Abstract
Retinal vascular disease is the most common cause of macular edema (ME). While there are several etiologies of vascular compromise and subsequent macular leakage, diabetic retinopathy is the most prevalent and continues to challenge ophthalmologists and frustrate patients due to its refractory nature. In response to this epidemic, diabetic ME (DME) along with cystoid ME (CME) have been areas of active investigation both in the clinic and the laboratory. Several decades of basic science research have revealed a growing and complex array of cytokine growth factors and proinflammatory mediators which are capable of inciting the cellular changes that result in accumulation of fluid within the retina. Much of this new molecular foundation provides the current and fundamental scaffold for understanding the pathologic process of ME while simultaneously identifying potential therapeutic targets. Whereas CME has classically been treated with corticosteroids and nonsteroidal antiinflammatory drugs, recent clinical studies have demonstrated improved visual outcomes for DME treatment with light focal/grid laser, corticosteroids and anti-vascular endothelial growth factor antibodies. Yet, each of these treatments has differential effects on the multifactorial mechanisms of ME. This article reviews the anatomical, cellular and molecular derangements associated with ME and highlights specific pathways targeted by current treatments.
Key Words: Retinal vascular disease; Macular edema, derangements
Introduction
Located in the central retina is the macula, a 5- to 6-mm-diameter region bordered by the vascular arcades and optic nerve, and noted for its yellowish appearance and high concentration of xanthophyll pigments. Within the central macula is the fovea, a small 1.5-mm-diameter area which is densely packed with cone photoreceptors, the specialized neurons that mediate color vision and fine spatial acuity. Any perturbation of the delicate cellular architecture or metabolic and signaling pathways of this precious biologic real estate can have devastating consequences on the quality of vision and life.
Macular edema (ME) is caused by extravasation of fluid and plasma components from blood vessels and/or derangements in cellular ion flux leading to the accumulation of intracellular and intercellular fluid in the outer plexiform and inner retinal layers. Patients suffering from ME present with symptoms of blurred or decreased central vision which can progress over a period of months to years, often with unyielding chronicity. ME commonly develops secondary to vascular insufficiency in disease states such as diabetic retinopathy (DR), branch and/or central retinal vein occlusion, ocular ischemic syndrome, radiation retinopathy, pseudophakia, age-related macular degeneration, uveitis, retinitis pigmentosa, ocular trauma or drug toxicity. Thus, ME may be considered the anatomic result of numerous pathologic processes that alter the blood flow, vascular integrity and fluidic balance in the neurosensory retina.
Clinical ME Phenotypes
Diabetic ME (DME) is defined by the Early Treatment Diabetic Retinopathy Study as retinal thickening and/or the presence of hard exudates within 1 disk diameter of the central macula. The severity of DME is graded by determining whether the disease parameters meet the criteria for clinically significant ME, defined as retinal thickening within 500 μm of the central macula, hard exudates within 500 μm of the central macula associated with thickening of the adjacent retina, or retinal thickening greater than 1 disk area within 1 disk diameter of the central macula. The disease phenotype is traditionally classified into focal and diffuse types, an important distinction as treatments vary accordingly. Focal ME is caused by small areas of retinal vascular abnormalities such as microaneurysms, which tend to leak fluid and lipoproteins into the surrounding tissue. In contrast, in the setting of diffuse ME, dilated retinal capillaries and/or intraretinal microvascular abnormalities allow for the widespread accumulation of intraretinal fluid throughout the macula.
While diabetes is the most common etiology for these clinical findings, other retinal vascular diseases such as those mentioned previously are equally capable of inducing similar retinal changes in focal and/or diffuse ME. Moreover, cystoid ME (CME) may also be associated with these disease states, especially those with robust inflammatory responses. To gain further understanding of the specific cellular changes that result in these various ME phenotypes, the infrastructural design elements of the blood-retinal barrier (BRB) must be addressed.
Biologic Mechanisms and Animal Models in ME
The BRB is anatomically divided into inner and outer partitions: the inner BRB is located at retinal endothelial cell tight junctions, whereas the outer BRB is formed by retinal pigment epithelium (RPE) cell tight junctions. These tight junctions are comprised of the transmembrane proteins occludin and claudin, which are arranged in an organizational network of cytoplasmic proteins [1] along with members of the recently designated junctional adhesion molecule family [2,3]. Their function is critical to controlling fluid, ion, molecular and cellular flux into the retinal parenchyma [4]. In a simplified model of ME, fluid may accumulate either in the intercellular or the intracellular compartments. It is suggested that intercellular edema is regulated by the integrity of the BRB, whereas intracellular edema is controlled by specific ion and fluid transporters on Müller cells and other neural retinal cell types [5,6]. These two fluid compartments are closely linked and likely cross-talk; thus, many investigations have focused on dissecting the molecular mechanisms of BRB behavior in order to determine potential pharmacologic targets for ME.
