Abstract
Background
Biological scaffolds must support a complex balance of resisting enzymatic degradation while promoting tissue remodeling. Thus, the purpose of this study was to evaluate the effects of in vitro enzymatic exposure on the mechanical properties of biological scaffolds. It was hypothesized that exposure to an enzyme solution would result in decreased tensile strength and that crosslinked scaffolds would resist enzymatic degradation more effectively than noncrosslinked scaffolds.
Methods
Nine scaffolds were evaluated (four porcine dermis: Permacol™, CollaMend™, Strattice™, XenMatrix™; two human dermis: AlloMax™, FlexHD®; two bovine pericardium: Veritas®, PeriGuard®; and one porcine small intestine submucosa: Surgisis™). Ten specimens (n = 10) were hydrated in saline at 37 °C and subjected to uniaxial testing to establish baseline properties. 50 specimens (n = 50) were incubated in collagenase solution at 37 °C for 2, 6, 12, 24, or 30 h (n = 10 each group) followed by uniaxial tensile testing.
Results
Tensile strength was significantly reduced after 30 h for CollaMend™, AlloMax™, Veritas®, Strattice™, XenMatrix™, Permacol™, and FlexHD® (p < 0.01), while PeriGuard® demonstrated a slight increase in tensile strength (p = 0.0188). Crosslinked bovine pericardium (PeriGuard®) maintained greater tensile strength than noncrosslinked bovine pericardium (Veritas®) throughout all exposure periods (p < 0.0001). Similarly, crosslinked porcine dermis (Permacol™) maintained greater tensile strength than non-crosslinked porcine dermis (Strattice™ and XenMatrix™) throughout all exposure periods (p < 0.0001).
Conclusions
Materials that deteriorate rapidly after in vitro enzymatic exposure may also deteriorate rapidly in vivo, particularly when exposed to a wound environment with elevated levels of matrix metalloproteinases. Permacol™, CollaMend™, Strattice™, FlexHD®, and Peri-Guard® survived the longest incubation period (30 h) and withstood mechanical testing. XenMatrix™, AlloMax™, Veritas®, and Surgisis™ degraded more quickly and did not survive the longer exposure periods. Scaffolds that maintain strength characteristics after in vitro collagenase exposure may be advantageous for long-term hernia repair scenarios where elevated enzyme levels are expected.
Keywords: Hernia repair, Biologic mesh, Tensile strength, Enzymes, Matrix metalloproteinases, Strain
Synthetic scaffolds such as polypropylene or expanded polytetrafluoroethylene are commonly utilized in hernia repair [1, 2] and urogynecological [3] applications. However, use of a synthetic material results in implantation of a permanent foreign body that elicits a chronic inflammatory response and is not remodeled by the host [4]. Biological scaffolds have recently emerged as an alternative to synthetic materials. Biological scaffolds are composed of the extracellular matrix (ECM) component of various human and animal tissues, including dermis, pericardium, and small intestine submucosa [5, 6].
The properties of these biological scaffolds are dependent upon the original species and type of tissue from which they are derived, as well as the processing that the scaffold undergoes [7]. All scaffolds undergo initial processing to render them acellular, but some are also chemically crosslinked. Crosslinking results in additional bonds between the collagen fibers of the ECM, which may improve the strength of the scaffold and/or prevent rapid loss of strength when exposed to enzymes such as collagenases and gelatinases in vivo [8].
An advantage of utilizing biological scaffolds rather than synthetic materials is that the ECM contains collagen, elastin, growth factors, and other components that encourage tissue remodeling at the repair site. Biological scaffolds should gradually degrade until all that is left behind is new, vascularized tissue [5]. Ultimately, no foreign material remains over the long term, so chronic inflammation is minimized. Another benefit is that biological scaffolds can be utilized in wounds that require rapid revascularization and clearance of bacteria [9].
Even with all of these benefits, biological scaffolds also present some major disadvantages, namely, extremely high cost compared to synthetic materials and potential early degradation of the scaffold, leading to failure of the repair [10]. Appropriate resistance to enzymatic degradation is critical because these scaffolds are often utilized in wound environments in which they are subjected to elevated levels of enzymes such as matrix metalloproteinases (MMPs) produced by inflammatory cells, predominantly neutrophils [11, 12]. The scaffolds must support a complex balance of resisting degradation and promoting cellular attachment, neotissue deposition, and angiogenesis until the repair site has healed.
MMPs are the enzymes responsible for breaking down ECM components such as collagen and elastin during normal wound healing and tissue remodeling. MMPs include collagenases and gelatinases such as MMP-1, -2, -8, and -9 [13]. Collagenases such as MMP-1 and MMP-8 are commonly detected in healing wounds [12]. MMP-1 is produced by fibroblasts and degrades type III collagen, while MMP-8 is produced by neutrophils and degrades type I collagen [14]. Gelatinases such as MMP-2 and MMP-9 are also produced by fibroblasts and neutrophils, respectively, and degrade collagen and elastin [13, 15]. Biological scaffold materials are composed of ECM, which is primarily type I and type III collagen with some elastin, growth factors, and other bioactive components.
MMPs such as collagenases and gelatinases are largely responsible for degrading biological scaffold materials in vivo. For this reason, a “crude” collagenase formulation (Sigma #C0130) was selected for the following experiments rather than a purified form of a single collagenase. This crude formulation comprised a mixture of collagenases and other proteases capable of degrading collagen [16]. It is thought that proteases and collagenases work together in vivo to remodel tissues, making this crude formulation an appropriate choice for these experiments.
As more biological scaffold materials are developed, it is critical that these materials are fully characterized for potential applications such as hernia repair and breast reconstruction. The processing that each scaffold undergoes (i.e., decellularization, crosslinking, and sterilization) plays a key role in the resulting properties of these materials and their suitability for long-term repair. Thus, the purpose of this study was to evaluate the effect of enzymatic degradation on the mechanical properties of a variety of biological scaffold materials. By investigating this effect on both crosslinked and noncrosslinked scaffolds derived from a variety of sources and types of tissues, we assessed how variables such as crosslinking and other processing conditions affect the scaffold’s ability to resist enzymatic degradation and maintain mechanical integrity. It was hypothesized that exposure to a collagenase solution would result in decreased mechanical properties over time and that crosslinked scaffolds would resist enzymatic degradation and retain initial mechanical properties more effectively than noncrosslinked scaffolds derived from the same source/type of tissue.
