Summary
Early psychologists, including Galton, Cattell, and Spearman proposed that intelligence and simple sensory discriminations are constrained by common neural processes, predicting a close link between them [1, 2]. However, strong supporting evidence for this hypothesis remains elusive. Although people with higher intelligence quotients (IQs) are quicker at processing sensory stimuli [1–5], these broadly replicated findings explain a relatively modest proportion of variance in IQ. Processing speed alone is, arguably, a poor match for the information processing demands on the neural system. Our brains operate on overwhelming amounts of information [6, 7], and thus their efficiency is fundamentally constrained by an ability to suppress irrelevant information [8–21]. Here, we show that individual variability in a simple visual discrimination task that reflects both processing speed and perceptual suppression [22] strongly correlates with IQ. High IQ individuals, although quick at perceiving small moving objects, exhibit disproportionately large impairments in perceiving motion as stimulus size increases. These findings link intelligence with low-level sensory suppression of large moving patterns—background-like stimuli that are ecologically less relevant [22–25]. We conjecture that the ability to suppress irrelevant and rapidly process relevant information fundamentally constrains both sensory discriminations and intelligence, providing an information-processing basis for the observed link.
Results
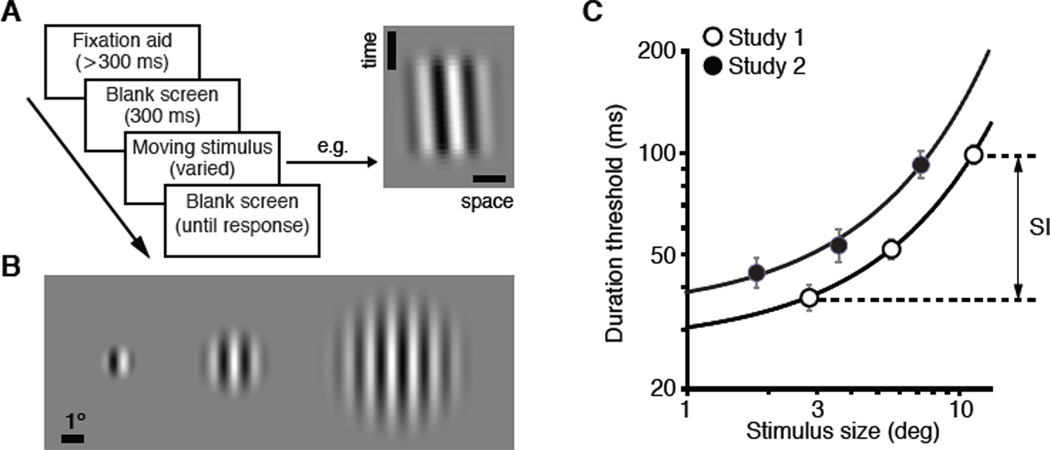
Motivated by fundamental roles of suppressive processes in neural function [8–21], we hypothesized that individual differences in a low-level visual task that reflects both processing speed and perceptual suppression should closely correlate with IQ. To estimate perceptual suppression, we used a simple visual task in which subjects identified motion direction of briefly presented grating stimuli [22] (Figure 1A; Supplemental Experimental Procedures). We adaptively adjusted stimulus duration to estimate the shortest exposure durations sufficient for threshold-level performance. This approach is analogous to conventional inspection time measures [2–4] and provides an estimate of perceptual processing speed. The critical manipulation was stimulus size (Figure 1B). We previously found that as stimulus size increases, motion direction of high contrast patterns becomes markedly harder to perceive [22] (Figure 1C). This counterintuitive result, termed spatial suppression, is believed to reflect inhibitory mechanisms that render motion selective neurons less responsive to large, background-like motion patterns; stimuli that are less likely to be perceptually relevant [22–28]. Importantly, subjects were not asked to suppress or ignore large moving stimuli, rather they were instructed to identify motion direction of each individually presented stimulus as accurately as possible. To quantify the strength of spatial suppression, we computed Suppression Index (SI) (Figure 1C), simply defined as the difference between the threshold for large stimuli and the threshold for small stimuli [27–30] (Figure 1C). Thus, SI indexes the degree of impairment in motion perception with increasing stimulus size.
Figure 1. Measurement of spatial suppression: task, stimuli and group level results.
(A) The sequence of events constituting a single trial. Subjects’ task was to simply identify motion direction of a briefly presented moving stimulus. The space-time plot illustrates a rightward moving stimulus. The depicted stimulus duration (53 ms) corresponds to the average threshold for the 3.6° stimulus size (see panel C). Vertical and horizontal scale bars are 10 ms and 1°, respectively.
