Abstract
Little is known about the pathophysiology of intracerebral haemorrhage that occurs during anticoagulant treatment. In observational studies, investigators have reported larger haematoma volumes and worse functional outcome in these patients than in those with intracerebral haemorrhage and a normal coagulation status. The need to prevent extensive haematoma enlargement by rapid reversal of the anticoagulation seems intuitive, although no evidence is available from randomised clinical trials. New oral anticoagulants, such as the direct thrombin inhibitor dabigatran and the factor Xa inhibitor rivaroxaban, have been approved recently; however, intracerebral haemorrhage during dabigatran or rivaroxaban anticoagulation has not been characterised, and whether anticoagulation reversal can be beneficial in this scenario is unknown. In a translational approach, new experimental models have been developed to study anticoagulation-associated intracerebral haemorrhage in more detail and to test treatment strategies. Vitamin k antagonists enlarge haematoma volumes and worsen functional outcome in animal models. Rapid reversal of anticoagulation in the experimental setting prevents prolonged haematoma expansion and improves outcome. The new oral anticoagulants increase intracerbral haemorrhage volumes less than does warfarin. Haemostatic approaches that have been used for vitamin k-associated intracerebral haemorrhage also seem to be effective in intracerebral haemorrhage associated with the new anticoagulants. These experimental studies are valuable for filling gaps in knowledge, but the results need careful translation into routine clinical practice.
Introduction
The long-term use of oral anticoagulants and antithrombotic drugs for the prevention of thrombotic and thromboembolic vascular events is increasing.1 Intracerebral bleeding is the most feared complication of these treatments. At symptom onset, about 20% of all patients with acute intracerebral haemorrhage are receiving anticoagulant treatment, and up to 30% take platelet inhibitors;2,3 by contrast, only about 6% of a population with similar characteristics and without intracerebral haemorrhage were on anticoagulants and roughly 23% took platelet inhibitors, which suggests that symptomatic intracerebral haemorrhage is more common in patients using these drugs.4 Since these drugs interfere with haemostasis, the assumption that such medications are associated with larger haematoma volumes and, subsequently, a worse functional outcome seems intuitive.5–7 Consequently, the rapid reversal of anticoagulation with concentrated coagulation factors or recombinant factor VIIa and the transfusion of platelets are potential treatment options to promote haemostasis and to reduce haematoma growth.8,9 In the past few years, several clinical case series and observational studies have addressed the pathophysiology of and treatment strategies in anticoagulation-associated intracerebral haemorrhage.3 All these studies were non-randomised and each only included a few patients, which precluded adequate control for confounding factors.10 However, such confounders seem to be crucial, since patients taking anticoagulants are unlikely to be identical in terms of clinical variables, such as concomitant diseases. Furthermore, large-scale randomised trials can rarely be performed because only a small proportion of patients qualify for study inclusion.11 Thus, many questions remain unanswered, and clear clinical data with strong supportive evidence are unlikely to be available soon.
This area of research could benefit from being addressed in a translational “from-bedside-to-bench-to-bedside” approach, since a standardised and randomised experimental setting might overcome some of the limitations associated with non-randomised clinical trials.12 This Review provides an overview of experimental studies in anticoagulation-associated intracerebral haemorrhage, and discusses their findings in the context of specific clinical questions.
Pretreatment with standard oral anticoagulants (vitamin K antagonists)
Effect on haematoma volume and outcome
Vitamin K antagonists decrease the concentration in plasma of the coagulation factors II, VII, IX, and X. Warfarin and phenprocoumon are the most commonly used drugs, with half-lives in plasma of 30–45 h and 156–172 h, respectively.13 The coagulation status in patients given vitamin K antagonists is monitored by use of the prothrombin time, a global coagulation test that measures time to clot after addition of a thromboplastin reagent to citrated plasma. To adjust for inter-laboratory variation, the international normalised ratio (INR) is computed from the sample prothrombin time, a control prothrombin time, and the international sensitivity index (a measure of the sensitivity of the thromboplastin reagent to reductions in the concentration of the vitamin K-dependent clotting proteins).14
The risk of symptomatic intracerebral haemorrhage during treatment with vitamin K antagonists is thought to be higher than 0·5% per year in patients with atrial fibrillation.15 Evidence suggests that patients with intracerebral haemorrhage taking vitamin K antagonists present with a larger haematoma size than do anticoagulation-naive patients,16–18 although some studies have suggested otherwise.19 At hospital admission, most patients with anticoagulation-associated intracerebral haemorrhage have INR values within the therapeutic range.20 Whether a correlation exists between anticoagulation intensity (assessed in terms of INR values) and haematoma size is a subject of debate,8,20 although raised INR values have been associated with a higher 30-day mortality rate.21 Other clinical data support the assumption that intracerebral haemorrhage in anticoagulated patients is characterised by a higher rate of delayed haematoma expansion and by a worse clinical outcome.19,17 Some studies in patients with anticoagulant-associated intracerebral haemorrhage suggest a cerebellar predilection, which—because of the crucial location and further haematoma expansion—leads to high mortality rates.22,23 By use of brain imaging, researchers have detected fluid (non-coagulated) blood within the haematoma, an expression of insufficient coagulation, and clot formation in the absence of vitamin K-dependent coagulation factors (figure 1).24 In non-anticoagulant intracerebral haemorrhage, thrombin production within the haematoma during clot formation induces apoptosis and disruption of the blood–brain barrier.25 Warfarin-associated intracerebral haemorrhage shows smaller relative early perihaematomal oedema than does intracerebral haemorrhage occurring under normal coagulation, perhaps because of the lower concentration of thrombin in the haematoma.26
Figure 1. Representative CT scans of intracerebral haemorrhage.
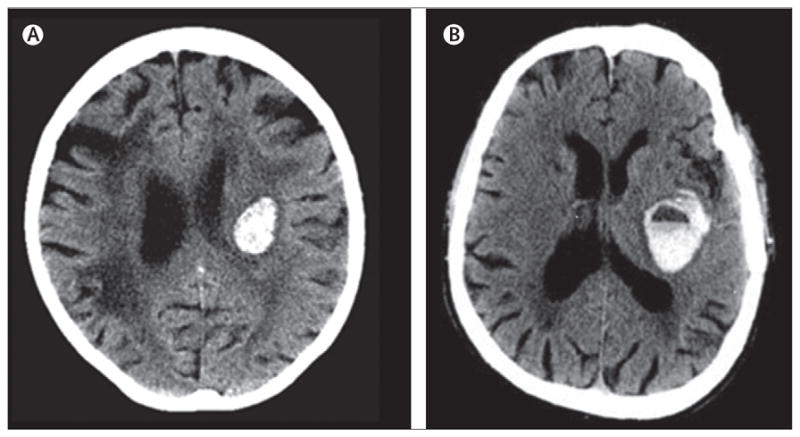
(A) This patient presented without medical history of anticoagulants. (B) This patient had raised international normalised ratio values and a positive history of vitamin K antagonist treatment at presentation. A fluid blood level is seen as a result of non-coagulated blood within the haematoma. (B) Reproduced from reference 24, by permission of the American Heart Association.
In addition to symptomatic intracerebral haemorrhage, cerebral microbleeds are also of interest in this context. There is a positive correlation between increasing rates of cerebral microbleeds and higher CHA2DS2VASc scores (these scores assess clinical attributes for prediction of embolic events in patients with atrial fibrillation), which suggests that they occur more frequently in patients with atrial fibrillation who are taking oral anticoagulants.27 Clinical data suggest that the occurrence of cerebral microbleeds in people receiving anticoagulation treatment increases the risk of anticoagulation-associated (symptomatic) intracerebral haemorrhage.28–30
An animal model of anticoagulation-associated intracerebral haemorrhage has been developed to enable study of the pathophysiology of the disease (figure 2). Mice were given warfarin dissolved in their drinking water, leading to a warfarin uptake of about 2 mg/kg per 24 h (table 1). After a 24-h feeding period, mean INR values ± SD increased from 0·8 ± 0·1 (controls) to 3·5 ± 0·9, whereas a 30-h feeding period led to supratherapeutic mean INR values of 7·2 ± 3·4.31 After warfarin withdrawal, INR values remained stable for 6 h and decreased to normal values within 18 h. A detailed analysis showed that the activities of all four vitamin K-dependent coagulation factors were reduced, mimicking full warfarin anticoagulation.34 Intracerebral haemorrhage was induced by collagenase injection, and haematoma volume was determined 24 h later with a quantitative photometric haemoglobin assay.31,39 Effective anticoagulation (mean INR 3·5 ± 0·9) increased haematoma volume by 2·5-fold, and supratherapeutic anticoagulation (mean INR 7·2 ± 3·4) by 3·1-fold (table 2). Anticoagulation also led to higher clot density in the haematoma than in controls, and non-coagulated blood was still reported in the haematoma 24 h after induction of intracerebral haemorrhage (figure 2). Although the total blood volume in the tissue (assessed by haemoglobin content measurement) increased in parallel with increasing INR values, the size of the haematoma (assessed by planimetry on brain slices) did not change consistently.31 Because clinical studies estimated the amount of blood that entered the brain by haematoma size measurements on brain imaging, this different approach could explain discrepant results for the effect of anticoagulants on bleeding volume. Additionally, strongly anticoagulated mice showed a higher rate between 2 and 24 h of haematoma enlargement than controls (controls −1·4%; anticoagulated to an INR of 3·5: +22·9%; to an INR of 7·2: +62·2%). Functional outcome was worse in anticoagulated animals than in control mice, and mortality rate increased in parallel with INR values.31
Figure 2. Collagenase-induced intracerebral haemorrhage in a murine model.