Disruption of the BRB may occur via the upregulation of multiple cytokines, including vascular endothelial growth factor-A (VEGF-A), which results in the phosphorylation of tight junction proteins and subsequent disassembly [7]. Advanced glycosylation end products, a molecular hallmark of diabetes, may also lead to breakdown of the BRB [8]. These events increase vascular permeability, clinically evident as subretinal fluid and ME. In the setting of ocular ischemia and inflammation, breakdown in the retinal endothelial cell tight junctions leads to massive third spacing in the outer plexiform layer of the neural retina, which presents in the clinic as CME. In order to dissect the mechanisms responsible for these varying biologic sequences, several animal models have been designed to approximate the pathobiology of ME in vivo.
Since only a few species such as nonhuman primates have a clinically identifiable macula, and since it is a chronic disease process, a good animal model of ME has proven difficult to recapitulate. There are models of postsurgical ME in monkeys that mimic important features of the human disease, but the model is limited by the cost and feasibility of performing large-scale nonhuman primate studies [9]. It has been shown that diabetic models in small animals including rats and mice also develop breakdown of the BRB, thereby providing systems in which to study molecular mediators of leakage and retinal edema [10,11]. An alternative approach to inducing ME is to administer intravitreal (IV) injections of recombinant molecular mediators which are suspected to play a part in the process of BRB breakdown. However, the utility of these techniques is limited by the metabolic clearance of recombinant proteins and an abbreviated biologic effect of these treatments. Studying the long-term effects of chronic cytokine upregulation requires the use of either sustained-release implants [12] or transgenic animals engineered with altered expression of the protein of interest [13].
Even with the limited number of animal models available to study ME, an enormous cache of data has been collected and important discoveries have been made. Both interleukin (IL)-1β and tumor necrosis factor (TNF)-α are potent mediators of leukocyte recruitment and BRB leakage in multiple models of ocular inflammation [14,15]. Further reports demonstrated that with IV administration of TNF-α, IL-1β or IL-8, but not of IL-6, BRB breakdown occurred in rats [16,17]. VEGF-A is a cytokine that potently induces vascular permeability, an effect that is transduced directly to endothelial cells via VEGF-A receptors or via platelet-activating factor (PAF) [18]. PAF is suggested to increase permeability via VEGF-A [19], nitric oxide [19] or prostaglandins (PG) [20].
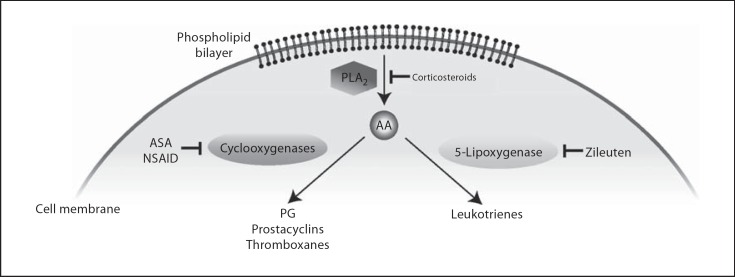
In the inflammatory pathway, the enzyme phospholipase A2 (PLA2) causes the release of arachidonic acid (AA) from phospholipids (fig. 1). Subsequently, cyclooxygenase converts AA to PG, which leads to breakdown of the BRB including vasodilation, increased capillary permeability from compromised endothelial tight junctions in the retinal capillaries, and decreased removal of fluid by the RPE [21]. Another product in this inflammatory cascade involves the enzyme 5-lipoxygenase, which alternately converts AA to leukotriene, a chemotactic agent that enhances vascular permeability and leukocyte activation. The pathophysiological role of eicosanoids in the eye is of great interest in ophthalmology because AA metabolites have been implicated in a number of ocular disorders including uveitis, CME and DR [22]. Stromal cell-derived factor-1 (SDF-1), a cytokine involved in cellular trafficking and angiogenesis, may also play a major role in the pathology of both ME and proliferative DR (PDR) [23].
Fig. 1.
Molecular pathways of inflammation via AA formation and downstream induction of cyclooxygenases and 5-lipoxygenase which generate PG and leukotrienes, respectively. While aspirin (ASA) and nonsteroidal antiinflammatory drugs (NSAID) inhibit PG, prostacyclin and thromboxane synthesis, corticosteroids act further upstream via inhibition of PLA2, which prevents the release of AA and activation of multiple downstream inflammatory cascades. Zileuton is a targeted 5-lipoxygenase antagonist.
In several studies investigating the aqueous and/or vitreous cytokine profile in patients with ME, an array of molecules was found to be upregulated: VEGF-A [24], erythropoietin [25], IL-6 [26], interferon (IFN)-γ [27], hepatocyte growth factor (HGF) [28], fibroblast growth factor 2 (FGF2) [29], PGE2 and carbonic anhydrase (CA) [30] (table 1). Many of these cytokines disrupt tight junctions at the BRB and have been shown to induce retinal leakage in animal models. Transforming growth factor-β1, which is also increased in ME, is released by hyalocytes residing in the vitreous cortex, inducing cellular contraction and biochemical changes in the vitreous [31,32]. These changes may induce tractional forces on the vitreomacular interface, causing ME via a mechanical mechanism. Other investigators are focused on the imbalance of pro- and antiangiogenic molecules as an important factor in the pathogenetic mechanism of ME. There are data to support this hypothesis as vitreous samples from patients with ME harbored increased VEGF-A accompanied by a reciprocal decrease in pigment epithelium-derived factor or soluble VEGF receptor-1 [28], both of which are potent antiangiogenic molecules. Still, while rigorous scientific investigation has revealed an array of cytokines and growth factors that may be critical in DME pathogenesis, studying patient responses to varying treatment regimens in the clinic has been equally, if not more, insightful.
