g., Supplemental Fig. 1C). This included responses to MSPDBL1, EBA140RIII-V, and PfRh4.2. For category 2 responses, high-level Abs were significantly associated with protection, but low-moderate Ab levels had little protective association (e.g., Supplemental Fig. 1J). This suggests that, for some Ags, there may be a high threshold level of Abs required for immune effector function or protective activity. This category included Ags such as MSP3 LSP, MSP6, EBA140RII, and PfRh2-2030. Most of the responses were category 3, in which moderate-high levels of Abs were associated with protection to a similar extent, but low-level Abs were not associated with protection (Supplemental Fig. 1A). This group included responses to the remaining merozoite Ags.
Assessing the additive effect of combination responses
Protective immunity may consist of combined responses to multiple merozoite proteins. We examined associations between responses to multiple Ags with protective immunity to determine whether additive effects are seen between responses to different Ags and whether these additive effects are common or restricted to specific combinations of Ag types. Because of the large number of possible Ag combinations (using 32 unique merozoite proteins in combinations that include one, two, or three Ags result in 4960 possible combinations), we focused on the potential additive effect of Ag combinations that are currently being tested in vaccine development, include multiple MSPs, include multiple micronemal and rhoptry proteins, and include MSPs plus micronemal/rhoptry proteins. In total, 82 combinations were examined for potential additive effects (Table IV; Supplemental Table III).
Table IV. Examples of Ab combinations and association with risk of malaria.
| Combinations |
uHR (95% CI) |
p Value |
|---|---|---|
| Additive combinations (n = 10) | ||
| MSP3/GLURP | 0.31 (0.11–0.92) | 0.035 |
| Rh5/MSP2 | 0.15 (0.04–0.65) | 0.011 |
| Rh5/AMA1 | 0.16 (0.04–0.71) | 0.015 |
| Rh5/MSP1-19 | 0.17 (0.04–0.72) | 0.016 |
| Rh5/EBA175F2 | 0.14 (0.03–0.59) | 0.007 |
| Rh5/EBA175RIII-V | 0.13 (0.03–0.54) | 0.005 |
| AMA1/MSP2 | 0.28 (0.08–0.98) | 0.047 |
| EBA175RIII-V/Rh2-2030/Rh4.2 | 0.06 (0.01–0.46) | 0.007 |
| EBA140RIII-V/Rh2-2030/Rh4.2 | 0.06 (0.01–0.47) | 0.007 |
| EBA140RIII-V/Rh4.2 | 0.07 (0.01–0.49) | 0.008 |
| Nonadditive combinations (n = 10) | ||
| MSP1-19/MSP2 | 0.47 (0.17–1.27) | 0.135 |
| MSP2/MSP3 | 0.46 (0.17–1.25) | 0.129 |
| MSP1-19/AMA1 | 0.47 (0.19–1.19) | 0.112 |
| AMA1/MSP1-19/MSP2 | 0.43 (0.16–1.14) | 0.09 |
| Rh5/Ripr | 0.26 (0.09–0.76) | 0.014 |
| EBA175RIII-V/Rh4.2 | 0.12 (0.03–0.51) | 0.004 |
| Rh2-2030/Rh4.2 | 0.15 (0.04–0.65) | 0.011 |
| EBA140RII/Rh2-2030/Rh4.2 | 0.15 (0.03–0.63) | 0.010 |
| EBA175F2/MSP1-19 | 0.38 (0.14–1.03) | 0.057 |
| MSP1-19/AMA1/EBA175F2 | 0.27 (0.09–0.79) | 0.017 |
Combined responses were examined using a summation of quartile responses (0, 1, 2, and 3, representing low to high) for each group. These combinations were then used to create three equal groups reflecting low-, intermediate-, and high-response scores. uHRs were calculated using Cox-regression comparing those with high versus low responses with the risk for symptomatic malaria over 6 mo of follow-up, with the analysis based on the first symptomatic episode only. Results for all Ab combinations that were assessed in this study are shown in Supplemental Table III. Examples shown represent 10 combinations with the strongest additive effect (additive combinations) and 10 representative examples of nonadditive combinations.
CI, Confidence interval.