(B) Three stimulus sizes used in Study 2. Only one stimulus was shown on each trial.
(C) The effect of stimulus size on duration thresholds for discriminating motion direction. Data were fit with an exponential model (a·ebx, R2 > 0.993). Slope, b, is 0.116 and 0.139 for Study 1 and Study 2, respectively. As detailed in Supplemental Experimental Procedures, raw threshold values cannot be compared across studies. Arrows illustrate the computation of SI, defined as the difference of log10 thresholds for large and small stimuli (SI = log10(large stimulus threshold) – log10(small stimulus threshold). Data are represented as mean ±SEM.
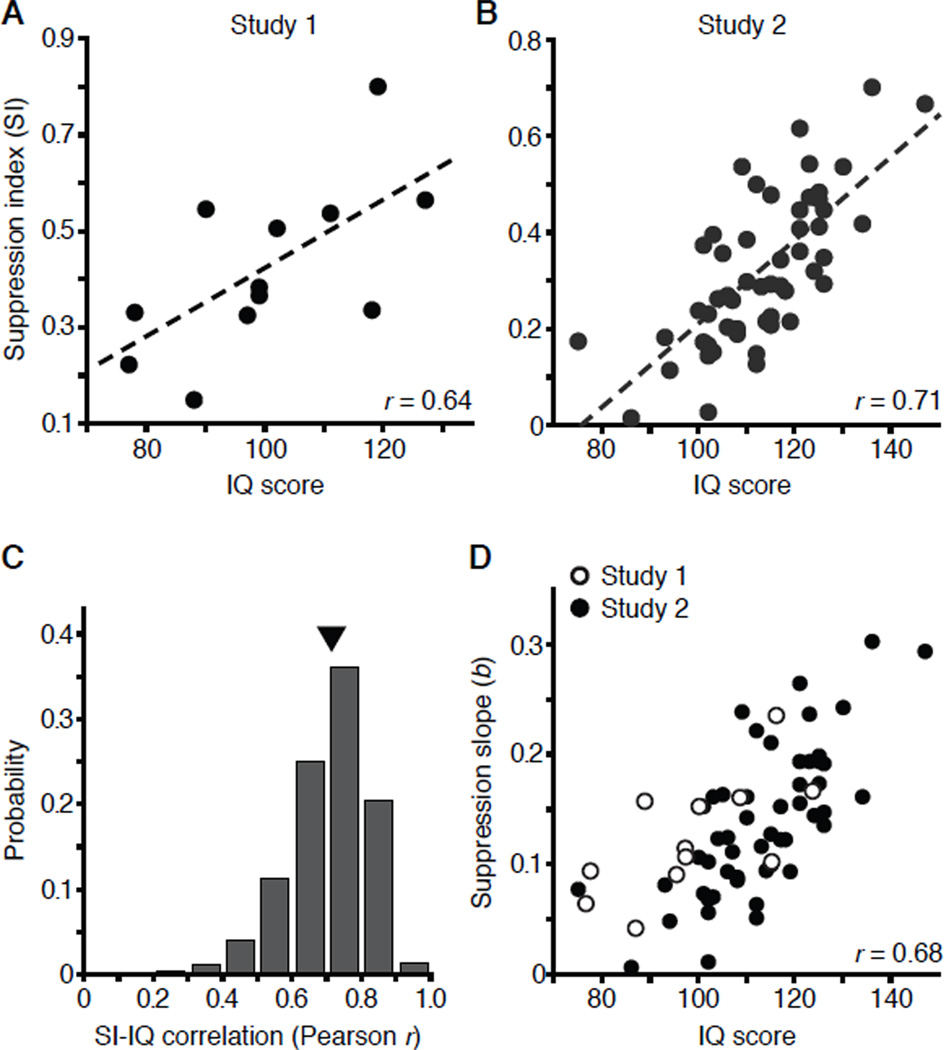
We first tested the hypothesized link between perceptual suppression and IQ in subjects who completed a short form Wechsler Adult Intelligence Scale III (WAIS-III) [31]. The results (Study 1) revealed a significant correlation between IQ and SI (Figure 2A; r = 0.64; P = 0.02). To test the robustness and replicability of this finding, in Study 2 we introduced several methodological and stimulus changes (Supplemental Experimental Procedures), including the administration of the full length WAIS-IV [32]. Again, we found that SI strongly correlates with IQ (Figure 2B; r = 0.71; P = 10−9; 95% CI = [0.55, 0.82]). The observed relationship between SI and IQ is considerably stronger than those reported for other sensory measures [2–4], and approaches in magnitude correlations between full scale IQ and WAIS-IV primary indexes (ranging between 0.72 and 0.86) [32].