Identifying which variable exerts the most influence over mechanical properties following enzymatic exposure will allow surgeons to determine which biological scaffold to utilize for a particular application. Many applications such as hernia repair and breast reconstruction require a scaffold to retain its initial strength for a relatively long period of time. Other applications such as dermal substitutes or materials utilized to reinforce an incision to prevent incisional hernia may tolerate some early loss of strength [17]. A thorough comparison of the resistance of each type of scaffold to enzymatic degradation will also improve surgeons’ understanding of which scaffolds are most at risk for rapid degradation when utilized in a wound environment with elevated levels of MMPs.
Materials and methods
Scaffolds evaluated
Nine biological scaffold materials were evaluated (porcine dermis: Permacol™, CollaMend™, Strattice™, XenMatrix™; human dermis: AlloMax™, FlexHD®; bovine pericardium: Veritas®, PeriGuard®; and porcine small intestine submucosa: Surgisis™) of which three are crosslinked (Permacol™, CollaMend™, and PeriGuard®). Details about the processing of each scaffold are given in Table 1 and have been described in detail previously [18].
Table 1.
Description of biologic scaffolds
| Trade name | Manufacturer | Crosslinked | Sterilization method | |
|---|---|---|---|---|
| Porcine dermis |
Permacol | Covidien, Norwalk, CT | Yes (hexamethylene diisocyanate) | Gamma irradiation |
| CollaMend FM |
C. R. Bard/Davol, Inc., Warwick, RI | Yes [1-ethyl-(3-dimethylaminopropyl)- carbodiimide hydrochloride (EDC)] |
Ethylene oxide | |
| Strattice, Firm |
LifeCell Corp., Branchburg, NJ | No | E-beam | |
| XenMatrix | C.R. Bard/Davol, Inc., Warwick, RI | No | E-beam | |
| Human dermis |
FlexHD, Thick |
Ethicon, Inc., Somerville, NJ | No | Decontamination with ethanol and peracetic acid |
| AlloMax | C. R. Bard/Davol, Inc., Warwick, RI | No | Low-dose gamma | |
| Bovine pericardium |
PeriGuard | Synovis Surgical Innovations, St. Paul, MN |
Yes (glutaraldehyde) | Ethanol and propylene oxide |
| Veritas | Synovis Surgical Innovations, St. Paul, MN |
No | E-beam | |
| Porcine small intestine submucosa |
Surgisis Biodesign | Cook Medical, Bloomington, IN | No | Ethylene oxide |
Specimen preparation
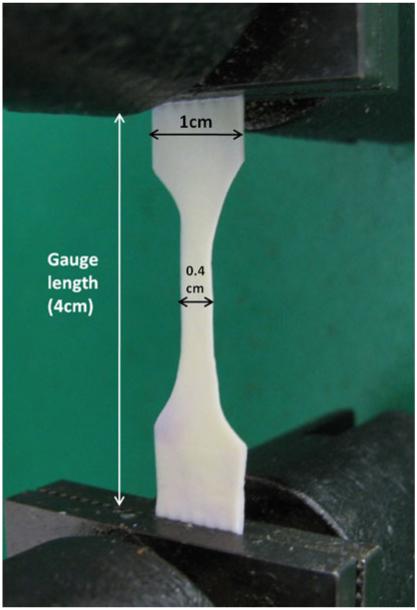
Sixty (n = 60) specimens were prepared of each scaffold type. The standard shape for a uniaxial tensile test specimen is a “dog bone” shape ~1.0 cm wide and 6.0 cm long with a narrowed central region ~0.4 cm wide and 1.5 cm long (scaled down from ASTM specification #D638-03) as shown in Fig. 1. A stainless-steel template and a scalpel were utilized to prepare each specimen to ensure consistency.
Fig. 1.
Biological scaffold specimen in the grips of the Instron® machine just prior to execution of a uniaxial tensile test
Mechanical evaluation
As described previously [19], 10 (n = 10) specimens of each scaffold were incubated in saline solution at 37 °C until fully hydrated (~2 h). These specimens comprised the “time zero” group and represent the mechanical properties of the scaffolds before implantation and without exposure to enzymes such as collagenases. 10 (n = 10) specimens of each scaffold were also incubated at 37 °C in a 0.1 M Tris buffer solution (pH 7.4) containing 0.05 M CaCl2 and 20 U/mL collagenase (Sigma #C0130) for each time point evaluated (2, 6, 12, 24, and 30 h). This particular concentration of collagenase and the duration of the incubation periods were selected based on preliminary studies (unpublished data) and do not correlate with a particular clinical scenario.
After the incubation period, the specimens were removed from the enzyme solution, blotted to remove excess solution, and subjected to uniaxial tensile testing on an Instron® 5542 material testing system (Instron, Nor-wood, MA). During uniaxial tensile testing, each specimen was oriented vertically in the Instron® machine with a 4.0-cm gauge length and ~1.0 cm of scaffold fastened in each pneumatic grip (upper and lower, 40 psi each) as shown in Fig. 1. Each specimen was then subjected to uniaxial tension at a rate of 300 mm/min until failure.
Tensile stress was calculated by dividing the load (force) that the specimen sustained (units = newton, N) during the tensile test by the cross-sectional area (units = square millimeters, mm2) of the specimen to yield tensile stress in units of megapascals (MPa), where 1 N/mm2 = 1 MPa. The cross-sectional area (mm2) of the central region of the specimen was calculated by multiplying the width of the central region (0.4 cm) by the scaffold thickness. Previously, the thickness of each scaffold was assessed in our laboratory via laser micrometry, and these values can be found in the published literature [18]. Tensile strain was also calculated by dividing the change in specimen length during the uniaxial tensile test by the original specimen length. Simply put, strain is a measure of the amount that the scaffold stretched during the tensile test. Finally, modulus of elasticity (also known as deformation modulus or Young’s modulus) was calculated by taking the slope of the line formed when stress was graphed versus strain. Modulus represents the elasticity of a material or its resistance against deformation.