Overall, 24 of the 82 combinations (29%) showed evidence of an additive effect on protective associations, defined as having an HR lower than any single response in the combination. Of note, this included combinations of EBA, PfRh2, and PfRh4 proteins, which indicated that additive effects and high responses to all three Ags were very strongly associated with protective immunity (e.g., EBA175RIII-V/Rh2-2030/Rh4.2 [combination 2], uHR = 0.06, Table IV). This may indicate the additional benefit of blocking ligands of alternate invasion pathways (10). A range of combinations that included PfRh5 also showed additive effects when combined with EBA175RIII-V (combination 11), EBA175F2 (combination 12), MSP2 (combination 14), AMA1 (combination 18), MSP1-42 (combination 19), and MSP1-19 (combination 21). MSP2 showed additive effects when combined with EBA175RIII-V (combination 24) and AMA1 (combination 44). The MSP3 (full-length)/GLURP combination also demonstrated additive effects (combination 47). Although the additive effects of combinations of responses were often modest, it should be noted that, in most cases, the single Ag responses already had strong associations with protective outcomes, making it difficult to further increase the strength of protective associations with combinations of responses. In some cases, the additive effect was small; therefore, the significance of these effects should be interpreted with caution.
It was interesting that most Ag combinations did not show an additional protective association when compared with single-Ag responses. Combinations that included multiple MSPs (e.g., combination 65, MSP1-19/MSP2) or combinations of MSPs with micronemal Ags (e.g., combination 64, MSP1-19/AMA1; or combination 77, MSP1-19/EBA175RII) did not show stronger protective associations than single-Ag responses (Supplemental Table III). Some Ab combinations that might target both proteins of a proposed protein–protein interaction (e.g., PfRh5/Ripr, combination 38, or AMA1/RON2) did not appear to be more strongly associated with protection than did either response alone. Similarly, several proposed vaccine combinations in clinical or preclinical testing did not show significantly stronger protective associations than did single-Ag responses. In some cases, the strength of the protective associations was very high (e.g., EBA140RIII-V, EBA175RIII-V, GAMA Ripr, Rh5, RALP1, RON2), such that there was little scope for an additive effect of another Ab response. These data suggest that the majority of Ab combinations do not have a strong additive or synergistic effect, but they identify a small number of Ag-response combinations that show additive effects and have very strong protective associations. These combinations may be particularly valuable for immune biomarker development and should be further investigated for vaccine potential.
Discussion
Current knowledge of the merozoite Ags targeted by human immunity is limited, and consequently, data to rationalize candidate Ags for vaccine development or as biomarkers of immunity and exposure are lacking. Very few merozoite Ags have been studied as immune targets in prospective human studies (6), and few studies compared multiple Ag responses in the same cohort (15–21). To address these gaps, we examined a large number of merozoite proteins that are biologically plausible targets of protective Abs, particularly those proteins with a defined role in erythrocyte invasion.
An important finding of our study was that the majority of the MSPs and apical proteins tested in this study were found to be targets of naturally acquired Abs and consistent with the characteristics of naturally acquired immunity (56–58). This was reflected by significantly higher reactivity of proteins to Abs from malaria-exposed individuals, but not malaria-naive individuals; Ab prevalence and/or levels being higher in older children; and Ab prevalence and/or levels being higher in children with active infection versus uninfected children. Thirteen Ags were found to have little reactivity with Abs, despite apparently adequate expression and effective coating in ELISA plates. Further studies are required to determine whether these Ags were truly nonimmunogenic, whether folding of these proteins was incorrect, or whether different allelic variants play a particularly important role for these Ags.
When we prospectively assessed Ab associations with protective immunity in our longitudinal cohort, it was striking that Abs to several newly described and understudied merozoite Ags were more strongly associated with protective immunity than were Abs to well-studied Ags or Ags that have already progressed to phase 1 or phase 2 human vaccine trials. Many of the Ags that progressed to clinical trials performed suboptimally or were not efficacious (59–61), and there is a strong need to identify and rationalize other Ags for vaccine development to obtain greater efficacy. An additional key finding was that Abs to microneme and rhoptry proteins were generally more strongly associated with protective immunity than were Abs to MSPs. This may reflect the key roles for apical organelle proteins in invasion; defined roles for MSPs are currently lacking (62). Considering that Ag-specific responses most strongly associated with protective outcomes are more likely to be causally related to protective immunity (63), Ags highlighted by our study should be considered for further evaluation as immune targets and potential vaccine candidates. High levels of Abs to proteins of the apical organelles, such as PfRh5, Ripr, EBAs, PfRh2, PfRh4, RON2, and GAMA, showed strong associations with protective immunity. The finding of naturally acquired responses to PfRh5 was especially interesting given a recent report suggesting that PfRh5 is not naturally immunogenic (64). Differences in the protein tested in the assay, expression levels in the parasite populations, or the human populations studied may account for these contrasting findings. A few understudied MSPs also showed strong-intermediate associations with protection (MSPDBL1, MSP9/ABRA, Pf38, MSP6). Overall, we found that responses to almost all of the 46 Ags were associated with protection from symptomatic malaria at some level, and most of these findings remained significant after adjusting for potential confounders. The high proportion of Ags associated with protection may reflect our strategy of selecting Ags that were likely to be targets of protective Abs because of their localization and known function. Protective associations were not adjusted for multiple comparisons for reasons outlined elsewhere (53). Therefore, associations that were weak or modest in strength or that were of borderline statistical significance should be interpreted with caution.