Table 1.
Inciting molecules in animal studies and intraocular cytokine profiles in patients with ME
| Inciting molecules in animal studies | |
| Prostaglandins | Cytokines |
| PGE1 | VEGF-A |
| PGE2 | P1GF |
| PGF2a | TNF-α |
| Leukotrienes | IL-1β |
| LTB4 | IGF-1 |
| LTC4 | IL-6 |
| LTD4 | SDF-1 |
| Nitric oxide | HGF |
| PAF | CA |
| PKC-β | |
| Intraocular cytokine profiles with ME | |
| Increases in: | Decreases in: |
| VEGF-A | TGF-β |
| FGF2 | sVEGFR-1 |
| Erythropoietin | |
| IL-6 | |
| IFN-Γ | |
| HGF | |
| PGE2 | |
| CA | |
P1GF = Placental growth factor; IGF-1 = insulin-like growth factor-1; PKC-ß = protein kinase C-ß; TGF-ß = transforming growth factor-ß; sVEGFR-1 = soluble VEGF receptor-1.
Treatment Responses in Patients with ME
The two classical types of laser treatment for ME are focal and grid. Focal laser treatment is used to treat focal ME with the aim of closing leaking microaneurysms. Grid laser treatment is used to treat diffuse ME and is applied to areas of retinal thickening in which there is diffuse leakage with the aim of producing a retinal burn of mild-to-moderate intensity. There have been several recent large studies comparing patient groups either treated with laser or corticosteroids with varying improvements in visual outcomes, depending on the patient's initial presentation, phakic status and whether the ME was refractory [33,34]. Other studies, some planned for large subject numbers and multicenter involvement, have now extended the treatment groups to compare treatment efficacies between laser photocoagulation, VEGF-A antagonists and corticosteroids.
In a recent study by Paccola et al. [35], 26 patients were split into treatment groups receiving either IV preservative-free triamcinolone (IV-TA; 4 mg) or bevacizumab (IV-B; 1.25 mg) for refractory DME, and were followed over a period of 6 months. A statistically significant improvement in both central retinal thickness (CRT) and best-corrected visual acuity (BCVA) was evident in both groups compared to baseline. However, the IV-TA group significantly outperformed the IV-B group at weeks 4 through 12 with respect to CRT and BCVA, with only a mild elevation in intraocular pressure and no cataract progression. In another study investigating similar treatment groups with bilateral DME (n = 28 eyes), IV-TA resulted in statistically significant improvement in BCVA and CRT compared to IV-B beginning at 4 weeks and extending to the study termination date at 6 months [36].
The enhanced treatment response of ME to corticosteroids compared to anti-VEGF-A therapy is not limited to refractory DME. In a study comparing IV-TA to IV-B in ME secondary to branch retinal vein occlusion, corticosteroid therapy had a significantly decreased rate of disease recurrence (7.6 vs. 26.0%) and a longer duration between treatments (12.6 ± 6.4 vs. 5.3 ± 3.1 months) [37]. To understand why corticosteroid treatment for DME may be advantageous when compared to targeted VEGF-A inhibition, it is necessary to further dissect the cellular and molecular mechanisms of intraretinal fluid accumulation within the retina.
Molecular Mechanisms of VEGF-A-Independent Retinal Vascular Permeability
As previously mentioned, there are many other factors besides VEGF-A that are capable of weakening the BRB and are upregulated in the vitreous of patients with ME. For example, although VEGF-A has a direct effect on vascular permeability, it has been reported that IL-1β and TNF-α can induce leakage independent of a VEGF-A blockade [38]. Therefore, we may presume that targeting VEGF-A alone in the clinical setting may only provide a partial inhibition of vascular leakage and allow other upregulated cytokines to counter the therapeutic efficacy and promote continued disease progression.