Degradation of the scaffold and loss of mechanical integrity were hypothesized to result in significantly reduced tensile strength, tensile strain, and modulus over time. As the collagenase enzymes digest the collagen structure of the ECM-based scaffolds, it is intuitive that the overall strength of the scaffold would be reduced (lower stress), that the scaffold would fail more quickly without stretching as much as at baseline (lower strain), and that it would deform more easily (lower modulus) than at baseline. Crosslinking introduces additional bonds into the collagen structure of the scaffold. Thus, crosslinked scaffolds are expected to display greater initial tensile strength and modulus and lower strain compared to noncrosslinked scaffolds. It was also hypothesized that crosslinked scaffolds would more effectively resist enzymatic degradation than noncrosslinked scaffolds, resulting in less pronounced changes in mechanical properties over time.
Statistical analysis
A one-way analysis of variance (ANOVA) was performed using Systat software ver. 12.0 (Systat Software, Inc., Chicago, IL), followed by a Fisher’s LSD post-test as appropriate. Statistical significance was set at the p < 0.05 level.
Results
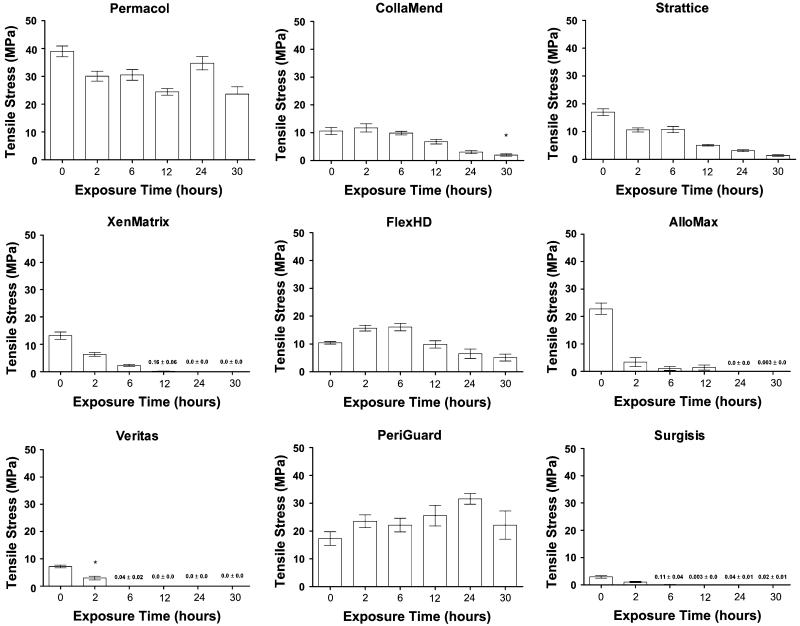
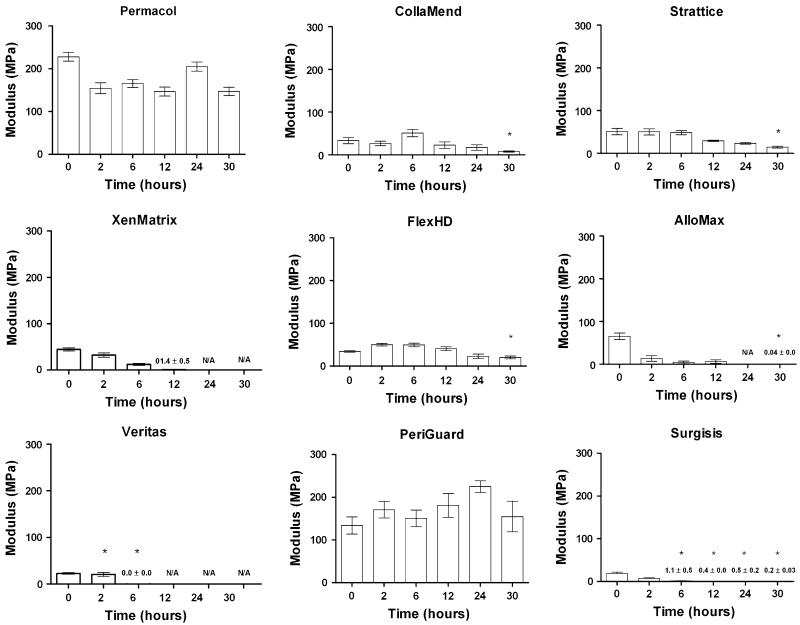
Rarely, mesh failures occurred near the grip rather than in the narrowed central region of the specimen. This occurred in one of 10 (10 %) of the Veritas® (2-h incubation only) and 1 of 10 (10 %) of the CollaMend™ (30-h incubation only) scaffolds. Data from these grip failures was excluded from statistical analysis. Values reported for these materials at these incubation times are based on nine data points rather than ten and are denoted with an asterisk above the bars in the graphs shown in Figs. 2, 3 and 4.
Fig. 2.
Tensile stress data (mean ± standard error of the mean) where asterisk indicates fewer than 10 specimens represented
Fig. 3.
Tensile strain data (mean ± standard error of the mean) where asterisk indicates fewer than 10 specimens represented and N/A indicates where a value could not be calculated due to disintegration of all specimens
Fig. 4.
Modulus data (mean ± standard error of the mean) where asterisk indicates fewer than 10 specimens represented and N/A indicates where a value could not be calculated due to disintegration of all specimens
In addition, some scaffolds were so degraded after one or many of the incubation periods that mechanical testing was impossible. Strengths of 0 MPa were recorded for these specimens, but strain and modulus could not be calculated under these circumstances. All of the Permacol™ and PeriGuard® scaffolds survived all of the incubation periods with 10 data points captured in each group, and the majority of the CollaMend™, Strattice™, and FlexHD® scaffolds survived each of the incubation periods. However, XenMatrix™, AlloMax™, Veritas®, and Surgisis™ did not survive some of the longer incubation periods. Thus, asterisks above the bars in the graphs in Figs. 2, 3 and 4 denote circumstances in which fewer than 10 data points are represented. Not available (N/A) is utilized to denote circumstances in which strain or modulus could not be calculated since the specimens were too degraded to evaluate mechanically.