Of further interest is that the protective association for different Ab levels (high, medium, or low) varied between Ags. These observations are important for future immune-epidemiological studies that examine associations between immune responses and protection from malaria, as well as for identifying potential Ab biomarkers of immunity. These results suggest that analyses that consider Ab levels are important for identifying protective associations, rather than simply classifying Ab responses as present or absent, which was often reported in the literature. It is also likely that Ab concentration plays a critical role in mediating protective immunity (65). This study provides preliminary evidence that the threshold level of Abs for protection may vary between Ags.
Our study identified combined Ab responses to two or three Ags that were very strongly associated with protective immunity, particularly Ab combinations for microneme and rhoptry Ags. This included combinations of EBA and PfRh Ags, which have emerged as promising vaccine candidates; it is likely that an effective vaccine will need to target multiple members of these invasion ligand families to maximize protective efficacy (40). Importantly, we found that only a small proportion of Ab combinations showed evidence of an additive effect on protective associations compared with the single-Ag responses, highlighting the need for careful selection of Ags for use as biomarkers of immunity and for vaccine development. It is likely that protective immunity targeting merozoites consists of a repertoire of responses targeting multiple Ags and that a multivalent vaccine will be required to induce an efficacious response. However, very few studies examined this issue in human populations, and only a small number of merozoite Ag combinations has been studied (16, 17, 48).
The strength of our approach was that we focused on Ags that are likely to be targets of protective Abs because of their localization on the merozoite surface or in the apical organelles and exposure during or before erythrocyte invasion. This approach was adopted rather than taking a genome-wide approach, which predominantly includes intracellular proteins, because the role of intracellular proteins as targets of protective or functionally active Abs is unclear. Although it was demonstrated that immune responses against intracellular proteins can induce inhibition of in vitro parasite growth by ADCI mechanisms (66, 67), we sought to focus on Ags that are likely to be direct targets of Abs to intact merozoites. Genome-wide approaches using high-throughput protein expression are also valuable and have identified Ags that may be important immune targets or biomarkers of immunity (15). An additional strength of our approach is that all recombinant proteins were assessed for quality, coating, and immunoreactivity before being evaluated as potential targets of protective humoral immunity in our cohort of PNG children. Lastly, our prospective cohort study design was important to investigate the temporal relationship between Abs and subsequent malaria risk, thereby allowing us to infer a causal relationship in the protective effects of Abs targeting merozoite Ags. All Ags in this study were based on the 3D7 reference sequence. Many of the Ags or antigenic regions that we used are highly conserved in sequence, but some are known to be polymorphic. Although it is likely that different allelic variants of Ags influence the protective activity of Abs to specific Ags, we did not find that assessing different allelic variants for the same Ag led to major variations in the protective association observed. For example, we found that protective associations were very similar for different alleles of MSP2, AMA1, and EBA175 (data not shown). This may due to the coacquisition of responses to multiple allelic variants of these Ags or to the presence of Abs targeting conserved epitopes.
The findings of this study are valuable for informing vaccine development in several ways. The demonstration that a merozoite protein is naturally immunogenic in humans and that Ab responses are prospectively associated with protective immunity support these Ags as vaccine candidates. Identifying Ags as potential vaccine candidates should also incorporate data from functional assays; indeed, some of the Ags studied were shown to generate functional Abs (in GIAs or ADCI assays) when used to immunize experimental animals, further supporting their contribution to protective immunity (Supplemental Table I). However, more functional studies using human Abs are urgently needed, because this knowledge is limited to a small number of merozoite Ags (10, 33, 41, 51, 68). The complementary roles of studies into naturally acquired immunity and functional assays are especially important given the limitations of animal models for P. falciparum. Many of the key Ags of P. falciparum are either not present in rodent malaria species or have major differences in structure and sequence, limiting their ability to be studied in animal models of human malaria. Additionally, Abs induced by immunization of animals can have important differences in affinity, specificity, and function from that seen in humans (69). Therefore, human studies are a crucial part of establishing evidence for a protective role of responses to specific Ags.