Figure 2. The relationship between spatial suppression and IQ.
(A, B) The relationship between SI and IQ in two studies.
(C) Results of a Monte Carlo simulation showing the distribution of SI-IQ correlations for 9999 random samples (with n = 15) from the data shown in panel B. The triangle indicates median correlation.
(D) Combined data from two studies with suppression strength estimated from slope, b, derived from exponential fits (a·ebx) to individual subject’s data.
To test the robustness of the SI-IQ link, we carried out a Monte Carlo simulation using Study 2 data. We generated 9999 data sets, each consisting of 15 subjects randomly sampled without replacement, and computed the SI-IQ correlation for each data set. The resultant correlation distribution (Figure 2C) is positively skewed with median r = 0.72 (95% CI = [0.43, 0.89]). Notably, nearly all (93.3%) of the obtained correlations were statistically significant, indicating that a relatively small sample size is sufficient to reveal the SI-IQ link.
The two studies presented here differ in methods and stimulus parameters (Supplemental Experimental Procedures), yet both reveal strong SI-IQ links. Stimulus size differences (Figure 1C), however, preclude a direct comparison of SI values. To circumvent this problem, we fit each subject’s data with a simple exponential model (a·ebx), where the scale parameter, a, determines the lower asymptote, while the slope, b, is an estimate of suppression strength that is not explicitly linked to specific stimulus sizes (Figure 1C). Importantly, for both data sets, the exponential slope and SI are highly correlated (r > 0.996). The combined distribution of slope estimates again reveals that as the stimulus size increases, high IQ is linked with increasing motion perception impairments (Figure 2D; r = 0.68; P = 10−10; 95% CI = [0.53, 0.80]).
Next, we examined the relationship between SI and WAIS-IV index scores (Study 2). Sensory measures tend to be better predictors of the performance aspects of IQ, often exhibiting weak or no relationship with verbal intelligence [33]. In contrast, SI is a good predictor of broad intellectual ability: correlations between SI and Verbal Comprehension, Perceptual Reasoning, Working Memory and Processing Speed Indexes were r = 0.69, 0.47, 0.49, and 0.50, respectively (10−3 > P > 10−8). For the purposes of magnitude comparison, we note that these relationships are in the same range as WAIS-IV inter-index correlations (ranging between 0.45–0.64) [32]. Additionally, SI strongly correlates with the General Ability Index (r = 0.69; P = 10−8), a WAIS-IV measure of general intellectual ability. Overall, we show that SI is strongly linked with a broad range of psychometric indices of intelligence.
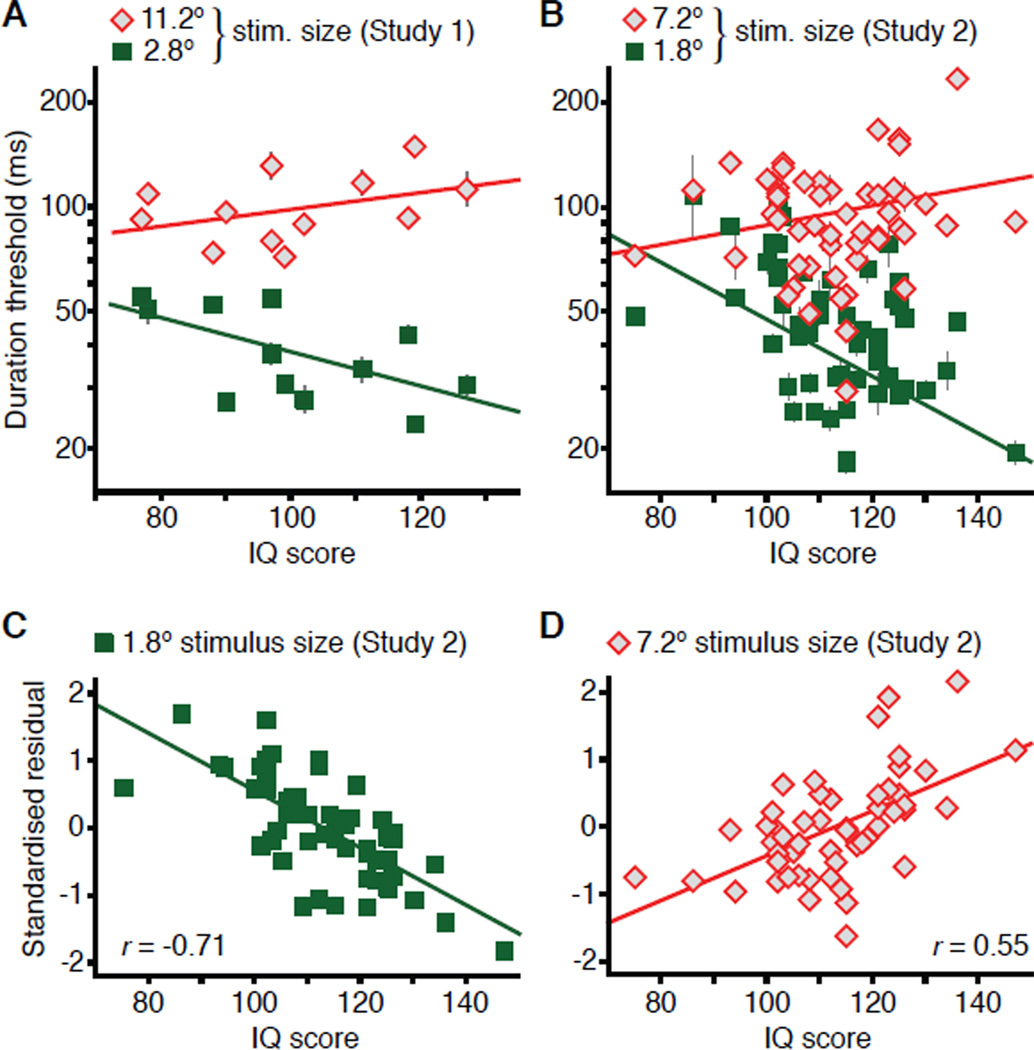