In DR, the loss of retinal vascular pericytes is suggested to be a major cause of focal areas of BRB breakdown and subsequent vascular leakage. When this occurs, there is extravasation of fluid and circulating inflammatory cells into the retinal layers. The process of leukocyte diapedesis itself induces local changes in postcapillary venule permeability, which may further exacerbate this inflammatory cascade [39]. Site-directed infiltration in areas of BRB breakdown occurs primarily via the expression of leukocyte adhesion proteins including platelet/ endothelial cell adhesion molecule-1, intercellular adhesion molecule (ICAM)-1 and E-selectin [40,41]. It is known that patients with DR have increased numbers of leukocytes within the retinal vasculature, and that a similar pattern of leukostasis can be prevented in a diabetic animal model via ICAM-1 inhibition [42]. Interestingly, ICAM-1, which is critical to leukocyte transmigration once bound to the endothelium, is selectively upregulated by VEGF-A, whereas P- and E-selectins, which are responsible for leukocyte adhesion during rolling, are not [43]. Yet, there are redundant mechanisms of leukocyte adhesion molecule induction as both IL-1β and TNF-α also upregulate ICAM-1. Thus, a preferred treatment for inhibiting the pathologic processes associated with DME would act upstream of VEGF-A, to reverse not only vascular permeability but also leukocyte adhesion. Corticosteroids would be expected to have increased treatment effects over the anti-VEGF-A compound as they suppress the activation of the inflammatory cascade at the upstream level of PLA2 and also inhibit leukostasis in the retina in a diabetic animal model [44] through inhibition of leukocyte rolling, adhesion and transmigration. In the clinic, the potent efficacy of corticosteroid therapy is demonstrated by the rapid resolution of ME, although an accompanying gain in BCVA may not occur due to irreversible damage of the neural retinal networks.
There are other highly effective treatments for ME including those that target CA, an enzyme that catalyzes the formation of water by dehydrating bicarbonate in a reversible reaction. CA inhibitors are well tolerated and very useful in patients with CME, often reversing the anatomic features within a matter of a few days. Clinicians and scientists have been intrigued by this remarkable efficacy, and thus the in vivofunction of CA has been thoroughly investigated with some fascinating results. Firstly, there are multiple reports that CA inhibition reduces macular leakage and edema, with additional data suggesting that CA inhibitors may increase oxygen tension and the retinal arteriole diameter [30,45]. In a study by Gao et al. [46], substantial levels of CA were detected in vitreous samples from patients with inactive PDR as well as moderate-to-severe nonproliferative DR. Upon IV injection of recombinant human CA into rat eyes, frank retinal vascular leakage and edema was evident within 30 min, with areas of focal vascular permeability observed at 48 h. These data were supported by in vivo optical coherence tomography imaging, also demonstrating diffuse retinal edema. In one of the experiments, animals administered both CA and VEGF-A demonstrated an additive effect in the amount of retinal vascular leakage observed. This is an important observation as VEGF-A-independent leakage may be a significant component of treatment response differentials observed between corticosteroid and anti-VEGF-A therapies.
Several other pathways have been discovered that modulate retinal vascular permeability independent of VEGF-A, including the receptor activator of nuclear factor-κB ligand, which acts via a nitric oxide-dependent mechanism [47,48]. Additionally, HGF, which is increased in vitreous samples from patients with ME, induced retinal vascular permeability via mitogen-activated protein kinase and phosphoinositide 3-kinase signaling cascades independent of VEGF-A [49]. RPE cells can also express HGF, which unravels the zona occludens (ZO-1) tight and adherens junctions that form the outer BRB, leading to decreased transepithelial resistance and fluid shifts in the photoreceptor-RPE interface [50]. In another study, the same capacity for disassembly of the RPE tight junctions was investigated with VEGF-A and anti-VEGF drugs, with the surprising result that these compounds do not disrupt the integrity of this cellular barrier [51] although these data have aroused controversy. All of these data speak to the importance of targeting beyond VEGF-A in order to achieve a maximal therapeutic response when treating ME.
Regulation and Prevention of Retinal Cell Edema and Death
As further molecular resolution has been gained in our understanding of vascular permeability and ME, it has been revealed that not only are the tight junctions of the BRB critical in the maintenance of retinal cell fluid balance, but there are multiple ion and water channels that also must be functioning in order to achieve this target osmolarity required for optimal retinal function. In CME, there are significant alterations in fluid compartmentalization with extreme shifts into Müller cells, resulting in intracellular edema, stretching of the cell body and intercellular cystoid spaces. Aquaporin 4 (AQP4) is an important transmembrane protein found on Müller cells that conducts water through the plasma membrane [52]. In an animal model of ocular ischemia, AQP4 activity caused deleterious fluid shifts in neural retinal cells, thus leading to cell death. Transgenic mice deficient in AQP4 have a significantly improved preservation of retinal function and minimal cell death after identical ischemic insults. Similarly, it has also been reported that retinal vein occlusion can result in caspase-dependent retinal cell apoptosis [53]. Thus, it would seem rational to target AQP4 in order to prevent this process. Interestingly, corticosteroids are able to regulate AQP4 expression and have been shown to decrease AQP4 protein in human neuronal isolates in vitro [54]. Corroborating these data, IV-TA was able to suppress Müller cell swelling associated with ME in multiple animal models [55,56]. Additionally, there are numerous other studies suggesting that corticosteroids are protective against retinal cell death [57,58,59]. Thus, in a current understanding of the pathobiology of ME, we may reasonably conclude that:
• RPE cell monolayer integrity or the vascular barrier can be disrupted by agents other than VEGF-A;
• edema and leakage due to inflammation are biochemically distinct from those induced by VEGF-A;
• therapy should aim to block permeability induced by both VEGF-A and inflammatory pathways.