Understanding naturally acquired human immunity to malaria is also important for reasons that extend beyond vaccine development. This study identified a broad array of biomarkers of human immunity that can aid in the development of low-cost serosurveillance tools for malaria. Such tools may guide control efforts by identifying populations at risk and evaluating the impact of malaria-control interventions (70). Furthermore, knowledge of human immunity to complex pathogens is extremely limited, and current knowledge is largely based on studies of much simpler organisms (mainly viruses and bacteria) that have smaller genomes and few target Ags. These studies are also important for contributing valuable reagents, data, and analytic approaches to the research community for defining targets of immunity and vaccine development. The many reagents generated in this work will be made available to other investigators in the field, and the database of Ab responses to multiple Ags will be accessible to other researchers to interrogate specific questions about the acquisition of Abs, associations among Abs and clinical data and outcomes, and relationships between multiple Ab responses. This will enable the standardization of studies across populations and ensure the generalizability of results. We believe that this will help to facilitate progress toward identifying immune targets and biomarkers of immunity, as well as prioritizing candidate Ags for vaccine development.
Major outcomes from this work are to advance specific Ags as candidates for malaria vaccine or biomarker development. Criteria for ranking and prioritizing merozoite vaccine candidates for further development include strong association of Ab responses with protective immunity in humans, relevant protective functional activity of immune responses (in vitro or in animal models), and a demonstrated important function for the protein in erythrocyte invasion (5). Considering these criteria, our new data presented in this article, and published findings on function, we propose that the following Ags should be prioritized as leading candidates for vaccine evaluation and development: EBA175, PfRh2, PfRh5, PfRh4, Ripr, MSPDBL1, EBA140, GAMA, and RON2. Other candidates may be prioritized as additional data on function and immune responses become available. Selecting Ags for development as biomarkers of immunity is based primarily on the strength of Ab-protective associations (alone or in combination), with consideration given to overall reactivity and established expression systems. Based on this, we propose the following as leading Ags for initial further evaluation in biomarker development: PfRh2, RALP1, PfRh4, EBA140, RON4/RON2, and RhopH1, and combinations of EBA175 with PfRh Ags.
In conclusion, these studies address an important gap in our knowledge of the targets of human immunity, understanding the potential of the many merozoite Ags as vaccine candidates, and identifying Ag-specific responses as biomarkers of immunity for the development of serosurveillance tools for malaria. These studies have identified sets of merozoite proteins of high priority for further evaluation as malaria vaccine candidates and development as biomarkers of immunity to malaria. Our findings help to define immunological principles underlying protective immunity that will facilitate the design and evaluation of new vaccines and malaria-surveillance tools.
Acknowledgments
We thank all study participants and the PNG Institute of Medical Research staff involved in the study, Robin Anders (La Trobe University, Bundoora, VIC, Australia) for providing recombinant MSP2 proteins and helpful advice and comments, and Annie Mo (National Institutes of Health) for providing recombinant EBA175R2 protein.
This work was supported by the National Health and Medical Research Council of Australia (a project grant and program grant, postgraduate research fellowship to J.S.R., a Training Award to F.J.I.F., and an Infrastructure for Research Institutes Support Scheme Grant); the Bill and Melinda Gates Foundation; the Australian Research Council (a Future Fellowship to J.G.B.); the Australia-India Strategic Research Fund of the Department of Innovation Industry Science and Research, Australia and Department of Biotechnology, India; Ministry of Education, Culture, Sports, Science and Technology Grants-in-Aid for Scientific Research (KAKENHI) (23117008); Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (KAKENHI) (23406007); the Ministry of Health, Labour, and Welfare, Japan (Grant H21-Chikyukibo-ippan-005); the Victorian State Government Operational Infrastructure Support; and the National Institutes of Health (to D.L.N.).
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The online version of this article contains supplemental material.