What drives the SI-IQ relationship? SI indexes the difference between one’s ability to perceive small and large moving stimuli (Figure 1C). Thus, the observed relationship indicates an interactive link between motion perception and IQ. Indeed, correlations between IQ and subjects’ ability to perceive motion of small and large stimuli were significantly different (Figure 3A, B; both z > 2.0, P < 0.04). As IQ rises, SI increases because of (a) faster processing of small stimuli coupled with (b) a diminishing ability to perceive large moving stimuli. Neither effect alone was sufficient to account for the observed SI-IQ link; only small stimulus thresholds in Study 2 significantly correlated with IQ (r = −0.46, P = 0.0005), indicating that performance with large stimuli is a key component of the SI-IQ link. Thus, we considered factors that may affect the correlation between IQ and large stimulus thresholds. The ability to perceive large moving stimuli is determined both by spatial suppression [22] and by nonspecific factors (e.g., general motion sensitivity and motivation). Such general factors tend to be positively correlated with IQ [3, 34], but they should affect motion perception regardless of stimulus size, allowing us to statistically control for nonspecific effects. First, to control for the shared variance between subjects’ performance with small and large stimuli, we computed semi-partial correlations between stimulus thresholds and IQ (Study 2). The results revealed significant, but opposite correlations between IQ and small (Figure 3C; sr = −0.71, P = 10−9) and large (Figure 3D; sr = 0.55, P = 10−5) stimulus thresholds. These results were further supported by a multiple linear regression analysis with thresholds for small and large stimuli as predictors of IQ scores (R2 = 0.52, F2,50 = 26.7, P = 10−8VIF < 1.7). High IQ was associated with lower thresholds for small moving stimuli (β = −0.92, t50 = −7.2, P = 10−9) and higher thresholds for large moving stimuli (β = 0.72, t50 = 5.6, P = 10−6). We found analogous results for the four WAIS-IV index scores (all R2 > 0.23, F2,50 > 7.55, P < 0.001), where high IQ was predicted by lower small stimulus thresholds (−0.89 < β < −0.58; all P < 0.006) and higher large stimulus thresholds (0.68 > β > 0.41; all P < 0.011). In conclusion, rather than being linked with an overall speeding of motion perception, we found that high IQ is associated with increasingly selective low-level sensory processing that favors smaller moving stimuli relative to large.
Figure 3. The relationship between motion discrimination thresholds and IQ.
(A, B) The relationship between IQ and duration thresholds for large (◇) and small (■) moving stimuli. Data are represented as mean ± SEM.
(C) The relationship between IQ (Study 2) and standardized residuals after regressing thresholds for the small stimulus on large stimulus thresholds.