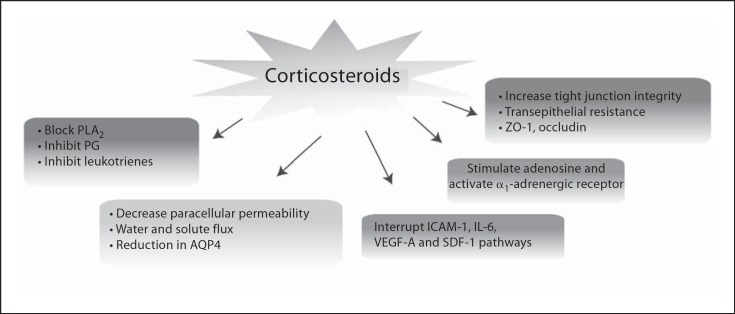
Corticosteroids which are widely available and safe meet most of these criteria by their in vivo multipronged action (fig. 2). Firstly, they inhibit PLA2 and the downstream production of PG and leukotrienes, thereby sig- nificantly suppressing AA-mediated inflammation. Secondly, several cytokine and adhesion signaling pathways are inhibited, including ICAM-1, IL-6, VEGF-A and SDF-1, all of which are associated with inflammation, vascular permeability and/or leukostasis. Thirdly, corticosteroids effectively reduce paracellular permeability in critical neural retinal cell types by decreasing AQP4 expression and altering ion and water flux. Furthermore, tight junction integrity is enhanced with corticosteroid treatment, as measured by transepithelial resistance and the presence of normal-appearing ZO-1-positive junctions.
Fig. 2.
Corticosteroids have a multipronged effect in vivo, leading to the suppression of several proinflammatory mechanisms.
The Unknown Effects of VEGF-A Inhibition
Beyond the rationale presented here and elsewhere that indicates a clinical benefit with the use of IV-TA as well as a scientific explanation for its enhanced biologic activity in the treatment of ME, there is another important area of interest that is currently being studied with anti-VEGF-A therapy. While there has been a huge advance in our fundamental knowledge of VEGF-A signaling and an unprecedented growth in the utilization of VEGF-A inhibitors in the clinic, there have been several studies which point to the role of VEGF-A in the survival of a multitude of retinal cell types. In an initial study, administration of IV or systemic VEGF-A antagonists resulted in retinal ganglion cell apoptosis approximately 8 weeks after treatment in a rat model [60]. Independent follow-up studies showed that endogenous VEGF-A is necessary for Müller and photoreceptor cell survival, and systemic blockade led to apoptosis of these cell types at 2–4 weeks after treatment in a mouse model [61]. In another mouse model, subretinal injection of a lentivirus encoding a VEGF-A antagonist caused significant photoreceptor degeneration at 6 months compared to controls [62]. Lastly, in a very recent study, it was found that a transgenic mouse strain in which the VEGF-A isoforms normally produced by the RPE were ablated developed choriocapillaris degeneration and RPE cell loss, suggesting that VEGF-A carries a survival function in the RPE-choroidal tissues as well as the neural retina [63].
Many groups have been studying whether similar effects occur in patients receiving frequent anti-VEGF-A treatments for age-related macular degeneration. In a small series of patients followed over the course of a year after treatment with ranibizumab every month for 3 months followed by as-needed dosing, a significant reduction in the mean retinal arteriolar diameter was observed that stabilized by day 90 and persisted after the study endpoint. In another clinical study, it was found that increased numbers of ranibizumab treatments correlated with specific neural retinal dysmorphic features. In this particular study, the inner segment/outer segment photoreceptor junction was often not visible using optical coherence tomography in patients receiving more frequent ranibizumab administrations, suggesting that retinal damage might occur with increased anti-VEGF-A exposure. Finally, in a study analyzing the efficacy of IV-TA versus that of IV-B for central retinal vein occlusion, minor differences in BCVA at 1 year were evident, yet ME did resolve with IV-TA and persisted with IV-B, which was significant at 6 months and persisted at 1 year [64]. However, in this particular study, final BCVA only differed in that 50% more patients lost 2 or more lines in the IV-B group compared to the IV-TA group. At this time, many questions remain as to whether anti-VEGF-A therapy results in a clinically relevant loss of critical retinal cells, but this important issue requires more critical analysis.
Conclusion
ME is the result of the molecular, cellular and anatomic alterations due to the multifactorial nature of retinal vascular diseases. Its pathogenesis involves the contribution of several molecular and physiological processes including inflammation, vascular permeability, angiogenesis and apoptosis. The two mainstays of treatment currently being utilized are grid/focal laser photocoagulation and IV-TA. As of late, many clinicians are resorting to VEGF-A antagonists for refractory cases of ME. Whereas laser treatment suppresses vascular permeability, and VEGF-A blockade inhibits vascular permeability and angiogenesis and likely leads to increased apoptosis, the only current therapy that addresses all of the upstream mechanisms known to be critical to the formation of ME are corticosteroids. Nonetheless, while we continue to challenge ourselves in the pursuit of an improved and targeted therapy to more directly treat the problem, corticosteroids will remain a potent, efficacious and widely available therapy for this epidemic that we are faced with.