Tensile stress (MPa)
Tensile stress data are depicted in Fig. 2. The maximum tensile stress that the specimen withstood before failure is referred to as “ultimate tensile strength.” Ultimate tensile strength was significantly reduced after 30 h of exposure to collagenase for CollaMend™, AlloMax™, Veritas®, Strattice™, XenMatrix™, Permacol™, and FlexHD® (p < 0.01 in all cases), while PeriGuard® demonstrated a slight increase in tensile strength (p = 0.0188), and no significant change in tensile strength was observed for Surgisis™ scaffolds. Crosslinked bovine pericardium (Peri-Guard®) maintained greater tensile strength than noncross-linked bovine pericardium (Veritas®) throughout all exposure periods (p < 0.0001). Similarly, crosslinked porcine dermis (Permacol™) maintained greater tensile strength than non-crosslinked porcine dermis (Strattice™ and XenMatrix™) throughout all exposure periods (p < 0.0001).
After the scaffolds were analyzed within each scaffold type for changes in ultimate tensile strength over time, the scaffolds were then grouped by tissue type and analyzed for differences between the ultimate tensile strength of cross-linked and noncrosslinked scaffolds of the same tissue type.
One of the crosslinked porcine dermis scaffolds (Permacol™) was significantly stronger than both non-crosslinked porcine dermis scaffolds (Strattice™ and XenMatrix™), as well as significantly stronger than the other crosslinked porcine dermis (CollaMend™) at all incubation periods evaluated (i.e., 0, 2, 6, 12, 24, and 30 h, p < 0.0001 in all cases).
The other crosslinked porcine dermis scaffold (Coll-aMend™) displayed mixed results, especially at the longer incubation times. For instance, CollaMend™ was shown to be significantly weaker than the noncrosslinked Strattice™ scaffold at time 0 (p = 0.0020). However, after exposure to collagenase, the strengths of CollaMend™ and Strattice™ were very similar at all subsequent incubation times (p >0.05 in all cases).
Prior to collagenase exposure, CollaMend™ and XenMatrix™ exhibited very similar ultimate tensile strength (p >0.05). However, CollaMend™ exhibited significantly greater ultimate tensile strength after 2 (p = 0.0090), 6 (p = 0.0003), and 12 (p = 0.0014) h of exposure compared to the values obtained for XenMatrix™. No significant differences were observed between Coll-aMend™ and XenMatrix™ after 24 or 30 h of exposure (p >0.05 in both cases).
The two noncrosslinked porcine dermis scaffolds (Strattice™ and XenMatrix™) exhibited very similar ultimate tensile strength prior to exposure to collagenase (p >0.05). However, Strattice™ exhibited significantly greater ultimate tensile strength after 2 (p = 0.0391), 6 (p < 0.0001), and 12 (p = 0.0173) h of exposure compared to the values obtained for XenMatrix™. No significant differences were observed between Strattice™ and XenMatrix™ after 24 or 30 h of exposure (p >0.05 in both cases).
The two noncrosslinked human dermis scaffolds also demonstrated significant differences between scaffold types. Initially, AlloMax™ exhibited significantly greater ultimate tensile strength than FlexHD® (p < 0.0001). However, after exposure to collagenase, FlexHD® exhibited significantly greater ultimate tensile strength than AlloMax™ after all subsequent exposure periods (p < 0.05 in all cases).
For the bovine pericardium scaffolds, the crosslinked scaffold (PeriGuard®) initially possessed significantly greater ultimate tensile strength and maintained significantly greater ultimate tensile strength throughout all of the exposure periods compared to the noncrosslinked scaffold (Veritas®, p < 0.0001 in all cases).
Tensile strain (%)
Tensile strain data are depicted in Fig. 3. Simply put, strain is a measure of the amount that the scaffold stretched during the tensile test. Tensile strain remained unchanged after exposure to collagenase for 30 h for Permacol™, AlloMax™, FlexHD®, PeriGuard®, and Surgisis™ scaffolds (p >0.05 in all cases), but the tensile strain of Strattice™ and Coll-aMend™ scaffolds was significantly reduced after 30 h of exposure to collagenase (p < 0.0001).
Once the scaffolds were analyzed within each scaffold type for changes in strain over time, the scaffolds were then grouped by tissue type and analyzed for differences in strain between crosslinked and noncrosslinked scaffolds of the same tissue type.
The crosslinked porcine dermis scaffold Permacol™ initially strained (stretched) less than both of the non-crosslinked porcine dermis scaffolds (Strattice™ and XenMatrix™). However, throughout the exposure periods, no significant differences were observed between Permacol™ and either of the noncrosslinked scaffolds except after 12 h of exposure in which Permacol™ strained more than XenMatrix™ (p = 0.0007).
The other crosslinked porcine dermis scaffold (Coll-aMend™) exhibited strain similar to that of both non-crosslinked porcine dermis scaffolds (Strattice™ and XenMatrix™) at the time of initial evaluation (i.e., time zero) prior to exposure to collagenase (p >0.05 in both cases). After 2 h of exposure, CollaMend™ strained significantly more than both Strattice™ (p = 0.0337) and XenMatrix™ (p = 0.0007). No significant differences were observed between these three scaffolds after 6 h of exposure (p >0.05 in all cases), but CollaMend™ again strained significantly more than both Strattice™ (p = 0.0079) and XenMatrix™ (p < 0.0001) after 12 h of exposure.
When comparing the two crosslinked porcine dermis (Permacol™ and CollaMend™), Permacol™ exhibited significantly lower strain than CollaMend™ throughout the early exposure periods and no difference after longer exposure periods. Significant differences were generally not observed between the two noncrosslinked porcine dermis (Strattice™ and XenMatrix™) regardless of exposure period. When comparing the two noncrosslinked human dermis scaffolds (FlexHD® and AlloMax™), no significant differences were detected between the baseline strain of these materials before exposure to collagenase (p >0.05). However, FlexHD® exhibited significantly greater strain than AlloMax™ after 2 (p = 0.0002), 6 (p < 0.0001), and 12 (p = 0.0009) h of exposure.
No significant differences were observed for the bovine pericardium scaffolds. The crosslinked bovine pericardium (PeriGuard®) exhibited significantly lower baseline strain before exposure to collagenase (p < 0.0001). However, after 2 or 6 h of exposure to collagenase, no significant differences were detected between these materials (p >0.05 in both cases).