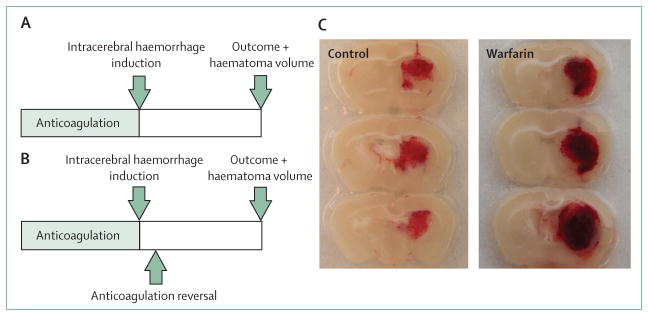
(A) Schematic experimental study protocol. After a defined period of anticoagulation, intracerebral haemorrhage is induced by stereotactic administration of collagenase into the right striatum. After the follow-up period, functional outcome and haematoma volume are measured. (B) Similar study design, but with reversal of anticoagulation after intracerebral haemorrhage induction to assess the effects of haemostatic agents. (C) Representative brain sections obtained 24 h after intracerebral haemorrhage induction in anticoagulation-naive (control) and warfarin-treated (warfarin) animals. Note larger haematoma size, higher clot density, and spots of non-coagulated blood in the warfarin-treated animal.
Table 1.
Effects on coagulation parameters of experimental anticoagulation and anticoagulation reversal
| Animal, strain | Anticoagulant | Dose | Haemostatic agent | Coagulation test and effect relative to controls* | |
|---|---|---|---|---|---|
| Vitamin K antagonists: anticoagulation | |||||
|
| |||||
| Foerch et al (2008)31 | Mice, CD-1 | Warfarin | Dose 1: 2 mg/kg per 24 h; dose 2: 2 mg/ kg/24 h for 30 h | None | PT. Dose 1: 4·4-fold increase; dose 2: 9-fold increase |
| Illanes et al (2010)32 | Mice, C57BL/6 | Warfarin | 0·4 mg/kg/24 h for 72 h | None | PT. 6·6-fold increase |
| Lauer et al (2011)33 | Mice, CD-1 | Warfarin | 2 mg/kg/24 h for 30 h | None | PT. 4·9-fold increase |
|
| |||||
| Vitamin K antagonists: anticoagulation reversal | |||||
|
| |||||
| Foerch et al (2009)34 | Mice, CD-1 | Warfarin | 2 mg/kg per 24 h | PCC 100 U/kg | PT. 4·0-fold decrease |
| Illanes et al (2011)35 | Mice, C57BL/6 | Warfarin | 0·4 mg/kg/24 h for 72 h | FFP 200 μL; PCC 100 U/kg; rFVIIa 3·5 mg/kg; rFVIIa 10 mg/kg; and TA 400 mg/kg | PT. FFP: 2·6-fold decrease; PCC: 5·1-fold decrease; rFVIIa 3·5 mg/kg: 1·5-fold decrease; rFVIIa 10 mg/kg: 1·7-fold decrease; TA: 1·15-fold decrease |
| Schlunk et al (2012)36 | Mice, CD-1 | Warfarin | 2 mg/kg per 24 h | PCC 100 U/kg; rFVIIa 1 mg/kg | PT. PCC: 3·1-fold decrease; rFVIIa: 4·7-fold decrease |
|
| |||||
| New oral anticoagulants: anticoagulation | |||||
|
| |||||
| Lauer et al (2011)33 | Mice, CD-1 | Dabigatran | Dose 1: 37·5 mg/kg p.o.; dose 2: 75·0 mg/kg p.o.; dose 3: 112·5 mg/kg p.o. | None | aPTT and dTT. Dose 1: aPTT 2·6-fold increase, dTT 6·6-fold increase; dose 2: aPTT 3·1-fold increase, dTT 7·3-fold increase; dose 3: aPTT 4·8-fold increase; dTT: 9·2-fold increase |
| Zhou et al (2011)37 | Mice, C57BL/6 | Dabigatran | Dose 1: 2·25 mg/kg i.p.; dose 2: 4·5 mg/kg i.p.; dose 3: 9 mg/kg i.p. | None | ECT and TVBT. Dose 1: ECT n.d., TVBT 1·5-fold increase; dose 2: ECT ≥7·5-fold increase, TVBT 20-fold increase; dose 3: ECT ≥7·5-fold increase; TVBT ≥20-fold increase |
| Zhou et al (2013)38 | Mice, C57BL/6 | Rivaroxaban | Dose 1: 10 mg/kg p.o.; dose 2: 30 mg/kg p.o. | None | PT. Dose 1: 2·6-fold increase; dose 2:3·5-fold increase |
|
| |||||
| New oral anticoagulants: anticoagulation reversal | |||||
|
| |||||
| Zhou et al (2011)37 | Mice, C57BL/6 | Dabigatran | 9 mg/kg i.p. | Dose 1: PCC 25 U/kg; dose 2: PCC 50 U/kg; dose 3: PCC 100 U/kg | TVBT. Dose 1: no change; dose 2: no change; dose 3: decrease |
| Zhou et al (2013)38 | Mice, C57BL/6 | Rivaroxaban | 30 mg/kg p.o. | PCC 100 U/kg; rFVIIa 1 mg/kg; and FFP 200 μL per mouse | PT. PCC: no change; rFVIIa: decrease; FFP: no change |
PT=prothrombin time. PCC=prothrombin complex concentrate. FFP=fresh frozen plasma. rFVIIa=recombinant factor VIIa. TA=tranexamic acid. p.o.=per os. aPTT=activated partial thromboplastin time. dTT=diluted thrombin time. i.p.=intraperitoneal. ECT=ecarin clotting time. TVBT=tail vein bleeding time. n.d.=not determined.
Controls were either non-anticoagulated (sham-treated) animals or anticoagulated animals that received sham treatment for anticoagulation reversal.
Table 2.