(D) Same as panel B, except that thresholds for the large stimulus were regressed on small stimulus thresholds.
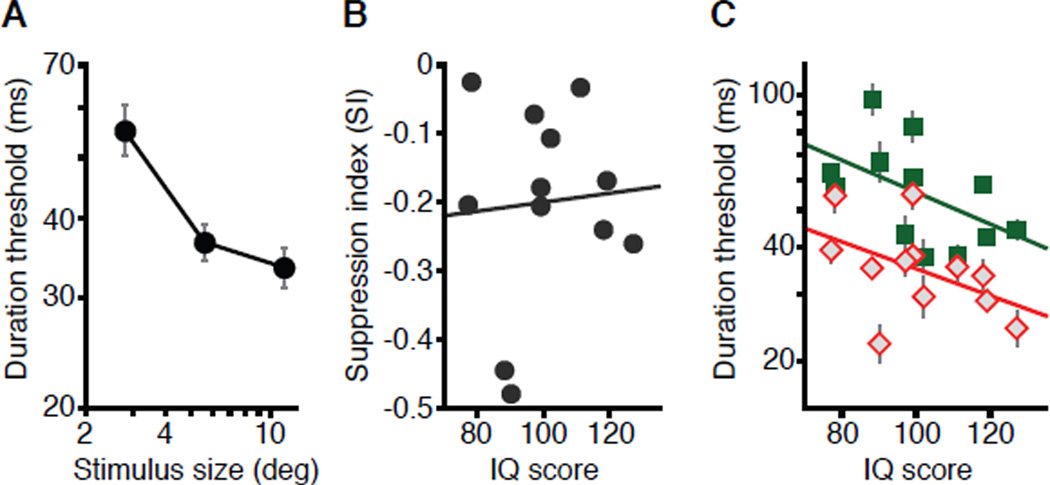
Lastly, we examined whether the observed link between motion perception and IQ is specific to suppressive processes or extends to other motion phenomena that change with increasing size. Spatial suppression is restricted to middle and high contrasts. As stimulus contrast decreases, spatial tuning of motion perception gradually shifts from spatial suppression to spatial summation [22], which is manifested as improved discriminability of low-contrast motions with increasing stimulus size [22, 35, 36]. Thus, by simply reducing stimulus contrast, we can measure perceptual discriminations under a regime largely unaffected by suppressive processes. Such low-contrast stimuli were tested in Study 1 (2.8% contrast, other methods unchanged). The results (Figure 4A) showed that as stimulus size increased, thresholds decreased (F2,22 = 20.7, P < 10−5), yielding pronounced spatial summation (i.e., negative SI). Confirming our hypothesis, spatial summation strength did not correlate with IQ (Figure 4B; r = 0.07; P = 0.82). Instead, for both large and small stimuli, subjects exhibited similar trends toward better performance with increasing IQ (Figure 4C; r = −0.48, −0.51; P = 0.10, 0.09). These results indicate that our main results (Figure 2) are specific to the suppressive effects that occur at supra-threshold contrasts.
Figure 4. The relationship between IQ and motion discrimination at low contrast.
(A) The effect of stimulus size on duration thresholds for discriminating motion direction at low contrast. Data are shown as mean ± SEM.
(B) The relationship between SI and IQ for low-contrast stimuli.
(C) The relationship between IQ and duration thresholds for large (◇) and small (■) moving stimuli at low contrast. Data are represented as mean ± SEM.
Discussion
Our findings endorse Sir Francis Galton and Charles Spearman’s original hypotheses [1] and reveal a close empirical link between sensory discriminations and intelligence. Since the early days of intelligence research, psychologists have hypothesized that sensory discriminations and intelligence are constrained by common underlying mechanisms [1, 2]. The proposed sensory correlates held promise of providing non-verbal, culture-fair measures of intelligence within a biologically constrained theoretical framework. Indeed, it is well established that IQ scores correlate with measures of inspection time: high IQ subjects require shorter stimulus exposure times to make simple perceptual judgments [1–5]. Similar results were found using reaction time measures [1–4]. These findings are intuitive—rapid information processing is important for both sensory discriminations and intelligence. However, the reported links were modest, with uncorrected correlations typically between 0.2 and 0.4 [2–4]. Still, there are indications that the underlying relationship between sensory discriminations and IQ is likely stronger than suggested by bivariate correlations. Structural equation modeling has revealed remarkably strong links (0.68 < r < 0.92) between two latent traits: general intelligence and general sensory discrimination [37, 38]. Moreover, basic sensory processing has been shown to account for intelligence variations in old age, suggesting that a common cause might underlie both cognitive and sensory declines in senescence [39].