Modulus (MPa)
Modulus data are depicted in Fig. 4. Modulus represents the elasticity of a material or its resistance against deformation. Modulus remained unchanged after 30 h of collagenase exposure for CollaMend™, XenMatrix™, AlloMax™, FlexHD®, PeriGuard®, Veritas®, and Surgisis™ scaffolds (p >0.05 in all cases). However, modulus was significantly reduced after the 30-h exposure period for both Permacol™ and Strattice™ scaffolds (p = 0.0164).
Once the scaffolds were analyzed within each scaffold type for changes in modulus over time, the scaffolds were then grouped by tissue type and analyzed for differences in modulus between crosslinked and noncrosslinked scaffolds of the same tissue type.
One of the crosslinked porcine dermis scaffolds (Permacol™) exhibited significantly greater modulus than both noncrosslinked porcine dermis scaffolds (Strattice™ and XenMatrix™) and the other crosslinked porcine dermis scaffold (CollaMend™) at all exposure periods evaluated (p < 0.0001 in all cases). CollaMend™ exhibited a modulus similar to that of both noncrosslinked porcine dermis scaffolds (Strattice™ and XenMatrix™) at all exposure times, except after 6 h for which CollaMend™ exhibited significantly higher modulus than XenMatrix™ (p = 0.0095). Similarly, the two noncrosslinked porcine dermis scaffolds (Strattice™ and XenMatrix™) exhibited similar moduli throughout all exposure periods except after 6 h for which Strattice™ exhibited significantly greater modulus than XenMatrix™ (p = 0.0158).
When comparing the two noncrosslinked human dermis scaffolds (FlexHD® and AlloMax™), FlexHD® initially exhibited significantly lower modulus than AlloMax™ after 0 h of exposure to collagenase (p = 0.0324). However, after 2, 6, and 12 h of exposure to collagenase, FlexHD® exhibited significantly greater modulus than AlloMax™ (p = 0.0132, p = 0.0025, and p = 0.0209, respectively).
For the bovine pericardium scaffolds, the crosslinked scaffold (PeriGuard®) initially possessed significantly greater modulus and maintained significantly greater modulus throughout all of the exposure periods compared to the noncrosslinked scaffold (Veritas®, p < 0.0001 in all cases).
Discussion
As more biological scaffold options are developed, it is critical that the properties of these materials and their suitability for potential applications such as hernia repair are fully characterized and understood. The processing that each scaffold undergoes (i.e., decellularization, crosslinking, and sterilization) plays a key role in the resulting scaffold properties and appropriateness for long-term repair. Decellularization is typically accomplished via chemical or enzymatic treatments to lyse the native cells present in the tissue, followed by a series of rinses to remove cellular components and debris [20]. A wide variety of decellularization protocols exist, and tissues derived from different species or different anatomical locations typically require unique combinations of decellularization agents. This information is generally withheld by manufacturers as proprietary, making it especially difficult to determine how the properties of the original tissue may have been impacted by the decellularization process. Crosslinking is an additional step that is sometimes added to the processing of biological scaffolds. Additional bonds are introduced into the collagen structure during crosslinking, improving tensile strength and resistance against enzymatic degradation. Similar to decellularization, crosslinking is accomplished through a variety of chemical agents such as carbodiimides [21-24], glutaraldehyde [25-27], and hexamethylene diisocyanate [25]. Sterilization represents the final step in the processing of biological scaffolds. Again, a variety of methods are employed, including ethanol treatment, gamma irradiation, ethylene oxide, and e-beam techniques. Of these three major steps, only crosslinking is intended to alter the original properties of the tissue that is being processed into a biological scaffold. However, it is possible that dehydration or unintentional crosslinking occurs during the decellularization and/or sterilization aspects of the process, ultimately impacting the final properties of the scaffold.
In addition to the processing variables of decellularization, crosslinking, and sterilization, scaffolds derived from different species (i.e., human, porcine, bovine), tissue types (i.e., dermis, pericardium, small intestine submucosa), or anatomical locations (i.e., back, arms, legs) exhibit a variety of properties based on their original physiological function. These differences, coupled with differences in processing techniques, lead to an abundance of variables and make it extremely difficult to determine which aspects are responsible for the final properties of the scaffold. Due to this abundance of variables, we chose to evaluate the effect of enzymatic degradation on the mechanical properties of a variety of biological scaffold materials. By evaluating multiple scaffolds of identical species/tissue type (e.g., porcine dermis) and crosslinking status (e.g., noncrosslinked), any differences observed between these particular scaffolds must be due to specific processing conditions such as decellularization and sterilization techniques unique to those scaffolds. In this way, we hoped to determine whether any other processing conditions besides crosslinking affect the scaffold’s ability to resist enzymatic degradation and maintain mechanical integrity. By investigating multiple crosslinked scaffolds of identical species/tissue type (e.g., porcine dermis), we also hoped to better determine the impact of different types of crosslinking agents on a scaffold’s ability to resist enzymatic degradation and maintain mechanical integrity.
Degradation of the scaffold and loss of mechanical integrity were hypothesized to result in significantly reduced tensile strength, tensile strain, and modulus over time. As the collagenase enzymes digest the collagen structure of the ECM-based scaffolds, it is intuitive that the overall strength of the scaffold would be reduced (lower stress), that the scaffold would fail more quickly without stretching as much as at baseline (lower strain), and that it would deform more easily (lower modulus) than at baseline. Crosslinking introduces additional bonds into the collagen structure of the scaffold. Thus, crosslinked scaffolds were expected to display greater initial tensile strength and modulus and lower strain compared to non-crosslinked scaffolds. It was also hypothesized that cross-linked scaffolds would more effectively resist enzymatic degradation than noncrosslinked scaffolds, resulting in less pronounced changes in mechanical properties over time. Scaffolds that are able to maintain their initial mechanical characteristics after prolonged exposure to a collagenase environment may be particularly advantageous for long-term repair scenarios such as hernia repair, especially when elevated enzyme levels are expected.
Beginning with the porcine dermis scaffolds, four scaffolds were evaluated, including two crosslinked (Permacol™ and CollaMend™) and two noncrosslinked (Strattice™ and XenMatrix™). Since all are derived from the same species and type of tissue, variables are limited. The only potential differences between the noncrosslinked porcine dermis scaffolds (Strattice™ and XenMatrix™) are the decellularization and sterilization techniques since neither are crosslinked. Both of these scaffolds are sterilized through e-beam techniques [18], so any differences observed between Strattice™ and XenMatrix™ scaffolds are due to the intricacies of their unique decellularization processes, which are withheld by the manufacturers as proprietary.