Effects on haematoma volumes of experimental anticoagulation and anticoagulation reversal
| Experimental model | Animal, strain | Anticoagulant | Dose | Haemostatic agent | Effect on haemorrhage volumes relative to controls* | |
|---|---|---|---|---|---|---|
| Vitamin K antagonists: anticoagulation | ||||||
|
| ||||||
| Foerch et al,31 (2008) | Collagenase injection | Mice, CD-1 | Warfarin | Dose 1: 2 mg/kg per 24 h; dose 2: 2 mg/kg/24 h for 30 h | None | Dose 1: increase; dose 2: large increase |
| Illanes et al,32 (2010) | Collagenase injection | Mice, C57BL/6 | Warfarin | 0·4 mg/kg/24 h for 72 h | None | Increase |
| Lauer et al,33 (2011) | Laser-induced haemorrhage | Mice, CD-1 | Warfarin | 2 mg/kg/24 h for 30 h | None | Increase |
|
| ||||||
| Vitamin K antagonists: reversal of anticoagulation | ||||||
|
| ||||||
| Foerch et al,34(2009) | Collagenase injection | Mice, CD-1 | Warfarin | 2 mg/kg per 24 h | PCC 100 U/kg | Decrease |
| Illanes et al,35 (2011) | Collagenase injection | Mice, C57BL/6 | Warfarin | 0·4 mg/kg/24 h for 72 h | FFP 200 μL; PCC 100 U/kg; rFVIIa 3·5mg/kg; rFVIIa 10 mg/kg; TA 400 mg/kg | FFP: large decrease; PCC: large decrease; rFVIIa (both doses): decrease; TA: decrease |
| Schlunk et al,36 (2012) | Collagenase injection | Mice, CD-1 | Warfarin | 2 mg/kg per 24 h | PCC 100 U/kg; rFVIIa 1 mg/kg | PCC: decrease; rFVIIa: decrease |
|
| ||||||
| New oral anticoagulants: anticoagulation | ||||||
|
| ||||||
| Lauer et al,33 (2011) | Collagenase injection | Mice, CD-1 | Dabigatran | Dose 1: 37·5 mg/kg p.o.; dose 2: 112·5 mg/kg p.o. | None | No change at either dose |
| Lauer et al,33 (2011) | Laser-induced haemorrhage | Mice, CD-1 | Dabigatran | 75 mg/kg p.o. | None | No change |
| Zhou et al,37 (2011) | Collagenase injection | Mice, C57BL/6 | Dabigatran | Dose 1: 2·25 mg/kg i.p.; dose 2: 4·5 mg/kg i.p.; dose 3: 9 mg/kg i.p. | None | Dose 1: no change; dose 2: increase; dose 3: large increase |
| Zhou et al,38 (2013) | Collagenase injection | Mice, C57BL/6 | Rivaroxaban | Dose 1: 10 mg/kg p.o.; dose 2: 30 mg/kg p.o. | None | Dose 1: no change; dose 2: increase |
|
| ||||||
| New oral anticoagulants: reversal of anticoagulation | ||||||
|
| ||||||
| Zhou et al,37 (2011) | Collagenase injection | Mice, C57BL/6 | Dabigatran | Dose 1: 4·5 mg/kg i.p.; dose 2: 9 mg/kg i.p. | FFP 200 μL; PCC 100 U/kg; rFVIIa 8 mg/kg | Dose 1 and FFP: decrease; dose 1 and PCC: large decrease; dose 1 and rFVIIa: no change. Dose 2 and FFP: no change; dose 2 and PCC: decrease; dose 2 and rFVIIa: no change |
| Zhou et al,38 (2013) | Collagenase injection | Mice, C57BL/6 | Rivaroxaban | 30 mg/kg p.o. | FFP 200 μL; PCC 100 U/kg; rFVIIa 8 mg/kg | Decrease |
|
| ||||||
| Antiplatelet drugs: antithrombotic treatment | ||||||
|
| ||||||
| Mihara et al,40 (2005) | Collagenase injection | Guineapigs | Aspirin or FK419 | Aspirin 1 mg/kg, 3 mg/kg, or 3·2 mg/kg; FK419 0·03 mg/kg, 0·06 mg/kg, or 0·12 mg/kg | None | No change for all doses |
| Lauer et al,41 (2011) | Collagenase injection | Mice, CD-1 | Aspirin, clopidogrel, and both combined | Aspirin 60 mg/kg; clopidogrel 22·5 mg/kg; or aspirin 60 mg/kg plus clopidrogrel 22·5 mg/kg | None | No change for all doses |
PCC=prothrombin complex concentrate. FFP=fresh frozen plasma. rFVIIa=recombinant factor VIIa. TA=tranexamic acid. p.o.=per os. i.p.=intraperitoneal.
Controls were either non-anticoagulated (sham-treated) animals or anticoagulated animals that received sham treatment for anticoagulation reversal.
Illanes and colleagues32 refined this model using a different mouse strain and a lower warfarin concentration in the drinking water (0·4 mg/kg per 24 h), which led to less variation in therapeutic INR values after 72 h of pretreatment. Furthermore, the authors reported that INR measurements done with a point-of-care device were as valid as routine laboratory testing, making possible the repeated assessment of the coagulation status in anticoagulated mice. Regarding the effects of warfarin anticoagulation on haematoma volume and functional outcome after intracerebral haemorrhage induction, the data were comparable to those obtained by Foerch and colleagues.31 Anticoagulation with INR values between 4 and 6 led to a more than twofold increase in haematoma volume (as established 48 h after intracerebral haemorrhage induction by T2* MRI imaging) and an increased mortality rate. However, discrepant results were reported for haematoma expansion. In the study by Illanes and colleagues,32 maximum haematoma size was reached by 6 h after intracerebral haemorrhage induction in the anticoagulation group, but at 24 h in the control group.32 By contrast, Foerch and colleagues31 reported prolonged haematoma expansion in warfarin-treated animals, with a substantial increase in bleeding volume between 2 and 24 h. Differences in the method of intracerebral haemorrhage volume assessment might have contributed to these conflicting results.31
Some studies offer very detailed insights into the molecular differences between anticoagulation-associated intracerebral haemorrhage and intracerebral haemorrhage occurring under normal coagulation. The direct injection of heparinised (anticoagulated) autologous blood and the injection of autologous blood in combination with the thrombin inhibitor argatroban both led to reduced formation of perihaematomal oedema compared with untreated autologous blood injections in a rat and a porcine model of intracerebral haemorrhage, probably because of reduced thrombin-mediated blood–brain barrier damage.42,43 However, haemoglobin breakdown products are known to play a part in secondary brain injury after intracerebral haemorrhage.5 Raised concentrations of haem and iron resulting from increased haematoma volumes in anticoagulation-associated intracerebral haemorrhage might induce more severe secondary damage cascades than those in non-coagulopathic intracerebral haemorrhage.
In an experimental model that uses tightly focused femtosecond laser pulses to injure the endothelium of targeted cerebral arterioles and trigger microbleeds,44,45 haemorrhage diameters increased by 1·7 times in mice given warfarin compared with non-anticoagulated controls (figure 3).33 However, expansion of these microhaemorrhages was only recorded during a short time window (roughly 1 min), and progress to large parenchymal bleeds was not reported.
Figure 3. In-vivo femtosecond laser-induced microhaemorrhage formation.

(A) In-vivo two-photon excited fluorescence (2PEF) image frames showing rapid expansion of the microhaemorrhage after irradiation of a penetrating arteriole about 100 μm beneath the cortical surface with a single, 800 nm wavelength, 100 fs duration, roughly 1 μJ energy laser pulse. Laser energy is only absorbed in the focal volume, leading to damage to and rupture of the vessel wall, but with no direct laser damage to surrounding tissue, thus producing a model of cortical microhaemorrhage. Fluorescently labelled blood plasma is red. The blood plasma and red blood cells (seen as dark shadows in the sea of fluorescent plasma) are pushed into the brain parenchyma. Neurons and astrocytes are labelled green with Oregon green BAPTA. (B) In-vivo 2PEF image stacks of fluorescently labelled blood plasma spanning a 20 μm depth centred at the microhaemorrhage origin in warfarin-treated animals and controls. Extravasated plasma is detected as diffuse fluorescence, in a halo surrounding the target vessel. The dark core immediately adjacent to the target vessel is filled with red blood cells. The warfarin-treated animal has a larger haematoma volume than the control. Image (A) reproduced from reference 46, by permission of OSA, the Optical Society.
In summary, strong experimental evidence suggests that effective anticoagulation with vitamin K antagonists leads to a pronounced enlargement of intracerebral haemorrhage volume, and that evidence is in line with several clinical studies. Animal data suggest that the method of haematoma volume measurement is crucial and is probably responsible for contradictory findings between clinical studies. Furthermore, experimental studies support the assumption that anticoagulation-associated intracerebral haemorrhage is characterised by poor functional outcome and prolonged haematoma expansion. Cerebral microbleeds do enlarge to some extent with vitamin K antagonist pretreatment, but have not been reported to expand into large parenchymal haemorrhages.
Efficacy of rapid anticoagulation reversal
The prolonged haematoma expansion attributed to anticoagulation-associated intracerebral haemorrhage provides a pathophysiological rationale for rapid haemostatic treatment.19,47 Treatment options for anticoagulation reversal include vitamin K, fresh frozen plasma, prothrombin complex concentrate, and recombinant factor VIIa. No evidence from randomised trials is yet available on whether or not the rapid reversal of anticoagulation in patients with acute anticoagulation-associated intracerebral haemorrhage improves functional outcome.24,48 Moreover, researchers have assessed the feasibility of a randomised trial on the efficacy of anticoagulation reversal in terms of clinical outcome, but the numbers needed to detect the assumed effect would be too high to allow such a trial to be undertaken.11 Observational studies based on few patients have focused on surrogate endpoints. The results suggest that prothrombin complex concentrate corrects increased INR values more rapidly than does fresh frozen plasma, whereas recombinant factor VIIa corrects INR values more reliably than does prothrombin complex concentrate.49–51 Furthermore, in patients with anticoagulation-associated intracerebral haemorrhage with elevated INR at hospital admission, the administration of prothrombin complex concentrate reduces INR values and haematoma volume and improves functional outcome.17,52 Different types of prothrombin complex concentrate are available, containing variable amounts of either three or four vitamin K-dependent coagulation factors. The four-factor concentrates contain factor VIIa and seem to correct INR most reliably.53 However, administration of recombinant factor VIIa alone does not increase concentrations of factors II, IX, or X. In non-anticoagulated patients with intracerebral haemorrhage, recombinant factor VIIa treatment did not have a beneficial effect on functional outcome, although it did reduce haematoma size.54
Animal models of intracerebral haemorrhage have been used to assess whether the rapid reversal of anticoagulation can prevent haematoma growth and poor functional outcome (figure 2). In a first approach, concentrated amounts of the vitamin K-dependent coagulation factors II, VII, IX, and X (prothrombin complex concentrate) were given to warfarin-treated mice 45 min after intracerebral haemorrhage induction. This resulted in substantially increased plasma activity of these factors and in normalised INR values. Haematoma volumes at 24 h were reduced by roughly 58%, and mortality rates were lower than those of saline-treated animals.34 In a similar investigation, fresh frozen plasma given systemically 30 min after intracerebral haemorrhage induction was as effective as prothrombin complex concentrate in reducing haematoma volumes, despite a higher volume load and a less pronounced INR reduction.35 Reduction of INR was reported to be a good predictor of reduced haematoma size. In contrast to prothrombin complex concentrate reversing anticoagulation by replacement of coagulation factors, recombinant factor VIIa at the site of injury is thought to lead to localised enhancement of thrombin generation and to a more stable clot structure.55 In non-anticoagulated rats with intracerebral haemorrhage, recombinant factor VIIa has been shown to reduce haematoma volumes, compared with animals without a haemostatic treatment.56 However, under effective anticoagulation, recombinant factor VIIa was far less powerful than prothrombin complex concentrate in reducing intracerebral haemorrhage volumes and INR values.35 Conflicting results have been reported from a study in which even though a lower dose was given (1 mg/kg vs 10 mg/kg), recombinant factor VIIa was just as effective as prothrombin complex concentrate in reducing haematoma growth and correcting INR values.36 The reason for this discrepancy is not clear. The timepoint of drug administration after intracerebral haemorrhage induction differs in these two studies, which could be a crucial variable. Additionally, the assessment of haematoma volume by photometric quantification of haemoglobin content as done in the second study might have been more sensitive than MRI planimetry to the effects of recombinant factor VIIa.