Why do our results exceed previously documented empirical links between IQ and sensory discriminations? By using time-limited stimuli, our approach incorporates the key feature found in studies that show the most consistent empirical links between IQ and sensory tasks [5]. Our results, however, show that while processing speed is indeed related to IQ, it alone is insufficient to account for the SI-IQ link. Rather, it is the relative inability to quickly perceive large moving stimuli (i.e., SI) that predicts variations in IQ scores. This finding supports the argument that rapid processing is of limited utility unless it is restricted to the most relevant information. This is critical for any system that operates on information that exceeds its processing capacity—a description that characterizes both perception and intelligence, and suggests a possible information-processing basis for the observed relationship. However, while information relevance in the context of intelligent cognition changes depending on task demands, the implicit assumption behind our perceptual results is that the relevance of different sizes of moving stimuli is predetermined. This assumption has ecological validity given that large moving stimuli are more likely to come from typically less relevant background motion [40]. Moreover, the insensitivity to background motion is built into responses of motion selective neurons as center-surround suppression [23]. A key exception occurs at low contrast levels where weakening of suppression is exhibited by both neural center-surround mechanisms [41] and behavioral motion sensitivity [22]. Paralleling this weakening of spatial suppression, we did not find a link between SI and IQ at low contrast (Figure 4).
While our results are the first report linking sensory suppression and intelligence, the central importance of inhibition in cognitive processing is well established [12–19, 42]. Working memory performance is predicted not by neural enhancement of task-relevant information, but rather by individual differences in neural suppression of distracters [12, 13]. The ability to ignore highly distracting items in working memory predicts individual differences in intelligence [18] and can account for differences in prefrontal cortex activity between low and high IQ individuals [16, 17]. Our results, while consistent with this framework, differ in important ways by implicating a very different form of neural suppression. Our subjects were not asked to ignore distracting stimuli or inhibit a prepotent response. Instead, our approach involves a low-level motion discrimination task that likely involves inhibitory center-surround receptive field mechanisms in cortical area MT [22–27]. We also considered the possibility that attentional differences might underlie our results. If high IQ individuals were somehow less attentive to large moving stimuli, such attentional effects, even if unconscious, would implicate top-down processes. We, however, find that explanation highly unlikely. Our subjects’ only task was to discriminate motion direction of a single stimulus presented in isolation. This absence of competing stimuli along with brief stimulus durations (~100ms) precludes most top-down attentional effects [9]. In Study 2, stimulus sizes were randomly interleaved, ruling out differences in sustained attention. Additionally, when stimuli were presented at low contrast, we found a positive trend between IQ and performance with large moving stimuli (Figure 4C)—a finding inconsistent with top-down attentional biases against large stimuli. Finally, as outlined above, the behavioral results reported here are believed to reflect neural center-surround suppression [22, 25, 27]. These suppressive mechanisms are found both in awake [24, 25] and anesthetized animals [23], further ruling out a possible role of attention.
Overall, our results highlight the fundamental importance of suppression in neural processing. Suppressive mechanisms play critical roles in low-level sensory processing, where they enable our perceptual systems to efficiently process an enormous amount of incoming sensory information [8, 10, 11]. Suppression plays an analogous role in intelligent cognition [15–17, 20, 21], contributing to overall neural efficiency [43]. While above we outlined an information-processing framework for the link between perceptual suppression and intelligence, we can only speculate about underlying neural mechanisms. Based on prior work [12–19, 42], we posit that the efficacy of neural suppression could provide a mechanistic explanation of our results. However, neural suppression is not a unitary mechanism but includes a broad range of inhibitory processes. Many such processes are only weakly related with one another and only some strongly predict IQ scores [44, 45], namely measures of attentional and working memory control over distracting information [16–18]. To determine whether SI is related to these higher-level suppressive processes, we measured working memory performance using a 3-back task that incorporates highly distracting lure targets (Supplemental Experimental Procedures). Consistent with earlier results [16–18], we found that subjects’ performance on distracting lure trials was correlated with IQ scores (r = 0.55, P = 0.001), while target and non-target trial performance did not (r < 0.25, P > 0.19). Notably, SI was correlated with lure trial performance (r = 0.43, P = 0.016), but not with performance on other types of trials (r < 0.23, P > 0.22). These results link the efficacy of low-level perceptual suppression with a measure of top-down suppression that has been linked with IQ. Of course, bottom-up visual and top-down working memory suppression involve very different neural mechanisms. However, we speculate that different biological instantiations of suppression might, at least in part, depend on similar underlying computations. One possible candidate is normalization, a divisive neural computation that may underlie operations in a wide range of brain systems, ranging from perceptual suppression to decision making [8].