- ADCI
- Ab-dependent cellular inhibition
- CSP
- circumsporozoite protein
- GIA
- growth-inhibition assay
- HR
- hazard ratio
- LM
- light microscopy
- MSP
- merozoite surface protein
- PNG
- Papua New Guinea(n)
- uHR
- unadjusted hazard ratio
- WGCF
- wheat germ cell–free expression system.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.World Health Organization 2011. World Malaria Report. World Health Organization, Geneva, Switzerland [Google Scholar]
- 2.Dondorp A. M., Yeung S., White L., Nguon C., Day N. P., Socheat D., von Seidlein L. 2010. Artemisinin resistance: current status and scenarios for containment. Nat. Rev. Microbiol. 8: 272–280 [DOI] [PubMed] [Google Scholar]
- 3.Dondorp A. M., Nosten F., Yi P., Das D., Phyo A. P., Tarning J., Lwin K. M., Ariey F., Hanpithakpong W., Lee S. J., et al. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361: 455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trape J. F., Tall A., Diagne N., Ndiath O., Ly A. B., Faye J., Dieye-Ba F., Roucher C., Bouganali C., Badiane A., et al. 2011. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect. Dis. 11: 925–932 [DOI] [PubMed] [Google Scholar]
- 5.Richards J. S., Beeson J. G. 2009. The future for blood-stage vaccines against malaria. Immunol. Cell Biol. 87: 377–390 [DOI] [PubMed] [Google Scholar]
- 6.Fowkes F. J., Richards J. S., Simpson J. A., Beeson J. G. 2010. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 7: e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S., McGREGOR I. A., Carrington S. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192: 733–737 [DOI] [PubMed] [Google Scholar]
- 8.Cowman A. F., Crabb B. S. 2006. Invasion of red blood cells by malaria parasites. Cell 124: 755–766 [DOI] [PubMed] [Google Scholar]
- 9.Blackman M. J., Scott-Finnigan T. J., Shai S., Holder A. A. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J. Exp. Med. 180: 389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Persson K. E., McCallum F. J., Reiling L., Lister N. A., Stubbs J., Cowman A. F., Marsh K., Beeson J. G. 2008. Variation in use of erythrocyte invasion pathways by Plasmodium falciparum mediates evasion of human inhibitory antibodies. J. Clin. Invest. 118: 342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen S., Butcher G. A., Crandall R. B. 1969. Action of malarial antibody in vitro. Nature 223: 368–371 [DOI] [PubMed] [Google Scholar]
- 12.Jiang L., Gaur D., Mu J., Zhou H., Long C. A., Miller L. H. 2011. Evidence for erythrocyte-binding antigen 175 as a component of a ligand-blocking blood-stage malaria vaccine. Proc. Natl. Acad. Sci. USA 108: 7553–7558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouharoun-Tayoun H., Attanath P., Sabchareon A., Chongsuphajaisiddhi T., Druilhe P. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J. Exp. Med. 172: 1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumaratilake L. M., Ferrante A., Jaeger T., Rzepczyk C. M. 1992. Effects of cytokines, complement, and antibody on the neutrophil respiratory burst and phagocytic response to Plasmodium falciparum merozoites. Infect. Immun. 60: 3731–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crompton P. D., Kayala M. A., Traore B., Kayentao K., Ongoiba A., Weiss G. E., Molina D. M., Burk C. R., Waisberg M., Jasinskas A., et al. 2010. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc. Natl. Acad. Sci. USA 107: 6958–6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osier F. H., Fegan G., Polley S. D., Murungi L., Verra F., Tetteh K. K., Lowe B., Mwangi T., Bull P. C., Thomas A. W., et al. 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect. Immun. 76: 2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenhouse B., Ho B., Hubbard A., Njama-Meya D., Narum D. L., Lanar D. E., Dutta S., Rosenthal P. J., Dorsey G., John C. C. 2011. Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. J. Infect. Dis. 204: 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soe S., Theisen M., Roussilhon C., Aye K. S., Druilhe P. 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72: 247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agak G. W., Bejon P., Fegan G., Gicheru N., Villard V., Kajava A. V., Marsh K., Corradin G. 2008. Longitudinal analyses of immune responses to Plasmodium falciparum derived peptides corresponding to novel blood stage antigens in coastal Kenya. Vaccine 26: 1963–1971 [DOI] [PubMed] [Google Scholar]