In summary, clinical studies in anticoagulated people showed that prothrombin complex concentrate and recombinant factor VIIa are efficacious in rapid correction of INR. Randomised experimental studies suggest that the rapid reversal of anticoagulation in acute intracerebral haemorrhage could prevent extensive haematoma formation and improve functional outcome. In that respect, data for prothrombin complex concentrate are more consistent than for recombinant factor VIIa.
Pretreatment with newly approved oral anticoagulants
Effect on haematoma volume and outcome
New anticoagulants with a steady bioavailability through oral delivery have been developed to overcome many of the disadvantages associated with vitamin K antagonists, such as the numerous drug and food interactions and the interindividual variations in drug response that require continuous laboratory monitoring. For example, the direct thrombin inhibitor dabigatran reversibly binds to the active site of the thrombin molecule, thereby inhibiting clot growth, platelet activation, and, indirectly, thrombin generation by preventing feedback activation loops. Dabigatran has a half-life in plasma of 12–17 h and does not require frequent coagulation monitoring in patients with healthy kidney function.57 By contrast with vitamin K antagonists, it has a very predictable pharmacological profile.58
The new anticoagulants dabigatran, rivaroxaban, and apixaban were at least as effective as warfarin in the prevention of ischaemic strokes in patients with atrial fibrillation and showed statistically significantly lower rates of intracerebral haemorrhage.59–61 Rivaroxaban, a direct factor Xa inhibitor, reduced rates of stroke or systemic embolism (absolute risk 1·7% per year) and rates of intracranial haemorrhage (0·5% per year) compared with the warfarin group (2·2% and 0·7% per year, respectively).60 Treatment with apixaban, another direct factor Xa inhibitor, resulted in reduced rates of stroke (1·19% per year) and a further reduction of haemorrhagic strokes (0·24% per year) compared with the warfarin group of the study (1·51% and 0·47% per year).61 In the RE-LY trial, dabigatran was non-inferior (110 mg) or superior (150 mg) to warfarin for the prevention of stroke in patients with non-valvular atrial fibrillation.59,62 Surprisingly, both dabigatran dose groups showed statistically significantly lower rates of intracranial haemorrhage than the warfarin group of the study. A subsequent analysis of intracranial haemorrhages in the RE-LY participants revealed notably lower rates of spontaneous intracerebral haemorrhage in patients assigned to dabigatran (150 mg 0·09% per year; 110 mg 0·08% per year) than in patients given warfarin (0·36% per year), whereas mortality in intracerebral haemorrhage was not reported to differ significantly among treatment groups.63 In the ROCKET AF trial, patients treated with rivaroxaban showed reduced rates of intracranial haemorrhage, whereas overall major bleeding rates did not differ from those in the warfarin group. Here and in the RE-LY trial, bleeding from gastrointestinal sites was more frequent in the groups given the new anticoagulants.59,60 The high concentration of these drugs at the site of absorption could be hypothesised to cause the increased rates of bleeding, and could partly explain the differences depending on the site of bleeding.
Impaired renal function could also lead to drug accumulation and an increased risk for haemorrhagic complications.64 A major concern is the scarce information about the optimum management of intracerebral haemorrhage developing during treatment with the newly approved anticoagulants.65 Additionally, specific clinical data for the effect of pretreatment with these new drugs on intracerebral haemorrhage volume, haematoma expansion, and functional outcome are scarce.
The experimental model of anticoagulation-associated intracerebral haemorrhage has been recently expanded to the new oral anticoagulants. Lauer and colleagues33 fed mice with three different doses of dabigatran (37·5 mg/kg, 75 mg/kg, and 112·5 mg/kg) with a gastric tube in 8 h intervals. This approach led to a dose-dependent prolongation of coagulation parameters (activated partial thromboplastin time) and to a pronounced prolongation of the thrombin time, which is particularly sensitive to dabigatran. Surprisingly, after induction of intracerebral haemorrhage by collagenase injection, haematoma volume in dabigatran-treated animals did not increase compared with non-anticoagulated controls. Similarly, laser-induced cerebral microbleeds were not enlarged as a result of dabigatran pretreatment, suggesting that this finding is not only linked to the collagenase model.33 Other investigators made similar observations: increased bleeding volumes were not found in mice with intracerebral haemorrhage and plasma concentrations of dabigatran below values judged as roughly five-times human therapeutic ranges.37 However, use of a parenteral application regimen of dabigatran at high doses—thereby overcoming its low bioavailability—resulted in dabigatran plasma concentrations of up to roughly ten-times human therapeutic values (about 850–1200 ng/mL peak concentrations) and prolonged the ecarin clotting time for at least 4 h, beyond the measurable range of 16·5 min. These large doses triggered an increased haematoma volume and worsened functional outcome after intracerebral haemorrhage induction.
Results from experimental studies have provided insights into the different pathophysiology of intracerebal haemorrhage occurring during warfarin or dabigatran anticoagulation. Whereas warfarin crudely affects coagulation by reducing the activity in plasma of factors II, VII, IX, and X, dabigatran directly interferes with factor IIa only,33 which might contribute to haemostasis in case of bleeding. It has been speculated that the high concentrations of tissue factor (factor III) that surround cerebral blood vessels lead to a tissue-specific coagulation activation via factor VIIa (which is not a target of direct thrombin inhibitors, but is reduced in warfarin anticoagulation).66 However, this assumption is questioned by the fact that rates of subdural haematomas were also significantly reduced in the RE-LY study.63 Interestingly, treatment with the direct thrombin inhibitor lepirudin in what was judged to be a therapeutic range did lead to increased experimental intracerebral haemorrhage volumes.33 The most striking pharmacological difference between dabigatran and lepirudin is the mode of thrombin inhibition. By contrast with lepirudin, which binds bivalently to the active site and one of two exosites, dabigatran binds only to the active site of the thrombin molecule, leaving the two exosites of thrombin available for interaction with other molecules of the haemostatic system.57 Furthermore, by contrast with the bivalent bonding by lepirudin, thrombin inhibition by dabigatran is reversible, and can be overcome in situations with increased thrombin release, such as in vascular injury.
Recently, the first data for a murine model of intracerebral haemorrhage under rivaroxaban anticoagulation were published. Rivaroxaban pretreatment (10 mg/kg and 30 mg/kg) statistically significantly prolonged the prothrombin time for several hours. No differences in intracerebral haemorrhage volumes between rivaroxaban-treated mice and controls were found for what might be judged standard doses of collagenase and rivaroxaban. However, after the size of the intracerebral bleedings was increased by injection of higher collagenase concentrations, the animals treated with the highest dose of rivaroxaban showed a statistically significant enlargement of haematomas and worse functional outcome.38
Together, these experimental studies suggest that the inhibition of only one coagulation factor (ie, with therapeutic dabigatran and rivaroxaban doses) might allow for feedback mechanisms within the clotting cascade to prevent haematoma enlargement, by contrast with anticoagulation by vitamin K antagonists. Results of in-vitro studies support this assumption, where deficiencies of the coagulation factors II, VII, and X led to delayed clot initiation, slowed clot propagation, and reduced clot strength.67 However, restoration of factor II to only a small proportion of normal concentrations resulted in clot initiation values similar to those in control plasma.