In conclusion, we report a strong link between low-level sensory discriminations and intelligence that is based on a simple visual task that involves reasonably well-understood neural mechanisms of motion processing, spatial suppression and evidence accumulation [22, 24, 25, 27, 46]. As such, SI provides a tractable paradigm for investigating sensory correlates of intelligence.
Supplementary Material
Highlights.
IQ scores are predicted by individual differences in sensory discriminations
High IQ is associated with motion perception impairments as stimulus size increases
The results link intelligence and low-level suppression of sensory information
Suppressive processes are a key constraint of both intelligence and perception
Acknowledgments
Funded by NIH EY019295 to D.T. and Core grant P30 EY001319. We thank Benjamin Hayden, Florian Jaeger, Steven Piantadosi, Jessica Cantlon, Philip Jaekl for comments on the manuscript and Heathman Nichols for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deary IJ. Sensory discrimination and intelligence: postmortem or resurrection. Am. J. Psychol. 1994;107:95–115. [PubMed] [Google Scholar]
- 2.Jensen AR. Clocking the mind: mental chronometry and individual differences. 1st Edition. Amsterdam: Elsevier; 2006. [Google Scholar]
- 3.Deary IJ. Intelligence. Annu. Rev. Psychol. 2012;63:453–482. doi: 10.1146/annurev-psych-120710-100353. [DOI] [PubMed] [Google Scholar]
- 4.Sheppard LD, Vernon PA. Intelligence and speed of information-processing: A review of 50 years of research. Pers. Indiv. Differ. 2008;44:535–551. [Google Scholar]
- 5.Deary IJ, McCrimmon RJ, Bradshaw J. Visual information processing and intelligence. Intelligence. 1997;24:461–479. [Google Scholar]
- 6.Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a nonsearch task. Percept. Psychophys. 1974;16:143–149. [Google Scholar]
- 7.Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends. Cogn. Sci. 2005;9:296–305. doi: 10.1016/j.tics.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat. Rev. Neurosci. 2012;13:51–62. doi: 10.1038/nrn3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu. Rev. Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz O, Simoncelli EP. Natural signal statistics and sensory gain control. Nat. Neurosci. 2001;4:819–825. doi: 10.1038/90526. [DOI] [PubMed] [Google Scholar]
- 11.Vinje WE, Gallant JL. Sparse coding and decorrelation in primary visual cortex during natural vision. Science. 2000;287:1273–1276. doi: 10.1126/science.287.5456.1273. [DOI] [PubMed] [Google Scholar]
- 12.Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nat. Neurosci. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- 13.Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. J. Neurosci. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane MJ, Bleckley MK, Conway AR, Engle RW. A controlled-attention view of working-memory capacity. J. Exp. Psychol. Gen. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- 15.Conway ARA, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends. Cogn. Sci. 2003;7:547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat. Neurosci. 2003;6:316–322. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- 17.Burgess GC, Gray JR, Conway AR, Braver TS. Neural mechanisms of interference control underlie the relationship between fluid intelligence and working memory span. J. Exp. Psychol. Gen. 2011;140:674–692. doi: 10.1037/a0024695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. J. Exp. Psychol. Gen. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- 19.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat. Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- 20.Dempster FN. Inhibitory processes: A neglected dimension of intelligence. Intelligence. 1991;15:157–173. [Google Scholar]
- 21.Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav. Brain Sci. 2007;30:135–154. doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- 22.Tadin D, Lappin JS, Gilroy LA, Blake R. Perceptual consequences of centre-surround antagonism in visual motion processing. Nature. 2003;424:312–315. doi: 10.1038/nature01800. [DOI] [PubMed] [Google Scholar]
- 23.Born RT, Tootell RBH. Segregation of global and local motion processing in primate middle temporal visual area. Nature. 1992;357:497–499. doi: 10.1038/357497a0. [DOI] [PubMed] [Google Scholar]
- 24.Born RT, Groh JM, Zhao R, Lukasewycz SJ. Segregation of object and background motion in visual area MT: Effects of microstimulation on eye movements. Neuron. 2000;26:725–734. doi: 10.1016/s0896-6273(00)81208-8. [DOI] [PubMed] [Google Scholar]