Differences between the other porcine dermis scaffolds (Permacol™ and CollaMend™) are more complex since these scaffolds are crosslinked with different crosslinking agents [hexamethylene diisocyanate and 1-ethyl-(3-dimethylaminopropyl)-carbodiimide hydrochloride, respectively]. Additionally, different sterilization methods are also utilized (gamma irradiation and ethylene oxide, respectively), and differences in the decellularization process are also likely but withheld by the manufacturers as proprietary [18]. Differences between Permacol™ and CollaMend™ could be due to any or all of these processing steps (i.e., decellularization, crosslinking, and sterilization).
In terms of initial mechanical properties, Permacol™ (crosslinked porcine dermis) was the strongest at time zero of all of the scaffolds evaluated, including all of the other porcine dermis scaffolds, and it remained significantly stronger than all other scaffold types over all of the exposure periods evaluated (i.e., 2, 6, 12, 24, and 30 h). Permacol™ did exhibit some slight loss of strength over time, but because it started out so much stronger than the others, it never dropped below any of the other scaffolds. Strain and modulus remained relatively unchanged for Permacol™, suggesting that Permacol™ scaffolds effectively resisted enzymatic degradation, ultimately preventing substantial changes in mechanical properties.
The other crosslinked porcine dermis scaffold (CollaMend™) was initially weaker than Permacol™ and one of the noncrosslinked porcine dermis scaffolds (Strattice™) and similar in tensile strength to the other noncrosslinked porcine dermis scaffold (XenMatrix™). However, Strattice™ and XenMatrix™ both demonstrated a pronounced loss of strength over time, while CollaMend™ remained unchanged until the later time points (24 and 30 h) when an overall significant decrease in strength was observed. Strain was significantly reduced after the 30-h exposure period, but modulus remained relatively unchanged throughout the experiment. This is intuitive since CollaMend™ is crosslinked. The additional bonds imparted during crosslinking slowed the degradation of the porcine dermis. However, Permacol™ (which is also crosslinked) still outperformed CollaMend™, indicating that the particular crosslinking agent or other aspects of the processing of the Permacol™ scaffolds (i.e., specific details of the decellularization or sterilization protocols) may allow for more effective maintenance of mechanical integrity in an environment with elevated enzyme levels.
Strattice™ and XenMatrix™ possessed similar tensile strength at time zero, but both lost a significant amount of strength over time. However, Strattice™ maintained its strength better than XenMatrix™ overall. XenMatrix™ was so degraded by 12 h that none of the specimens could be evaluated mechanically at 12, 24, or 30 hours, and the strength was recorded as 0 MPa for these exposure periods. Strattice™ scaffolds were measureable even after 30 h, demonstrating that this scaffold resisted degradation more effectively than XenMatrix™ even though neither are crosslinked. Again, the only difference between these two scaffolds is the decellularization protocol. These results indicate that some aspect of the decellularization protocol for Strattice™ improves the ability of a noncrosslinked porcine dermis material to resist enzymatic degradation and maintain initial tensile strength for a longer period of time. It is possible that unintentional crosslinking occurs during the processing of Strattice™ scaffolds, leading to improved resistance to enzymatic degradation. Strain was significantly reduced after 30 h of exposure to collagenase solution for both Strattice™ and XenMatrix™, indicating that after sufficient exposure, both noncrosslinked porcine dermis are degraded enough to impact their ability to stretch. Modulus remained relatively unchanged for XenMatrix™, but modulus was decreased overall after 30 h of exposure for Strattice™ scaffolds. However, it should be noted that the modulus for Strattice™ was initially much higher than that for XenMatrix™. Thus, even after a significant reduction, the modulus for Strattice™ was still greater than that for XenMatrix™ overall. This correlates well with what was observed for the tensile strength values and adds support to the argument that some aspect of the processing of Strattice™ impacts its mechanical properties more favorably than the processing of XenMatrix™.
Two noncrosslinked human dermis-derived scaffolds were also evaluated (FlexHD® and AlloMax™). Processing variables were limited to decellularization and “decontamination” techniques since human tissue-derived scaffolds do not require terminal sterilization. Decellularization techniques were withheld by the manufacturers as proprietary, but FlexHD® is decontaminated with an ethanol and peracetic acid solution, and AlloMax™ is subjected to a low-dose gamma treatment, making these the key differences between the scaffolds in terms of processing variables [18]. However, because these scaffolds are derived from human tissues, many other variables exist such as donor variables (i.e., age, sex, body mass index, history of smoking, history of diabetes, and other comorbidities) or anatomical harvest location (i.e., back, arms, legs). It is likely that there is a difference in the properties of dermis derived from a young, healthy, physically fit donor compared to an elderly, obese, diabetic donor or even between dermis harvested from the back versus the legs of the same donor. The abundance of variables makes it especially difficult to compare human dermis-derived scaffolds and makes it particularly frustrating to surgeons as their surgical technique cannot be altered to overcome these variables.
Although AlloMax™ initially possessed almost twice the tensile strength of FlexHD® at time zero, AlloMax™ lost a significant amount of tensile strength after only brief exposure to collagenase (i.e., 2 and 6 h) and became immeasurable at the later time points (24 and 30 h). Meanwhile, the tensile strength of FlexHD® remained relatively unchanged over time, suggesting that differences in processing variables allow FlexHD® to more effectively resist degradation than AlloMax™. Of course, donor variables such as age, sex, comorbidities, and tissue harvest variables (time of harvest as well as site) cannot be ignored. In addition, FlexHD® exhibited greater strain and greater modulus than AlloMax™ in the early exposure periods (i.e., 2, 6, and 12 h).
Two bovine pericardium-derived scaffolds were also evaluated (Veritas® and PeriGuard®). The PeriGuard® scaffold is crosslinked via glutaraldehyde treatment, while the Veritas® scaffold is not crosslinked. Sterilization occurs through e-beam treatment for the Veritas® scaffold and through ethanol and propylene oxide treatment for the PeriGuard® scaffold [18]. Decellularization is likely similar since both are derived from the same species/tissue type and are processed by the same manufacturer, although this information was withheld by the manufacturer as proprietary. Thus, differences between Veritas® and PeriGuard® are likely attributable primarily to the presence or absence of glutaraldehyde crosslinking treatment and potentially impacted somewhat by different sterilization techniques.