In summary, clinical studies provide strong evidence that intracerebral haemorrhage incidence rates are lower with long-term treatment with the new anticoagulants than with warfarin treatment. Very few clinical data are available that characterise intracerebral haemorrhage developing under the new anticoagulants. Mortality rates in patients with dabigatran-associated intracerebral haemorrhage were reported to be similar to those in patients with warfarin-associated intracerebral haemorrhage. Conversely, experimental studies consistently suggest that dabigatran and rivaroxaban at therapeutic doses might have a less detrimental effect on functional outcome and lead to less expansion of haematoma than warfarin. The specific and reversible inhibition of only one coagulation factor by the new anticoagulants, compared with the broad effect of vitamin K antagonists on the coagulation cascade, is probably responsible for these differences. Very high plasma concentrations of dabigatran and rivaroxaban might nevertheless be associated with large intracerebal haemorrhage volumes. Contrary to what has been shown for warfarin, cerebral microbleeds triggered during dabigatran treatment do not seem to enlarge.
Effectiveness of rapid anticoagulation reversal
No specific antidotes have been established for the new oral anticoagulants. For direct thrombin inhibitors, activated charcoal can adsorb dabigatran in vitro and can be considered early (about 1–2 h) after ingestion of the drug to prevent its absorption. Additionally, haemodialysis and haemofiltration could lead to more rapid clearance of dabigatran.68 Protamine sulphate and vitamin K are not expected to counteract the anticoagulant effects of dabigatran in the case of an acute bleeding event.69 Use of prothrombin complex concentrate infusion to increase the activity of procoagulant factors did not reduce prolonged activated partial thromboplastin time, thrombin time, or ecarin clotting time in patients given dabigatran.70 However, in the event of acute bleeding, administration of prothrombin complex concentrate and, hence, of large amounts of thrombin, might overcome direct thrombin inhibition. In healthy people, prothrombin complex concentrate could immediately reverse prolonged prothrombin time after anticoagulation with the factor Xa inhibitor rivaroxaban.70 However, reversed clotting times in factor Xa-inhibited patients do not guarantee sufficient haemostasis. To answer the question of whether haemostasis can be sufficiently restored, clinical data are warranted.71 A dabigatran-directed neutralising antibody is under development.72
Several systemic haemostatic drugs (eg, desmopressin, aprotinin, tranexamic acid, and aminocaproic acid) did not reverse prolonged surface bleeding in an animal model after treatment with a direct thrombin inhibitor.73 However, other agents are more promising: in an in-vitro thrombelastographic model, the administration of recombinant factor VIIa and activated prothrombin complex concentrate statistically significantly shortened the thrombin inhibitor-induced prolongation of clot initiation.74 In vivo, the administration of recombinant factor VIIa reduced prolonged bleeding time and blood loss in a template tail-bleeding model in rats given high doses of the thrombin inhibitor melagatran. Gavage of activated prothrombin complex concentrate was even more effective. Notably, although they partly restored sufficient haemostasis, activated prothrombin complex concentrates could not shorten the prolonged activated partial thromboplastin time.75
In the murine model of intracerebral haemorrhage during high-dose dabigatran treatment, both fresh frozen plasma and prothrombin complex concentrate reduced haematoma expansion.37 However, at an even higher tested dose of dabigatran, only prothrombin complex concentrate was effective. Additionally, prothrombin complex concentrate was also the only haemostatic drug to improve functional outcome. Administration of the coagulation factors II, VII, IX, and X (ie, prothrombin complex concentrate treatment) might improve thrombin generation by enhancing feedback loops within the coagulation cascade that overcome thrombin inhibition. In this model, recombinant factor VIIa did not reduce haematoma size. However, it could shorten the prothrombin time and reduce intracerebral haemorrhage volume in animals given rivaroxaban. Prothrombin complex concentrate and fresh frozen plasma prevented haematoma expansion at least as effectively as did recombinant factor VII, but did not significantly affect the prolonged prothrombin time.38 Prothrombin complex concentrate was given in increasing doses and a dose-dependent effect was recorded.
In summary, administration of procoagulant factors does have different effects on clotting times after direct thrombin inhibition and factor Xa inhibition in the clinical setting. Clotting times may remain prolonged after dabigatran anticoagulation, whereas the effects of rivaroxaban anticoagulation can be immediately and completely reversed. Standard coagulation tests might not be able to predict success of haemostatic treatment in thrombin inhibitor-induced anticoagulation. In the murine model, both fresh frozen plasma and prothrombin complex concentrate reduced haematoma size in intracerebral haemorrhage during supratherapeutic dabigatran and rivaroxaban anticoagulation. Although ineffective in dabigatran-treated anticoagulated animals, recombinant factor VII was able to reduce haematoma enlargement after anticoagulation with rivaroxaban. These findings suggest a potential benefit of reversal strategies and should be investigated further.
Pretreatment with antiplatelet agent
Effect on haematoma volume and outcome
Platelets have a central role in the haemostatic system. They adhere to the site of injury, aggregate, and provide a procoagulant surface for the rapid formation of a haemostatic plug.76 Thus, one can logically assume that an intracerebral haemorrhage during antiplatelet treatment will be associated with prolonged haematoma expansion and larger haematoma volumes. Several observational studies have associated the previous use of antiplatelet drugs with raised mortality rates,77,78 worse functional outcome,2 increased early haematoma growth,79 and an increased need for craniotomy.80 Furthermore, the combined treatment of aspirin and clopidogrel caused enlarged haematoma volumes and a higher mortality rate than aspirin single therapy in patients with spontaneous intracerebral haemorrhage.81 By contrast, other investigators could not show that the previous use of antiplatelet drugs was associated with increased rates of haematoma expansion or worse outcome, particularly after adjustment for confounding factors.82–85
Small studies in which the effect of platelet infusion after onset of intracerebral haemorrhage was investigated in patients with a history of antiplatelet drug use did not show a statistically significant benefit after spontaneous intracerebral haemorrhage, although platelet activity increased after platelet transfusion.86–88 A larger, randomised clinical trial investigating this subject is underway.89
The question of whether antiplatelet pretreatment increases haematoma volume and worsens functional outcome has been addressed in the mouse model of collagenase-induced intracerebral haemorrhage.41 Oral pretreatment with antiplatelet drugs (aspirin or clopidogrel) in drinking water reduced platelet activity below rates comparable to those recorded in patients with long-term platelet inhibition.90 Although collected bleeding volumes after cutting the distal tip of the animal’s tail increased in antiplatelet-treated mice, larger intracerebral haematoma volumes and a worsened functional outcome were not reported, even in the group receiving a combined treatment of aspirin and clopidogrel.41 This finding is consistent with comparable experiments done in guinea pigs using low to high aspirin doses and the glycoprotein IIb/III antagonist FK419.40 Interestingly, in both intracerebral haemorrhage models, antiplatelet pretreatment de facto affected haemostasis, in terms of ear-bleeding time or tail-bleeding volumes. This finding suggests tissue-specific and disease-specific reactions, and roles for platelets after vascular injury. Platelets provide a necessary surface for the promotion and regulation of thrombin release.91 Brain pericytes lining intracerebral blood vessels can also provide the surface and express procoagulant proteins for interaction with the prothrombinase complex. In the presence of normal concentrations of prothrombin, they contribute substantially to thrombin production.92 In acute intracerebral haemorrhage, such mechanisms could help to compensate for impaired platelet function caused by antiplatelet treatment.
In summary, experimental evidence supports those clinical studies that did not show a detrimental effect of antiplatelet pretreatment on intracerebral haemorrhage volume. Therefore, transfusion of platelets seems unlikely to affect haematoma volume and functional outcome.
Conclusions
Many confounding factors make it difficult to assess the true effects of anticoagulant and antithrombotic pretreatment on intracerebral haemorrhage in the clinical setting.11 However, the experimental setting allows for testing with a randomised design. Our Review discusses the results of preclinical research on anticoagulation-associated intracerebral haemorrhage in the context of specific clinical questions. Table 1 summarises the change in coagulation parameters achieved with different anticoagulant and antiplatelet treatments (and the effects of efforts to reverse anticoagulation) in experimental animals, whereas table 2 draws attention to the effects of these manipulations on haematoma volume in experimental intracerebral haemorrhage.
The translation of experimental findings into human beings should be done with caution. The animal models have several important shortcomings. On the one hand, the collagenase model causes easily reproducible intracerebral haemorrhage formation in the basal ganglia, which closely mimics deep intracerebral haematoma formation in human beings. On the other hand, collagenase injection is associated with chaotic and widespread destruction of vascular tissue, whereas primary intracerebral haemorrhage in human beings typically originates from a distinct lesion of a small perforating artery deep in the brain.93 Furthermore, collagenase is a metalloproteinase that itself has side-effects, such as an inflammatory reaction that can interfere with haemostasis.94 The replication of experiments with the new microhaemorrhage model, which uses laser-induced rupture of targeted cerebral arterioles,44,45 could be one way to avoid model-dependent biases in animal research. The murine and the human haemostatic system are reportedly comparable in many ways, but some molecular differences exist.95 Additionally, intracerebral bleeds occur predominantly in elderly patients with comorbidities such as arterial hypertension and diabetes,84 but most of the aforementioned studies were done in young and healthy mice. Although all these factors limit the validity of translational research with regard to specific clinical questions, we believe that important insights into the pathophysiology of anticoagulation-associated intracerebral haemorrhage and into potential treatment strategies can be derived successfully from these models.