- 25.Churan J, Khawaja FA, Tsui JMG, Pack CC. Brief motion stimuli preferentially activate surround-suppressed neurons in macaque visual area MT. Curr. Biol. 2008;18:R1051–R1052. doi: 10.1016/j.cub.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Tadin D, Lappin JS. Linking psychophysics and physiology of center-surround interactions in visual motion processing. In: Jenkin MRM, Harris LR, editors. Seeing spatial form. Oxford: Oxford University Press; 2005. pp. 279–314. [Google Scholar]
- 27.Tadin D, Silvanto J, Pascual-Leone A, Battelli L. Improved motion perception and impaired spatial suppression following disruption of cortical area MT/V5. J. Neurosci. 2011;31:1279–1283. doi: 10.1523/JNEUROSCI.4121-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betts LR, Taylor CP, Sekuler AB, Bennett PJ. Aging reduces center-surround antagonism in visual motion processing. Neuron. 2005;45:361–366. doi: 10.1016/j.neuron.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 29.Tadin D, Kim J, Doop ML, Gibson C, Lappin JS, Blake R, Park S. Weakened center-surround interactions in visual motion processing in schizophrenia. J. Neurosci. 2006;26:11403–11412. doi: 10.1523/JNEUROSCI.2592-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foss-Feig JH, Tadin D, Schauder KB, Cascio CJ. A substantial and unexpected enhancement of motion perception in autism. J. Neurosci. doi: 10.1523/JNEUROSCI.1608-12.2013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Axelrod BN. Validity of the Wechsler Abbreviated Scale of Intelligence and other very short forms of estimating intellectual functioning. Assessment. 2002;9:17–23. doi: 10.1177/1073191102009001003. [DOI] [PubMed] [Google Scholar]
- 32.Psychological Corporation. WAIS-IV technical and interpretive manual. San Antonio, TX: Pearson; 2008. [Google Scholar]
- 33.Crawford JR, Deary IJ, Allan KM, Gustafsson J. Evaluating competing models of the relationship between inspection time and psychometric Intelligence. Intelligence. 1998;26:27–42. [Google Scholar]
- 34.Duckworth AL, Quinn PD, Lynam DR, Loeber R, Stouthamer-Loeber M. Role of test motivation in intelligence testing. Proc. Natl. Acad. Sci. U.S.A. 2011;108:7716–7720. doi: 10.1073/pnas.1018601108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watson AB, Turano K. The optimal motion stimulus. Vision Res. 1995;35:325–336. doi: 10.1016/0042-6989(94)00182-l. [DOI] [PubMed] [Google Scholar]
- 36.Anderson SJ, Burr DC. Spatial summation properties of directionally selective mechanisms in human vision. J. Opt. Soc. Am. A. 1991;8:1330–1339. doi: 10.1364/josaa.8.001330. [DOI] [PubMed] [Google Scholar]
- 37.Deary IJ, Bell PJ, Bell AJ, Campbell ML, Fazal ND. Sensory discrimination and intelligence: testing Spearman's other hypothesis. Am. J. Psychol. 2004;117:1–18. [PubMed] [Google Scholar]
- 38.Meyer CS, Hagmann-von Arx P, Lemola S, Grob A. Correspondence between the general ability to discriminate sensory stimuli and general intelligence. J Individ Differ. 2010;31:46–56. [Google Scholar]
- 39.Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol. Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- 40.Regan D. Human perception of objects. Sunderland, MA: Sinauer Press; 2000. [Google Scholar]
- 41.Pack CC, Hunter JN, Born RT. Contrast dependence of suppressive influences in cortical area MT of alert macaque. J. Neurophysiol. 2005;93:1809–1815. doi: 10.1152/jn.00629.2004. [DOI] [PubMed] [Google Scholar]
- 42.Ophir E, Nass C, Wagner AD. Cognitive control in media multitaskers. Proc. Natl. Acad. Sci. U.S.A. 2009;106:15583–15587. doi: 10.1073/pnas.0903620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neubauer AC, Fink A. Intelligence and neural efficiency. Neurosci. Biobehav. Rev. 2009;33:1004–1023. doi: 10.1016/j.neubiorev.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Friedman NP, Miyake A. The relations among inhibition and interference control functions: a latentvariable analysis. J. Exp. Psychol. Gen. 2004;133:101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- 45.Friedman NP, Miyake A, Corley RP, Young SE, DeFries JC, Hewitt JK. Not all executive functions are related to intelligence. Psych. Sci. 2006;17:172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- 46.Gold JI, Shadlen MN. The neural basis of decision making. Annu. Rev. Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.