PeriGuard® was initially much stronger than Veritas® at time zero and maintained its initial strength, strain, and modulus characteristics after 30 h of exposure to collagenase. However, Veritas® lost a significant amount of tensile strength and ability to stretch early on and could not be evaluated mechanically after 6, 12, 24, or 30 h. These results suggest that crosslinking bovine pericardium with glutaraldehyde effectively prevents rapid enzymatic degradation and loss of mechanical integrity of bovine pericardium tissue in an in vitro environment that simulates exposure to elevated enzyme levels in vivo.
Surgisis™ was the ninth scaffold evaluated during this study. This material falls into a unique category as it is currently the only porcine small intestine submucosa— derived product on the market for hernia repair applications. This scaffold is decellularized according to a proprietary protocol, is not crosslinked, and is sterilized through ethylene oxide treatments [18]. Surgisis™ was the only scaffold that did not exhibit any significant change in tensile strength, strain, and modulus over time. However, the initial tensile strength, strain, and modulus of this material were very low (the lowest of all of the scaffolds evaluated), and it was completely degraded and immeasurable after 6, 12, 24, and 30 h. These results indicate that this scaffold may not be appropriate for clinical scenarios in which elevated enzyme levels are expected.
The results of this study revealed that, in general, crosslinking improved the ability of biological tissue-based scaffolds to resist enzymatic degradation and maintain initial mechanical properties. This was particularly evident in scaffolds such as crosslinked porcine dermis (Permacol™) and crosslinked bovine pericardium (PeriGuard®), which possessed greater initial tensile strength than their noncrosslinked counterparts. These particular scaffolds maintained their initial tensile strength after prolonged exposure to an enzymatic solution. However, the results of this study also revealed that widespread generalizations cannot be made between all noncrosslinked versus all crosslinked scaffolds. The results demonstrated substantial differences between noncrosslinked scaffolds derived from different species and types of tissues, as well as between scaffolds that differ only in the details of the decellularization protocol.
Although the results of this study are compelling, it is important to point out a few limitations. First, the experiments presented here represent an in vitro model of an extremely complex in vivo environment. It is impossible to fully capture all aspects of the in vivo environment,particularly those patient-specific factors such as comorbidities which may impact the wound-healing response and alter the environment encountered by the scaffold.
Second, the correlation between the 20-U/mL collagenase concentration used here and typical in vivo levels of collagenases has not yet been established. This level was chosen based on preliminary experiments and does not represent a particular clinical scenario.
Third, measuring the strength of these scaffolds after degradation by an enzyme solution alone ignores the mechanical strength that would be imparted during in vivo remodeling in which the patient’s cells would deposit collagen and other ECM proteins at the repair site. Scaffold degradation and neotissue deposition are complimentary in vivo, and this in vitro model does not capture these complexities. Furthermore, it was cost-prohibitive to pursue biaxial evaluation of the mechanical properties of these biological scaffolds due to the large specimen size required for ball burst testing (7.5 × 7.5 cm) versus uniaxial testing (1 × 6 cm). Ball burst testing would provide an even better approximation of the conditions of the human abdomen due to the biaxial nature of this test and should be pursued in future studies.
Fourth, the specimens were cut from the sheets of scaffolds in such a way as to maximize the number of specimens prepared from each sheet and to reduce waste of extremely costly materials. Thus, the specimens were not all oriented in the same direction. Because these scaffolds are derived from biological tissues, it is likely that the collagen fibers making up the original tissues are oriented in a particular direction due to the physiological function of the tissue or the anatomical location from which the scaffold was derived. However, this fiber alignment pattern is not readily detectable once the tissues have been processed and packaged, so no attempt was made to evaluate these effects in this study.
Finally, multiple sheets of each type of scaffold were utilized to prepare the 60 specimens needed for this study, and no attempt was made to differentiate specimens cut from one sheet versus another sheet. It is likely that the mechanical properties of the scaffolds differ slightly based on specimen orientation relative to the original fiber structure of the tissue and potentially even between different sheets or lots of the same scaffold type due to differences between donors or tissue harvest location. By not controlling these variables, the specimens in this study represent the variety of scaffold properties available to the surgeon in actual clinical experience when these scaffolds are utilized in the operating room.
Overall, this in vitro model provided the first insights into how processing and donor variables impact the mechanical properties of a variety of biological scaffolds and improved understanding of which scaffolds are most at risk for rapid degradation when utilized in a wound environment with elevated levels of MMPs.
Conclusions
Materials that deteriorate rapidly after in vitro enzymatic exposure may also deteriorate rapidly in vivo, particularly when exposed to a wound environment with elevated levels of MMPs. The results of this study demonstrated that Permacol™, CollaMend™, Strattice™, FlexHD®, and PeriGuard® survived the longest incubation period (30 h) and withstood mechanical testing at each time point in this in vitro model of enzymatic exposure. However, XenMatrix™, AlloMax™, Veritas®, and Surgisis™ degraded more quickly and did not survive the longer in vitro exposure periods.
Acknowledgments
This research was supported by research grants from Covidien (Norwalk, CT), the Musculoskeletal Transplant Foundation (Edison, NJ), the Washington University Institute for Minimally Invasive Surgery (St. Louis, MO), and an NIH/NHLBI Training Grant (5 T35 HL007815-16). Permacol™ scaffolds were donated by Covidien (Norwalk, CT). The authors also acknowledge the contributions of Evan G. Buettmann to this study.
Footnotes
A. H. Annor and M. E. Tang contributed equally to this work.
Presented at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) Annual Meeting, San Diego, CA, March 7–10, 2012.