Conflicts of Interest
The authors do not have any conflict of interest that may be relevant to the present work.
References
- 1.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47(suppl 2):S253–S262. doi: 10.1016/s0039-6257(02)00387-9. [DOI] [PubMed] [Google Scholar]
- 2.Daniele LL, Adams RH, Durante DE, Pugh EN Jr, Philp NJ. Novel distribution of junctional adhesion molecule-C in the neural retina and retinal pigment epithelium. J Comp Neurol. 2007;505:166–176. doi: 10.1002/cne.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo Y, Fukuhara M, Weitzman M, Rizzolo LJ. Expression of JAM-A, AF-6, PAR-3 and PAR-6 during the assembly and remodeling of RPE tight junctions. Brain Res. 2006;1110:55–63. doi: 10.1016/j.brainres.2006.06.059. [DOI] [PubMed] [Google Scholar]
- 4.Erickson KK, Sundström JM, Antonetti DA. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103–117. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- 5.Yanoff M, Fine BS, Brucker AJ, Eagle RC., Jr Pathology of human cystoid macular edema. Surv Ophthalmol. 1984;28(suppl):505–511. doi: 10.1016/0039-6257(84)90233-9. [DOI] [PubMed] [Google Scholar]
- 6.Fine BS, Brucker AJ. Macular edema and cystoid macular edema. Am J Ophthalmol. 1981;92:466–481. doi: 10.1016/0002-9394(81)90638-3. [DOI] [PubMed] [Google Scholar]
- 7.Harhaj NS, Felinski EA, Wolpert EB, et al. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–5115. doi: 10.1167/iovs.06-0322. [DOI] [PubMed] [Google Scholar]
- 8.Kaji Y, Usui T, Ishida S, et al. Inhibition of diabetic leukostasis and blood-retinal barrier breakdown with a soluble form of a receptor for advanced glycation end products. Invest Ophthalmol Vis Sci. 2007;48:858–865. doi: 10.1167/iovs.06-0495. [DOI] [PubMed] [Google Scholar]
- 9.Tso MO, Shih CY. Experimental macular edema after lens extraction. Invest Ophthalmol Vis Sci. 1977;16:381–392. [PubMed] [Google Scholar]
- 10.Cunha-Vaz J, Faria de Abreu JR, Campos AJ. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975;59:649–656. doi: 10.1136/bjo.59.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonetti DA, Barber AJ, Khin S, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- 12.Ozaki H, Hayashi H, Vinores SA, et al. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res. 1997;64:505–517. doi: 10.1006/exer.1996.0239. [DOI] [PubMed] [Google Scholar]
- 13.Vinores SA, Derevjanik NL, Ozaki H, Okamoto N, Campochiaro PA. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc Ophthalmol. 1999;97:217–228. doi: 10.1023/a:1002136712070. [DOI] [PubMed] [Google Scholar]
- 14.Forrester JV, Liversidge J, Dua HS, et al. Experimental autoimmune uveoretinitis: a model system for immunointervention – a review. Curr Eye Res. 1992;11(suppl):33–40. doi: 10.3109/02713689208999509. [DOI] [PubMed] [Google Scholar]
- 15.Caspi RR, Roberge FG, Chan CC, et al. A new model of autoimmune disease: experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–1495. [PubMed] [Google Scholar]
- 16.Ferrick MR, Thurau SR, Oppenheim MH, et al. Ocular inflammation stimulated by intravitreal interleukin-8 and interleukin-1. Invest Ophthalmol Vis Sci. 1991;32:1534–1539. [PubMed] [Google Scholar]
- 17.Bamforth SD, Lightman S, Greenwood J. The effect of TNF-alpha and IL-6 on the permeability of the rat blood-retinal barrier in vivo. Acta Neuropathol. 1996;91:624–632. doi: 10.1007/s004010050476. [DOI] [PubMed] [Google Scholar]
- 18.Sirois MG, Edelman ER. VEGF effect on vascular permeability is mediated by synthesis of platelet-activating factor. Am J Physiol. 1997;272:H2746–H2756. doi: 10.1152/ajpheart.1997.272.6.H2746. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed A, Dearn S, Shams M, et al. Localization, quantification, and activation of platelet-activating factor receptor in human endometrium during the menstrual cycle: PAF stimulates NO, VEGF, and FAKpp125. FASEB J. 1998;12:831–843. doi: 10.1096/fasebj.12.10.831. [DOI] [PubMed] [Google Scholar]
- 20.de Lima WT, Kwasniewski FH, Sirois P, Jancar S. Studies on the mechanism of PAF-induced vasopermeability in rat lungs. Prostaglandins Leukot Essent Fatty Acids. 1995;52:245–249. doi: 10.1016/0952-3278(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 21.Mishima H, Masuda K, Miyake K. The putative role of prostaglandins in cystoid macular edema. Prog Clin Biol Res. 1989;312:251–264. [PubMed] [Google Scholar]
- 22.Abdel-Latif AA. Release and effects of prostaglandins in ocular tissues. Prostaglandins Leukot Essent Fatty Acids. 1991;44:71–82. doi: 10.1016/0952-3278(91)90186-9. [DOI] [PubMed] [Google Scholar]