- 20.Villard V., Agak G. W., Frank G., Jafarshad A., Servis C., Nébié I., Sirima S. B., Felger I., Arevalo-Herrera M., Herrera S., et al. 2007. Rapid identification of malaria vaccine candidates based on alpha-helical coiled coil protein motif. PLoS ONE 2: e645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meraldi V., Nebié I., Tiono A. B., Diallo D., Sanogo E., Theisen M., Druilhe P., Corradin G., Moret R., Sirima B. S. 2004. Natural antibody response to Plasmodium falciparum Exp-1, MSP-3 and GLURP long synthetic peptides and association with protection. Parasite Immunol. 26: 265–272 [DOI] [PubMed] [Google Scholar]
- 22.Michon P., Cole-Tobian J. L., Dabod E., Schoepflin S., Igu J., Susapu M., Tarongka N., Zimmerman P. A., Reeder J. C., Beeson J. G., et al. 2007. The risk of malarial infections and disease in Papua New Guinean children. Am. J. Trop. Med. Hyg. 76: 997–1008 [PMC free article] [PubMed] [Google Scholar]
- 23.Smith D. B., Johnson K. S. 1988. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67: 31–40 [DOI] [PubMed] [Google Scholar]
- 24.Tsuboi T., Takeo S., Arumugam T. U., Otsuki H., Torii M. 2010. The wheat germ cell-free protein synthesis system: a key tool for novel malaria vaccine candidate discovery. Acta Trop. 114: 171–176 [DOI] [PubMed] [Google Scholar]
- 25.Gao X., Yeo K. P., Aw S. S., Kuss C., Iyer J. K., Genesan S., Rajamanonmani R., Lescar J., Bozdech Z., Preiser P. R. 2008. Antibodies targeting the PfRH1 binding domain inhibit invasion of Plasmodium falciparum merozoites. PLoS Pathog. 4: e1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayner J. C., Galinski M. R., Ingravallo P., Barnwell J. W. 2000. Two Plasmodium falciparum genes express merozoite proteins that are related to Plasmodium vivax and Plasmodium yoelii adhesive proteins involved in host cell selection and invasion. Proc. Natl. Acad. Sci. USA 97: 9648–9653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Triglia T., Thompson J., Caruana S. R., Delorenzi M., Speed T., Cowman A. F. 2001. Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax. Infect. Immun. 69: 1084–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaur D., Singh S., Singh S., Jiang L., Diouf A., Miller L. H. 2007. Recombinant Plasmodium falciparum reticulocyte homology protein 4 binds to erythrocytes and blocks invasion. Proc. Natl. Acad. Sci. USA 104: 17789–17794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez M., Lustigman S., Montero E., Oksov Y., Lobo C. A. 2008. PfRH5: a novel reticulocyte-binding family homolog of plasmodium falciparum that binds to the erythrocyte, and an investigation of its receptor. PLoS ONE 3: e3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baum J., Chen L., Healer J., Lopaticki S., Boyle M., Triglia T., Ehlgen F., Ralph S. A., Beeson J. G., Cowman A. F. 2009. Reticulocyte-binding protein homologue 5 - an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int. J. Parasitol. 39: 371–380 [DOI] [PubMed] [Google Scholar]
- 31.Thompson J. K., Triglia T., Reed M. B., Cowman A. F. 2001. A novel ligand from Plasmodium falciparum that binds to a sialic acid-containing receptor on the surface of human erythrocytes. Mol. Microbiol. 41: 47–58 [DOI] [PubMed] [Google Scholar]
- 32.Gilberger T. W., Thompson J. K., Triglia T., Good R. T., Duraisingh M. T., Cowman A. F. 2003. A novel erythrocyte binding antigen-175 paralogue from Plasmodium falciparum defines a new trypsin-resistant receptor on human erythrocytes. J. Biol. Chem. 278: 14480–14486 [DOI] [PubMed] [Google Scholar]
- 33.Hodder A. N., Crewther P. E., Anders R. F. 2001. Specificity of the protective antibody response to apical membrane antigen 1. Infect. Immun. 69: 3286–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto H., Takeo S., Maier A. G., Sattabongkot J., Cowman A. F., Tsuboi T. 2012. Antibodies against a Plasmodium falciparum antigen PfMSPDBL1 inhibit merozoite invasion into human erythrocytes. Vaccine 30: 1972–1980 [DOI] [PubMed] [Google Scholar]
- 35.Arumugam T. U., Takeo S., Yamasaki T., Thonkukiatkul A., Miura K., Otsuki H., Zhou H., Long C. A., Sattabongkot J., Thompson J., et al. 2011. Discovery of GAMA, a Plasmodium falciparum merozoite micronemal protein, as a novel blood-stage vaccine candidate antigen. Infect. Immun. 79: 4523–4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kocken C. H., Withers-Martinez C., Dubbeld M. A., van der Wel A., Hackett F., Valderrama A., Blackman M. J., Thomas A. W. 2002. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect. Immun. 70: 4471–4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z. G., Yu W. G., Qiu W. S., Zhao H. M. 2006. Immunogenicity of C-terminus of Plasmodium falciparum merozoite surface protein 1 expressed as a non-glycosylated polypeptide in yeast. Acta Biochim. Biophys. Sin. (Shanghai) 38: 403–409 [DOI] [PubMed] [Google Scholar]