Disclosures Dr. Deeken is a consultant for Atrium Medical Corporation and C.R. Bard/Davol, Inc., and has received honoraria from Covidien and Musculoskeletal Transplant Foundation, as well as grant support from Atrium Medical Corporation, Covidien, Kensey Nash Corporation, and Musculoskeletal Transplant Foundation. Dr. Matthews is a consultant for Atrium Medical Corporation and Ethicon, Inc. He also receives honoraria and research/equipment support from Atrium Medical Corporation, Ethicon Endo-Surgery, Karl Storz Endoscopy, Stryker Endoscopy, and W.L. Gore & Associates, Inc. Margaret M. Frisella is a consultant for Atrium Medical Corporation and receives honoraria from W.L. Gore & Associates. Afua H. Annor, Michael E. Tang, Chi Lun Pui, and Gregory C. Ebersole have no conflicts of interest or financial ties to disclose.
References
- 1.Earle DB, Mark LA. Prosthetic material in inguinal hernia repair: how do I choose? Surg Clin N Am. 2008;88:179–201. doi: 10.1016/j.suc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Coda A, Botto-Micca F, Quaglino F, Ramellini G. In vivo tissue reaction to different prosthetic materials in abdominal wall hernia repair. Hernia. 2000;4:206–211. [Google Scholar]
- 3.Jakus SM, Shapiro A, Hall CD. Biologic and synthetic graft use in pelvic surgery: a review. Obstet Gynecol Surv. 2008;63:253–266. doi: 10.1097/OGX.0b013e318166fb44. [DOI] [PubMed] [Google Scholar]
- 4.Klinge U, Klosterhalfen B, Muller M, Schumpelick V. Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg. 1999;165:665–673. doi: 10.1080/11024159950189726. [DOI] [PubMed] [Google Scholar]
- 5.Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 7.Cook JL, Fox DB, Kuroki K, Jayo M, De Deyne PG. In vitro and in vivo comparison of five biomaterials used for orthopedic soft tissue augmentation. Am J Vet Res. 2008;69:148–156. doi: 10.2460/ajvr.69.1.148. [DOI] [PubMed] [Google Scholar]
- 8.Charulatha V, Rajaram A. Influence of different cross-linking treatments on the physical properties of collagen membranes. Biomaterials. 2003;24:759–767. doi: 10.1016/s0142-9612(02)00412-x. [DOI] [PubMed] [Google Scholar]
- 9.Milburn ML, Holton LH, Chung TL, Li EN, Bochicchio GV, Goldberg NH, Silverman RP. Acellular dermal matrix compared with synthetic implant material for repair of ventral hernia in the setting of peri-operative Staphylococcus aureus implant contamination: a rabbit model. Surg Infect (Larchmt) 2008;9:433–442. doi: 10.1089/sur.2007.044. [DOI] [PubMed] [Google Scholar]
- 10.Blatnik J, Jin J, Rosen M. Abdominal hernia repair with bridging acellular dermal matrix—an expensive hernia sac. Am J Surg. 2008;196:47–50. doi: 10.1016/j.amjsurg.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Wright JB, Lam K, Buret AG, Olson ME, Burrell RE. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen. 2002;10:141–151. doi: 10.1046/j.1524-475x.2002.10308.x. [DOI] [PubMed] [Google Scholar]
- 12.Nwomeh BC, Liang HX, Cohen IK, Yager DR. MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers. J Surg Res. 1999;81:189–195. doi: 10.1006/jsre.1998.5495. [DOI] [PubMed] [Google Scholar]
- 13.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair Regen. 1998;6:127–134. doi: 10.1046/j.1524-475x.1998.60206.x. [DOI] [PubMed] [Google Scholar]
- 15.Corbel M, Boichot E, Lagente V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz J Med Biol Res. 2000;33:749–754. doi: 10.1590/s0100-879x2000000700004. [DOI] [PubMed] [Google Scholar]
- 16.Harper E. Collagenases. Annu Rev Biochem. 1980;49:1063–1078. doi: 10.1146/annurev.bi.49.070180.005215. [DOI] [PubMed] [Google Scholar]
- 17.van der Veen V, van der Wal MB, van Leeuwen MC, Ulrich MM, Middelkoop E. Biological background of dermal substitutes. Burns. 2010;36:305–321. doi: 10.1016/j.burns.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Deeken CR, Eliason BJ, Pichert MD, Grant SA, Frisella MM, Matthews BD. Differentiation of biologic scaffold materials through physicomechanical, thermal, and enzymatic degradation techniques. Ann Surg. 2012;255(3):595–604. doi: 10.1097/SLA.0b013e3182445341. [DOI] [PubMed] [Google Scholar]
- 19.Pui CL, Tang ME, Annor AH, Ebersole GC, Frisella MM, Matthews BD, Deeken CR. Effect of repetitive loading on the mechanical properties of biological scaffold materials. J Am Coll Surg. 2012 doi: 10.1016/j.jamcollsurg.2012.03.006. in press. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Damink LHHO, Dijkstra PJ, vanLuyn MJA, vanWachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765–773. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 22.Billiar K, Murray J, Laude D, Abraham G, Bachrach N. Effects of carbodiimide crosslinking conditions on the physical properties of laminated intestinal submucosa. J Biomed Mater Res. 2001;56:101–108. doi: 10.1002/1097-4636(200107)56:1<101::aid-jbm1074>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. In vitro degradation of dermal sheep collagen cross-linked using a water-soluble carbodiimide. Biomaterials. 1996;17:679–684. doi: 10.1016/0142-9612(96)86737-8. [DOI] [PubMed] [Google Scholar]
- 24.Abraham GA, Murray J, Billiar K, Sullivan SJ. Evaluation of the porcine intestinal collagen layer as a biomaterial. J Biomed Mater Res. 2000;51:442–452. doi: 10.1002/1097-4636(20000905)51:3<442::aid-jbm19>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Khor E. Methods for the treatment of collagenous tissues for bioprostheses. Biomaterials. 1997;18:95–105. doi: 10.1016/s0142-9612(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 26.HardinYoung J, Carr RM, Downing GJ, Condon KD, Termin PL. Modification of native collagen reduces antigenicity but preserves cell compatibility. Biotechnol Bioeng. 1996;49:675–682. doi: 10.1002/(SICI)1097-0290(19960320)49:6<675::AID-BIT9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Courtman DW, Errett BF, Wilson GJ. The role of crosslinking in modification of the immune response elicited against xenogenic vascular acellular matrices. J Biomed Mater Res. 2001;55:576–586. doi: 10.1002/1097-4636(20010615)55:4<576::aid-jbm1051>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]