- 23.Butler JM, Guthrie SM, Koc M, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noma H, Minamoto A, Funatsu H, et al. Intravitreal levels of vascular endothelial growth factor and interleukin-6 are correlated with macular edema in branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2006;244:309–315. doi: 10.1007/s00417-004-1087-4. [DOI] [PubMed] [Google Scholar]
- 25.Friedman EA, Brown CD, Berman DH. Erythropoietin in diabetic macular edema and renal insufficiency. Am J Kidney Dis. 1995;26:202–208. doi: 10.1016/0272-6386(95)90175-2. [DOI] [PubMed] [Google Scholar]
- 26.Noma H, Funatsu H, Yamasaki M, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005;140:256–261. doi: 10.1016/j.ajo.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Rothova A. Inflammatory cystoid macular edema. Curr Opin Ophthalmol. 2007;18:487–492. doi: 10.1097/ICU.0b013e3282f03d2e. [DOI] [PubMed] [Google Scholar]
- 28.Patel JI, Tombran-Tink J, Hykin PG, Gregor ZJ, Cree IA. Vitreous and aqueous concentrations of proangiogenic, antiangiogenic factors and other cytokines in diabetic retinopathy patients with macular edema: implications for structural differences in macular profiles. Exp Eye Res. 2006;82:798–806. doi: 10.1016/j.exer.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto T. Cell biology of hyalocytes. Nippon Ganka Gakkai Zasshi. 2003;107:866–882. discussion 883. [PubMed] [Google Scholar]
- 30.Wolfensberger TJ. The role of carbonic anhydrase inhibitors in the management of macular edema. Doc Ophthalmol. 1999;97:387–397. doi: 10.1023/a:1002143802926. [DOI] [PubMed] [Google Scholar]
- 31.Sebag J, Buckingham B, Charles MA, Reiser K. Biochemical abnormalities in vitreous of humans with proliferative diabetic retinopathy. Arch Ophthalmol. 1992;110:1472–1476. doi: 10.1001/archopht.1992.01080220134035. [DOI] [PubMed] [Google Scholar]
- 32.Ikuno Y, Kazlauskas A. TGF-β1-dependent contraction of fibroblasts is mediated by the PDGF-α receptor. Invest Ophthalmol Vis Sci. 2002;43:41–46. [PubMed] [Google Scholar]
- 33.Diabetic Retinopathy Clinical Research Network A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–1449. doi: 10.1016/j.ophtha.2008.06.015. 1449.e1–1449.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diabetic Retinopathy Clinical Research Network. Beck RW, Edwards AR, Aiello LP, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–251. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paccola L, Costa RA, Folgosa MS, et al. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study) Br J Ophthalmol. 2008;92:76–80. doi: 10.1136/bjo.2007.129122. [DOI] [PubMed] [Google Scholar]
- 36.Shimura M, Nakazawa T, Yasuda K, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008;145:854–861. doi: 10.1016/j.ajo.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 37.Byun YJ, Roh MI, Lee SC, Koh HJ. Intravitreal triamcinolone acetonide versus bevacizumab therapy for macular edema associated with branch retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 2010, E-pub ahead of print. [DOI] [PubMed]
- 38.Saishin Y, Takahashi K, Melia M, Vinores SA, Campochiaro PA. Inhibition of protein kinase C decreases prostaglandin-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003;195:210–219. doi: 10.1002/jcp.10238. [DOI] [PubMed] [Google Scholar]
- 39.Xu H, Dawson R, Crane IJ, Liversidge J. Leukocyte diapedesis in vivo induces transient loss of tight junction protein at the blood-retina barrier. Invest Ophthalmol Vis Sci. 2005;46:2487–2494. doi: 10.1167/iovs.04-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh DC, Bula DV, Miller JW, Gragoudas ES, Arroyo JG. Expression of leukocyte adhesion molecules in human subfoveal choroidal neovascular membranes treated with and without photodynamic therapy. Invest Ophthalmol Vis Sci. 2004;45:2368–2373. doi: 10.1167/iovs.03-0981. [DOI] [PubMed] [Google Scholar]
- 41.Xu H, Forrester JV, Liversidge J, Crane IJ. Leukocyte trafficking in experimental autoimmune uveitis: breakdown of blood-retinal barrier and upregulation of cellular adhesion molecules. Invest Ophthalmol Vis Sci. 2003;44:226–234. doi: 10.1167/iovs.01-1202. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto K, Khosrof S, Bursell SE, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci USA. 1999;96:10836–10841. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu M, Perez VL, Ma N, et al. VEGF increases retinal vascular ICAM-1 expression in vivo. Invest Ophthalmol Vis Sci. 1999;40:1808–1812. [PubMed] [Google Scholar]