- 38.Sahar T., Reddy K. S., Bharadwaj M., Pandey A. K., Singh S., Chitnis C. E., Gaur D. 2011. Plasmodium falciparum reticulocyte binding-like homologue protein 2 (PfRH2) is a key adhesive molecule involved in erythrocyte invasion. PLoS ONE 6: e17102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tham W. H., Wilson D. W., Reiling L., Chen L., Beeson J. G., Cowman A. F. 2009. Antibodies to reticulocyte binding protein-like homologue 4 inhibit invasion of Plasmodium falciparum into human erythrocytes. Infect. Immun. 77: 2427–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopaticki S., Maier A. G., Thompson J., Wilson D. W., Tham W. H., Triglia T., Gout A., Speed T. P., Beeson J. G., Healer J., Cowman A. F. 2011. Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect. Immun. 79: 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Egan A. F., Burghaus P., Druilhe P., Holder A. A., Riley E. M. 1999. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol. 21: 133–139 [DOI] [PubMed] [Google Scholar]
- 42.Kauth C. W., Woehlbier U., Kern M., Mekonnen Z., Lutz R., Mücke N., Langowski J., Bujard H. 2006. Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J. Biol. Chem. 281: 31517–31527 [DOI] [PubMed] [Google Scholar]
- 43.Kennedy M. C., Wang J., Zhang Y., Miles A. P., Chitsaz F., Saul A., Long C. A., Miller L. H., Stowers A. W. 2002. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect. Immun. 70: 6948–6960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arumanayagam P., San S. J. 1972. Health trends in three Asian countries—Ceylon, Malaysia and Singapore. Int. J. Epidemiol. 1: 101–109 [DOI] [PubMed] [Google Scholar]
- 45.Pandey K. C., Singh S., Pattnaik P., Pillai C. R., Pillai U., Lynn A., Jain S. K., Chitnis C. E. 2002. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol. Biochem. Parasitol. 123: 23–33 [DOI] [PubMed] [Google Scholar]
- 46.Narum D. L., Haynes J. D., Fuhrmann S., Moch K., Liang H., Hoffman S. L., Sim B. K. 2000. Antibodies against the Plasmodium falciparum receptor binding domain of EBA-175 block invasion pathways that do not involve sialic acids. Infect. Immun. 68: 1964–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crowther J. R. 2009. The ELISA Guidebook. Humana Press, New York, NY [Google Scholar]
- 48.Richards J. S., Stanisic D. I., Fowkes F. J., Tavul L., Dabod E., Thompson J. K., Kumar S., Chitnis C. E., Narum D. L., Michon P., et al. 2010. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin. Infect. Dis. 51: e50–e60 [DOI] [PubMed] [Google Scholar]
- 49.Reiling L., Richards J. S., Fowkes F. J., Barry A. E., Triglia T., Chokejindachai W., Michon P., Tavul L., Siba P. M., Cowman A. F., et al. 2010. Evidence that the erythrocyte invasion ligand PfRh2 is a target of protective immunity against Plasmodium falciparum malaria. J. Immunol. 185: 6157–6167 [DOI] [PubMed] [Google Scholar]
- 50.Stanisic D. I., Richards J. S., McCallum F. J., Michon P., King C. L., Schoepflin S., Gilson P. R., Murphy V. J., Anders R. F., Mueller I., Beeson J. G. 2009. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect. Immun. 77: 1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiling L., Richards J. S., Fowkes F. J., Wilson D. W., Chokejindachai W., Barry A. E., Tham W. H., Stubbs J., Langer C., Donelson J., et al. 2012. The Plasmodium falciparum erythrocyte invasion ligand Pfrh4 as a target of functional and protective human antibodies against malaria. PLoS ONE 7: e45253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin E., Kiniboro B., Gray L., Dobbie S., Robinson L., Laumaea A., Schöpflin S., Stanisic D., Betuela I., Blood-Zikursh M., et al. 2010. Differential patterns of infection and disease with P. falciparum and P. vivax in young Papua New Guinean children. PLoS ONE 5: e9047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perneger, T. V. 1998. What's wrong with Bonferroni adjustments. BMJ 316: 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. ApiLoc. A database of published protein sub-cellular localisation in Apicomplexa.Available at: http://apiloc.biochem.unimelb.edu.au/apiloc/apiloc. Accessed: February 20, 2013.