In the future, further refinement and adjustment of the experimental models of anticoagulation-associated intracerebral haemorrhage will be necessary to improve their translational utility. A big step forward would be to use animal models in which intracerebral haemorrhage occurs spontaneously, with no need to induce bleeding with the highly artificial collagenase injection or by laser rupture of a vessel. For example, stroke-prone spontaneous hypertensive rats develop intracerebral haemorrhage after being fed a salt-enriched diet.96 These animals can be put on long-term anticoagulation to study the risk for, behaviour of, and outcome after intracerebral haemorrhage. Use of the existing intracerebral haemorrhage models in aged animals, or animal models of typical comorbidities found in human beings, such as diabetes or hypertension, would also represent important progress. More experimental studies of apixaban, dabigatran, and rivaroxaban are needed to identify similarities and differences in intracerebral haemorrhage pathophysiology in the three anticoagulation treatments. Still, bleeding behaviour in the scenario of intracerebral haemorrhage is still largely unknown for these drugs, as is their response to haemostatic treatment.97 In fact, no head-to-head comparison has been done between thrombin inhibitors and factor Xa inhibitors, so an experimental study could be a first step forward. In the clinical setting, observational studies and registries are needed to assess the effects of the new anticoagulants on haemorrhage volume, haematoma expansion, mortality, and functional outcome. These studies are also needed for haemostatic approaches, which have mainly been merely transferred from reversal of the effects of vitamin K antagonists on coagulation to treating complications of new oral anticoagulants. For example, the administration of prothrombin complex concentrate is supposed to compensate for the systemic deficit of the vitamin K-dependent coagulation factors. From a pharmacological perspective, the strategy to increase factors II, VII, IX, and X by prothrombin complex concentrate or factor VII by factor VIIa does not seem to be entirely congruent with the selective inhibition of coagulation factors IIa or Xa by the new anticoagulants. Such treatment would not eliminate the active compound from the system. However, once eliminated, it might leave the system in a potentially harmful hypercoagulative state. Furthermore, haemostatic approaches and intensive blood pressure control have both shown promising results in patients with intracerebral haemorrhage; however, interaction between the two might occur in combined treatment.98 This finding might also apply to anticoagulant-associated intracerebral haemorrhage, since rheology is known to affect haemostasis.99
Despite all major limitations that should be taken into account regarding the translation of experimental findings into clinical routine, the clear trends reported here show that experimental research could help to fill knowledge gaps in areas in which the pathophysiology needs to be explored, and in situations when randomised clinical trials are difficult.
Search strategy and selection criteria.
We identified studies from a PubMed search with the following search term: “(animal OR mouse OR rat OR pig OR rabbit OR dog OR guinea pig OR cat) AND (anticoagulation OR warfarin OR dabigatran OR apixaban OR rivaroxaban OR acetyl-salicylic acid OR clopidogrel) AND (intracerebral hemorrhage OR cerebral hemorrhage)”. Only reports published in English from January, 1990, to January, 2013, were selected. Additional studies were selected from the reference lists of these articles. All publications were jointly assessed by the authors for being of relevance to the objectives of this Review.
Footnotes
Contributors
AL and CF reviewed the literature, selected relevant publications, and wrote the Review. WP reviewed the literature and wrote the Review. CBS and EHL wrote the Review.
Conflicts of interest
We declare that we have no conflicts of interest.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–75. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Stead LG, Jain A, Bellolio MF, et al. Effect of anticoagulant and antiplatelet therapy in patients with spontaneous intra-cerebral hemorrhage: does medication use predict worse outcome? Clin Neurol Neurosurg. 2010;112:275–81. doi: 10.1016/j.clineuro.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Flaherty ML. Anticoagulant-associated intracerebral hemorrhage. Semin Neurol. 2010;30:565–72. doi: 10.1055/s-0030-1268866. [DOI] [PubMed] [Google Scholar]
- 4.Vernooij MW, Haag MDM, van der Lugt A, et al. Use of antithrombotic drugs and the presence of cerebral microbleeds: the Rotterdam Scan Study. Arch Neurol. 2009;66:714–20. doi: 10.1001/archneurol.2009.42. [DOI] [PubMed] [Google Scholar]
- 5.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 6.Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–81. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 7.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–93. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 8.Aguilar MI, Hart RG, Kase CS, et al. Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc. 2007;82:82–92. doi: 10.4065/82.1.82. [DOI] [PubMed] [Google Scholar]
- 9.Mayer SA, Davis SM, Skolnick BE, et al. Can a subset of intracerebral hemorrhage patients benefit from hemostatic therapy with recombinant activated factor VII? Stroke. 2009;40:833–40. doi: 10.1161/STROKEAHA.108.524470. [DOI] [PubMed] [Google Scholar]
- 10.Elliott J, Smith M. The acute management of intracerebral hemorrhage: a clinical review. Anesth Analg. 2010;110:1419–27. doi: 10.1213/ANE.0b013e3181d568c8. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty ML, Adeoye O, Sekar P, et al. The challenge of designing a treatment trial for warfarin-associated intracerebral hemorrhage. Stroke. 2009;40:1738–42. doi: 10.1161/STROKEAHA.108.538462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkman MA, Allan SM, Parry-Jones AR. Experimental intracerebral hemorrhage: avoiding pitfalls in translational research. J Cereb Blood Flow Metab. 2011;31:2135–51. doi: 10.1038/jcbfm.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rane A, Lindh JD. Pharmacogenetics of anticoagulants. Hum Genomics Proteomics. 2010;2010:754919. doi: 10.4061/2010/754919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsh J, Poller L. The international normalized ratio. A guide to understanding and correcting its problems. Arch Intern Med. 1994;154:282–88. doi: 10.1001/archinte.154.3.282. [DOI] [PubMed] [Google Scholar]
- 15.Friberg L, Rosenqvist M, Lip GYH. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298–307. doi: 10.1161/CIRCULATIONAHA.111.055079. [DOI] [PubMed] [Google Scholar]
- 16.Franke CL, de Jonge J, van Swieten JC, op de Coul AA, van Gijn J. Intracerebral hematomas during anticoagulant treatment. Stroke. 1990;21:726–30. doi: 10.1161/01.str.21.5.726. [DOI] [PubMed] [Google Scholar]
- 17.Huttner HB, Schellinger PD, Hartmann M, et al. Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke. 2006;37:1465–70. doi: 10.1161/01.STR.0000221786.81354.d6. [DOI] [PubMed] [Google Scholar]
- 18.Flaherty ML, Tao H, Haverbusch M, et al. Warfarin use leads to larger intracerebral hematomas. Neurology. 2008;71:1084–89. doi: 10.1212/01.wnl.0000326895.58992.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flibotte JJ, Hagan N, O’Donnell J, Greenberg SM, Rosand J. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology. 2004;63:1059–64. doi: 10.1212/01.wnl.0000138428.40673.83. [DOI] [PubMed] [Google Scholar]
- 20.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–84. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 21.Fang MC, Go AS, Chang Y, et al. Thirty-day mortality after ischemic stroke and intracranial hemorrhage in patients with atrial fibrillation on and off anticoagulants. Stroke. 2012;43:1795–99. doi: 10.1161/STROKEAHA.111.630731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flaherty ML, Haverbusch M, Sekar P, et al. Location and outcome of anticoagulant-associated intracerebral hemorrhage. Neurocrit Care. 2006;5:197–201. doi: 10.1385/NCC:5:3:197. [DOI] [PubMed] [Google Scholar]
- 23.Toyoda K, Yasaka M, Nagata K, et al. Antithrombotic therapy influences location, enlargement, and mortality from intracerebral hemorrhage. The Bleeding with Antithrombotic Therapy (BAT) Retrospective Study. Cerebrovasc Dis. 2009;27:151–59. doi: 10.1159/000177924. [DOI] [PubMed] [Google Scholar]