- 44.Tamura H, Miyamoto K, Kiryu J, et al. Intravitreal injection of corticosteroid attenuates leukostasis and vascular leakage in experimental diabetic retina. Invest Ophthalmol Vis Sci. 2005;46:1440–1444. doi: 10.1167/iovs.04-0905. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen DB, Koch Jensen P, la Cour M, et al. Carbonic anhydrase inhibition increases retinal oxygen tension and dilates retinal vessels. Graefes Arch Clin Exp Ophthalmol. 2005;243:163–168. doi: 10.1007/s00417-003-0817-3. [DOI] [PubMed] [Google Scholar]
- 46.Gao BB, Clermont A, Rook S, et al. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007;13:181–188. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- 47.Min JK, Cho YL, Choi JH, et al. Receptor activator of nuclear factor (NF)-κB ligand (RANKL) increases vascular permeability: impaired permeability and angiogenesis in eNOS-deficient mice. Blood. 2007;109:1495–1502. doi: 10.1182/blood-2006-06-029298. [DOI] [PubMed] [Google Scholar]
- 48.Kim YM, Kim YM, Lee YM, et al. TNF-related activation-induced cytokine (TRANCE) induces angiogenesis through the activation of Src and phospholipase C (PLC) in human endothelial cells. J Biol Chem. 2002;277:6799–6805. doi: 10.1074/jbc.M109434200. [DOI] [PubMed] [Google Scholar]
- 49.Clermont AC, Cahill M, Salti H, et al. Hepatocyte growth factor induces retinal vascular permeability via MAP kinase and PI-3 kinase without altering retinal hemodynamics. Invest Ophthalmol Vis Sci. 2006;47:2701–2708. doi: 10.1167/iovs.05-0071. [DOI] [PubMed] [Google Scholar]
- 50.Jin M, Barron E, He S, Ryan SJ, Hinton DR. Regulation of RPE intercellular junction integrity and function by hepatocyte growth factor. Invest Ophthalmol Vis Sci. 2002;43:2782–2790. [PubMed] [Google Scholar]
- 51.Peng S, Adelman RA, Rizzolo LJ. VEGF and anti-VEGF drugs have minimal effects on the permeability or selectivity of RPE tight junctions. Invest Ophthalmol Vis Sci. 2010;51:3216–3225. doi: 10.1167/iovs.09-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iandiev I, Pannicke T, Reichel MB, et al. Expression of aquaporin-1 immunoreactivity by photoreceptor cells in the mouse retina. Neurosci Lett. 2005;388:96–99. doi: 10.1016/j.neulet.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 53.Donati G, Kapetanios A, Dubois-Dauphin M, Pournaras CJ. Caspase-related apoptosis in chronic ischaemic microangiopathy following experimental vein occlusion in mini-pigs. Acta Ophthalmol. 2008;86:302–306. doi: 10.1111/j.600-0420.2007.01044.x. [DOI] [PubMed] [Google Scholar]
- 54.Salaria S, Chana G, Caldera F, et al. Microarray analysis of cultured human brain aggregates following cortisol exposure: implications for cellular functions relevant to mood disorders. Neurobiol Dis. 2006;23:630–636. doi: 10.1016/j.nbd.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 55.Uckermann O, Kutzera F, Wolf A, et al. The glucocorticoid triamcinolone acetonide inhibits osmotic swelling of retinal glial cells via stimulation of endogenous adenosine signaling. J Pharmacol Exp Ther. 2005;315:1036–1045. doi: 10.1124/jpet.105.092353. [DOI] [PubMed] [Google Scholar]
- 56.Pannicke T, Iandiev I, Wurm A, et al. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 2006;55:633–639. doi: 10.2337/diabetes.55.03.06.db05-1349. [DOI] [PubMed] [Google Scholar]
- 57.Wenzel A, Grimm C, Seeliger MW, et al. Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest Ophthalmol Vis Sci. 2001;42:1653–1659. [PubMed] [Google Scholar]
- 58.She H, Nakazawa T, Matsubara A, et al. Photoreceptor protection after photodynamic therapy using dexamethasone in a rat model of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2008;49:5008–5014. doi: 10.1167/iovs.07-1154. [DOI] [PubMed] [Google Scholar]
- 59.Hao W, Wenzel A, Obin MS, et al. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet. 2002;32:254–260. doi: 10.1038/ng984. [DOI] [PubMed] [Google Scholar]
- 60.Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saint-Geniez M, Maharaj AS, Walshe TE, et al. Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murakami Y, Ikeda Y, Yonemitsu Y, et al. Inhibition of choroidal neovascularization via brief subretinal exposure to a newly developed lentiviral vector pseudotyped with Sendai viral envelope proteins. Hum Gene Ther. 2009;21:199–209. doi: 10.1089/hum.2009.102. [DOI] [PubMed] [Google Scholar]
- 63.Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D’Amore PA. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA. 2009;106:18751–18756. doi: 10.1073/pnas.0905010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tao Y, Hou J, Jiang YR, Li XX, Jonas JB. Intravitreal bevacizumab vs triamcinolone acetonide for macular oedema due to central retinal vein occlusion. Eye (Lond) 2010;24:810–815. doi: 10.1038/eye.2009.220. [DOI] [PubMed] [Google Scholar]