- 55.Fowkes F. J., McGready R., Cross N. J., Hommel M., Simpson J. A., Elliott S. R., Richards J. S., Lackovic K., Viladpai-Nguen J., Narum D., et al. 2012. New insights into acquisition, boosting, and longevity of immunity to malaria in pregnant women. J. Infect. Dis. 206: 1612–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baird J. K. 1995. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol. Today (Regul. Ed.) 11: 105–111 [DOI] [PubMed] [Google Scholar]
- 57.Doolan D. L., Dobaño C., Baird J. K. 2009. Acquired immunity to malaria. Clin. Microbiol. Rev. 22: 13–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bull P. C., Lowe B. S., Kaleli N., Njuga F., Kortok M., Ross A., Ndungu F., Snow R. W., Marsh K. 2002. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J. Infect. Dis. 185: 1688–1691 [DOI] [PubMed] [Google Scholar]
- 59.Ogutu B. R., Apollo O. J., McKinney D., Okoth W., Siangla J., Dubovsky F., Tucker K., Waitumbi J. N., Diggs C., Wittes J., et al. MSP-1 Malaria Vaccine Working Group 2009. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS ONE 4: e4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thera M. A., Doumbo O. K., Coulibaly D., Laurens M. B., Ouattara A., Kone A. K., Guindo A. B., Traore K., Traore I., Kouriba B., et al. 2011. A field trial to assess a blood-stage malaria vaccine. N. Engl. J. Med. 365: 1004–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lawrence G., Cheng Q. Q., Reed C., Taylor D., Stowers A., Cloonan N., Rzepczyk C., Smillie A., Anderson K., Pombo D., et al. 2000. Effect of vaccination with 3 recombinant asexual-stage malaria antigens on initial growth rates of Plasmodium falciparum in non-immune volunteers. Vaccine 18: 1925–1931 [DOI] [PubMed] [Google Scholar]
- 62.Boyle M. J., Wilson D. W., Beeson J. G. 2013. New approaches to studying Plasmodium falciparum merozoite invasion and insights into invasion biology. Int. J. Parasitol. 43: 1–10 [DOI] [PubMed] [Google Scholar]
- 63.Hill A. B. 1965. The Environment and Disease: Association or Causation? Proc. R. Soc. Med. 58: 295–300 [PMC free article] [PubMed] [Google Scholar]
- 64.Douglas A. D., Williams A. R., Illingworth J. J., Kamuyu G., Biswas S., Goodman A. L., Wyllie D. H., Crosnier C., Miura K., Wright G. J., et al. 2011. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nat Commun 2: 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saul A. 1987. Kinetic constraints on the development of a malaria vaccine. Parasite Immunol. 9: 1–9 [DOI] [PubMed] [Google Scholar]
- 66.Olugbile S., Kulangara C., Bang G., Bertholet S., Suzarte E., Villard V., Frank G., Audran R., Razaname A., Nebie I., et al. 2009. Vaccine potentials of an intrinsically unstructured fragment derived from the blood stage-associated Plasmodium falciparum protein PFF0165c. Infect. Immun. 77: 5701–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kulangara C., Luedin S., Dietz O., Rusch S., Frank G., Mueller D., Moser M., Kajava A. V., Corradin G., Beck H. P., Felger I. 2012. Cell biological characterization of the malaria vaccine candidate trophozoite exported protein 1. PLoS ONE 7: e46112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilson D. W., Fowkes F. J., Gilson P. R., Elliott S. R., Tavul L., Michon P., Dabod E., Siba P. M., Mueller I., Crabb B. S., Beeson J. G. 2011. Quantifying the importance of MSP1-19 as a target of growth-inhibitory and protective antibodies against Plasmodium falciparum in humans. PLoS ONE 6: e27705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miura K., Zhou H., Diouf A., Moretz S. E., Fay M. P., Miller L. H., Martin L. B., Pierce M. A., Ellis R. D., Mullen G. E., Long C. A. 2009. Anti-apical-membrane-antigen-1 antibody is more effective than anti-42-kilodalton-merozoite-surface-protein-1 antibody in inhibiting Plasmodium falciparum growth, as determined by the in vitro growth inhibition assay. Clin. Vaccine Immunol. 16: 963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drakeley C., Cook J. 2009. Chapter 5. Potential contribution of sero-epidemiological analysis for monitoring malaria control and elimination: historical and current perspectives. Adv. Parasitol. 69: 299–352 [DOI] [PubMed] [Google Scholar]