- 24.Dowlatshahi D, Butcher KS, Asdaghi N, et al. Poor prognosis in warfarin-associated intracranial hemorrhage despite anticoagulation reversal. Stroke. 2012;43:1812–17. doi: 10.1161/STROKEAHA.112.652065. [DOI] [PubMed] [Google Scholar]
- 25.Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38:759–62. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- 26.Levine JM, Snider R, Finkelstein D, et al. Early edema in warfarin-related intracerebral hemorrhage. Neurocrit Care. 2007;7:58–63. doi: 10.1007/s12028-007-0039-3. [DOI] [PubMed] [Google Scholar]
- 27.Song T, Kim J, Lee HS, et al. The frequency of cerebral microbleeds increases with CHADS(2) scores in stroke patients with non-valvular atrial fibrillation. Eur J Neurol. 2012 doi: 10.1111/ene.12003. published online Oct 11. [DOI] [PubMed] [Google Scholar]
- 28.Wong KS, Chan YL, Liu JY, Gao S, Lam WWM. Asymptomatic microbleeds as a risk factor for aspirin-associated intracerebral hemorrhages. Neurology. 2003;60:511–13. doi: 10.1212/01.wnl.0000046583.40125.20. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Ryu W, Roh J. Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology. 2009;72:171–76. doi: 10.1212/01.wnl.0000339060.11702.dd. [DOI] [PubMed] [Google Scholar]
- 30.Lovelock CE, Cordonnier C, Naka H, et al. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke. 2010;41:1222–28. doi: 10.1161/STROKEAHA.109.572594. [DOI] [PubMed] [Google Scholar]
- 31.Foerch C, Arai K, Jin G, et al. Experimental model of warfarin-associated intracerebral hemorrhage. Stroke. 2008;39:3397–404. doi: 10.1161/STROKEAHA.108.517482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illanes S, Zhou W, Heiland S, Markus Z, Veltkamp R. Kinetics of hematoma expansion in murine warfarin-associated intracerebral hemorrhage. Brain Res. 2010;1320:135–42. doi: 10.1016/j.brainres.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Lauer A, Cianchetti FA, van Cott EM, et al. Anticoagulation with the oral direct thrombin inhibitor dabigatran does not enlarge hematoma volume in experimental intracerebral hemorrhage. Circulation. 2011;124:1654–62. doi: 10.1161/CIRCULATIONAHA.111.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foerch C, Arai K, van Cott EM, van Leyen K, Lo EH. Rapid reversal of anticoagulation reduces hemorrhage volume in a mouse model of warfarin-associated intracerebral hemorrhage. J Cereb Blood Flow Metab. 2009;29:1015–21. doi: 10.1038/jcbfm.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Illanes S, Zhou W, Schwarting S, Heiland S, Veltkamp R. Comparative effectiveness of hemostatic therapy in experimental warfarin-associated intracerebral hemorrhage. Stroke. 2011;42:191–95. doi: 10.1161/STROKEAHA.110.593541. [DOI] [PubMed] [Google Scholar]
- 36.Schlunk F, van Cott EM, Hayakawa K, Pfeilschifter W, Lo EH, Foerch C. Recombinant activated coagulation factor VII and prothrombin complex concentrates are equally effective in reducing hematoma volume in experimental warfarin-associated intracerebral hemorrhage. Stroke. 2012;43:246–49. doi: 10.1161/STROKEAHA.111.629360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou W, Schwarting S, Illanes S, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594–99. doi: 10.1161/STROKEAHA.111.624650. [DOI] [PubMed] [Google Scholar]
- 38.Zhou W, Zorn M, Nawroth P, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with rivaroxaban. Stroke. 2013 doi: 10.1161/STROKEAHA.112.675231. published online Jan 22. [DOI] [PubMed] [Google Scholar]
- 39.Choudhri TF, Hoh BL, Solomon RA, Connolly ES, Pinsky DJ. Use of a spectrophotometric hemoglobin assay to objectively quantify intracerebral hemorrhage in mice. Stroke. 1997;28:2296–302. doi: 10.1161/01.str.28.11.2296. [DOI] [PubMed] [Google Scholar]
- 40.Mihara K, Aoki T, Moriguchi A, et al. Prohemorrhagic and bleeding time activities of recombinant tissue plasminogen activator, heparin, aspirin, and a glycoprotein IIb/IIIa antagonist. J Neurotrauma. 2005;22:1362–73. doi: 10.1089/neu.2005.22.1362. [DOI] [PubMed] [Google Scholar]
- 41.Lauer A, Schlunk F, van Cott EM, Steinmetz H, Lo EH, Foerch C. Antiplatelet pretreatment does not increase hematoma volume in experimental intracerebral hemorrhage. J Cereb Blood Flow Metab. 2011;31:1736–42. doi: 10.1038/jcbfm.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Z, Qu F, Zhang C. Systemic administration of argatroban inhibits protease-activated receptor-1 expression in perihematomal tissue in rats with intracerebral hemorrhage. Brain Res Bull. 2011;86:235–38. doi: 10.1016/j.brainresbull.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 43.Xi G, Wagner KR, Keep RF, et al. Role of blood clot formation on early edema development after experimental intracerebral hemorrhage. Stroke. 1998;29:2580–86. doi: 10.1161/01.str.29.12.2580. [DOI] [PubMed] [Google Scholar]
- 44.Nishimura N, Schaffer CB, Friedman B, Tsai PS, Lyden PD, Kleinfeld D. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat Methods. 2006;3:99–108. doi: 10.1038/nmeth844. [DOI] [PubMed] [Google Scholar]
- 45.Rosidi NL, Zhou J, Pattanaik S, et al. Cortical microhemorrhages cause local inflammation but do not trigger widespread dendrite degeneration. PLoS One. 2011;6:e26612. doi: 10.1371/journal.pone.0026612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cianchetti FA, Nishimura N, Schaffer CB. Biomedical Optics. OSA Technical Digest; 2010. Stimulus-evoked calcium transients in somatosensory cortex are inhibited after a nearby microhemorrhage. paper JMA93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leira R, Dávalos A, Silva Y, et al. Early neurologic deterioration in intracerebral hemorrhage: predictors and associated factors. Neurology. 2004;63:461–67. doi: 10.1212/01.wnl.0000133204.81153.ac. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein JN, Thomas SH, Frontiero V, et al. Timing of fresh frozen plasma administration and rapid correction of coagulopathy in warfarin-related intracerebral hemorrhage. Stroke. 2006;37:151–55. doi: 10.1161/01.STR.0000195047.21562.23. [DOI] [PubMed] [Google Scholar]
- 49.Makris M, Greaves M, Phillips WS, Kitchen S, Rosendaal FR, Preston EF. Emergency oral anticoagulant reversal: the relative efficacy of infusions of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thromb Haemost. 1997;77:477–80. [PubMed] [Google Scholar]
- 50.Boulis NM, Bobek MP, Schmaier A, Hoff JT. Use of factor IX complex in warfarin-related intracranial hemorrhage. Neurosurgery. 1999;45:1113–18. doi: 10.1097/00006123-199911000-00020. [DOI] [PubMed] [Google Scholar]
- 51.Pinner NA, Hurdle AC, Oliphant C, Reaves A, Lobo B, Sills A. Treatment of warfarin-related intracranial hemorrhage: a comparison of prothrombin complex concentrate and recombinant activated factor VII. World Neurosurg. 2010;74:631–35. doi: 10.1016/j.wneu.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 52.Kuwashiro T, Yasaka M, Itabashi R, et al. Effect of prothrombin complex concentrate on hematoma enlargement and clinical outcome in patients with anticoagulant-associated intracerebral hemorrhage. Cerebrovasc Dis. 2011;31:170–76. doi: 10.1159/000321766. [DOI] [PubMed] [Google Scholar]
- 53.Voils SA, Baird B. Systematic review: 3-factor versus 4-factor prothrombin complex concentrate for warfarin reversal: does it matter? Thromb Res. 2012;6:833–40. doi: 10.1016/j.thromres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 55.Monroe DM. Further understanding of recombinant activated factor VII mode of action. Semin Hematol. 2008;45:S7–S11. doi: 10.1053/j.seminhematol.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 56.Kawai N, Nakamura T, Nagao S. Early hemostatic therapy using recombinant factor VIIa in a collagenase-induced intracerebral hemorrhage model in rats. Acta Neurochir Suppl. 2006;96:212–17. doi: 10.1007/3-211-30714-1_46. [DOI] [PubMed] [Google Scholar]
- 57.Di Nisio M, Middeldorp S, Büller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028–40. doi: 10.1056/NEJMra044440. [DOI] [PubMed] [Google Scholar]
- 58.Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008;47:285–95. doi: 10.2165/00003088-200847050-00001. [DOI] [PubMed] [Google Scholar]
- 59.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 60.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 61.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 62.Weimar C, Hohnloser SH, Eikelboom JW, Diener H. Preventing cardioembolic stroke in atrial fibrillation with dabigatran. Curr Neurol Neurosci Rep. 2012;12:17–23. doi: 10.1007/s11910-011-0229-4. [DOI] [PubMed] [Google Scholar]
- 63.Hart RG, Diener H, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43:1511–17. doi: 10.1161/STROKEAHA.112.650614. [DOI] [PubMed] [Google Scholar]
- 64.Eriksson BI, Dahl OE, Ahnfelt L, et al. Dose escalating safety study of a new oral direct thrombin inhibitor, dabigatran etexilate, in patients undergoing total hip replacement: BISTRO I. J Thromb Haemost. 2004;2:1573–80. doi: 10.1111/j.1538-7836.2004.00890.x. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe M, Siddiqui FM, Qureshi AI. Incidence and management of ischemic stroke and intracerebral hemorrhage in patients on dabigatran etexilate treatment. Neurocrit Care. 2012;16:203–09. doi: 10.1007/s12028-011-9591-y. [DOI] [PubMed] [Google Scholar]
- 66.del Zoppo GJ, Eliasziw M. New options in anticoagulation for atrial fibrillation. N Engl J Med. 2011;365:952–53. doi: 10.1056/NEJMe1107516. [DOI] [PubMed] [Google Scholar]
- 67.Nielsen VG, Cohen BM, Cohen E. Effects of coagulation factor deficiency on plasma coagulation kinetics determined via thrombelastography: critical roles of fibrinogen and factors II, VII, X and XII. Acta Anaesthesiol Scand. 2005;49:222–31. doi: 10.1111/j.1399-6576.2005.00602.x. [DOI] [PubMed] [Google Scholar]
- 68.Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259–68. doi: 10.2165/11318170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 69.van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–27. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 70.Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–79. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 71.Battinelli EM. Reversal of new oral anticoagulants. Circulation. 2011;124:1508–10. doi: 10.1161/CIRCULATIONAHA.111.054510. [DOI] [PubMed] [Google Scholar]
- 72.Van Ryn J, Litzenburger T, Gan G, Coble K, Schurer J. In vitro characterization, pharmacokinetics and reversal of supratherapeutic doses of dabigatran-induced bleeding in rats by a specific antibody fragment antidote to dabigatran. American Heart Association Scientific Sessions 2012; Los Angeles. Nov 3–7, 2012; Oral presentation 9928. [Google Scholar]
- 73.Elg M, Carlsson S, Gustafsson D. Effects of agents, used to treat bleeding disorders, on bleeding time prolonged by a very high dose of a direct thrombin inhibitor in anesthesized rats and rabbits. Thromb Res. 2001;101:159–70. doi: 10.1016/s0049-3848(00)00398-4. [DOI] [PubMed] [Google Scholar]
- 74.Sørensen B, Ingerslev J. A direct thrombin inhibitor studied by dynamic whole blood clot formation. Haemostatic response to ex-vivo addition of recombinant factor VIIa or activated prothrombin complex concentrate. Thromb Haemost. 2006;96:446–53. [PubMed] [Google Scholar]
- 75.Elg M, Carlsson S, Gustafsson D. Effect of activated prothrombin complex concentrate or recombinant factor VIIa on the bleeding time and thrombus formation during anticoagulation with a direct thrombin inhibitor. Thromb Res. 2001;101:145–57. doi: 10.1016/s0049-3848(00)00397-2. [DOI] [PubMed] [Google Scholar]
- 76.Broos K, Feys HB, De Meyer SF, Vanhoorelbeke K, Deckmyn H. Platelets at work in primary hemostasis. Blood Rev. 2011;25:155–67. doi: 10.1016/j.blre.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Roquer J, Rodríguez Campello A, Gomis M, Ois A, Puente V, Munteis E. Previous antiplatelet therapy is an independent predictor of 30-day mortality after spontaneous supratentorial intracerebral hemorrhage. J Neurol. 2005;252:412–16. doi: 10.1007/s00415-005-0659-5. [DOI] [PubMed] [Google Scholar]
- 78.Saloheimo P, Ahonen M, Juvela S, Pyhtinen J, Savolainen E, Hillbom M. Regular aspirin-use preceding the onset of primary intracerebral hemorrhage is an independent predictor for death. Stroke. 2006;37:129–33. doi: 10.1161/01.STR.0000196991.03618.31. [DOI] [PubMed] [Google Scholar]
- 79.Naidech AM, Jovanovic B, Liebling S, et al. Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke. 2009;40:2398–401. doi: 10.1161/STROKEAHA.109.550939. [DOI] [PubMed] [Google Scholar]
- 80.Naidech AM, Rosenberg NF, Bernstein RA, Batjer HH. Aspirin use or reduced platelet activity predicts craniotomy after intracerebral hemorrhage. Neurocrit Care. 2011;15:442–46. doi: 10.1007/s12028-011-9557-0. [DOI] [PubMed] [Google Scholar]
- 81.Campbell PG, Yadla S, Sen AN, Jallo J, Jabbour P. Emergency reversal of clopidogrel in the setting of spontaneous intracerebral hemorrhage. World Neurosurg. 2011;76:100–04. doi: 10.1016/j.wneu.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 82.Moussouttas M, Malhotra R, Fernandez L, et al. Role of antiplatelet agents in hematoma expansion during the acute period of intracerebral hemorrhage. Neurocrit Care. 2010;12:24–29. doi: 10.1007/s12028-009-9290-0. [DOI] [PubMed] [Google Scholar]
- 83.Sansing LH, Messe SR, Cucchiara BL, Cohen SN, Lyden PD, Kasner SE. Prior antiplatelet use does not affect hemorrhage growth or outcome after ICH. Neurology. 2009;72:1397–402. doi: 10.1212/01.wnl.0000342709.31341.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foerch C, Sitzer M, Steinmetz H, Neumann-Haefelin T. Pretreatment with antiplatelet agents is not independently associated with unfavorable outcome in intracerebral hemorrhage. Stroke. 2006;37:2165–67. doi: 10.1161/01.STR.0000231842.32153.74. [DOI] [PubMed] [Google Scholar]
- 85.Thompson BB, Béjot Y, Caso V, et al. Prior antiplatelet therapy and outcome following intracerebral hemorrhage: a systematic review. Neurology. 2010;75:1333–42. doi: 10.1212/WNL.0b013e3181f735e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Creutzfeldt CJ, Weinstein JR, Longstreth WT, Becker KJ, McPharlin TO, Tirschwell DL. Prior antiplatelet therapy, platelet infusion therapy, and outcome after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2009;18:221–28. doi: 10.1016/j.jstrokecerebrovasdis.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ducruet AF, Hickman ZL, Zacharia BE, et al. Impact of platelet transfusion on hematoma expansion in patients receiving antiplatelet agents before intracerebral hemorrhage. Neurol Res. 2010;32:706–10. doi: 10.1179/174313209X459129. [DOI] [PubMed] [Google Scholar]
- 88.Naidech AM, Liebling SM, Rosenberg NF, et al. Early platelet transfusion improves platelet activity and may improve outcomes after intracerebral hemorrhage. Neurocrit Care. 2012;16:82–87. doi: 10.1007/s12028-011-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Gans K, de Haan RJ, Majoie CB, et al. PATCH: platelet transfusion in cerebral haemorrhage: study protocol for a multicentre, randomised, controlled trial. BMC Neurol. 2010;10:19. doi: 10.1186/1471-2377-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koltai K, Feher G, Kenyeres P, et al. Relation of platelet aggregation and fibrinogen levels to advancing age in aspirin- and thienopyridine-treated patients. Clin Hemorheol Microcirc. 2008;40:295–302. [PubMed] [Google Scholar]
- 91.Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22:1381–89. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- 92.Bouchard BA, Shatos MA, Tracy PB. Human brain pericytes differentially regulate expression of procoagulant enzyme complexes comprising the extrinsic pathway of blood coagulation. Arterioscler Thromb Vasc Biol. 1997;17:1–9. doi: 10.1161/01.atv.17.1.1. [DOI] [PubMed] [Google Scholar]
- 93.Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–07. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- 94.Smyth SS, McEver RP, Weyrich AS, et al. Platelet functions beyond hemostasis. J Thromb Haemost. 2009;7:1759–66. doi: 10.1111/j.1538-7836.2009.03586.x. [DOI] [PubMed] [Google Scholar]
- 95.Tsakiris DA, Scudder L, Hodivala-Dilke K, Hynes RO, Coller BS. Hemostasis in the mouse (Mus musculus): a review. Thromb Haemost. 1999;81:177–88. [PubMed] [Google Scholar]
- 96.Lee JM, Zhai G, Liu Q, et al. Vascular permeability precedes spontaneous intracerebral hemorrhage in stroke-prone spontaneously hypertensive rats. Stroke. 2007;38:3289–91. doi: 10.1161/STROKEAHA.107.491621. [DOI] [PubMed] [Google Scholar]
- 97.Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol. 2012;87 (suppl 1):S141–45. doi: 10.1002/ajh.23202. [DOI] [PubMed] [Google Scholar]
- 98.Steiner T, Petersson J, Al-Shahi Salman R, et al. European research priorities for intracerebral haemorrhage. Cerebrovasc Dis. 2011;32:409–19. doi: 10.1159/000330653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ruggeri ZM. Platelet adhesion under flow. Microcirculation. 2009;16:58–83. doi: 10.1080/10739680802651477. [DOI] [PMC free article] [PubMed] [Google Scholar]


