Abstract
Background & Aims
Among the ethnic groups age standardized incidence rate of colorectal cancer (CRC) is highest among African-Americans. The majority of CRC arise from preexisting adenoma. It is shown that 30% of the US adult population has adenomas. The potential risk of malignant transformation in adenomas differs by specific pathologic and clinical characteristics that we aimed to study in AAs.
Material and Methods
All pathologic reports (150,000) in Howard University Hospital from 1959 to 2006 were reviewed manually. Those pathology reports compatible with the colorectal polyps were carefully reviewed and selected by a GI pathologist. All cases with cancer then excluded from the list. Data then were entered into excel and checked for missing data and duplications. Differences in right side and left side polyps for sex, histology, clinical symptoms were assessed by chi-2 test.
Results
A total number of 5013 colorectal polyps were diagnosed in this period that include 47% male, with mean age (SD) of 63 (12). Half of cases were diagnosed in 2001–2006. Tubular adenoma was the most frequent pathology (73%). The highest frequency of right sided polyps was observed in 1990’s (56%). Left sided polyps were younger (P<0.000), more hyperplasic (23% vs. 5%; P<0.000) and more frequent in female (56% vs. 52%; P=0.02) compared to right sided. The frequency of right sided adenoma significantly increases from 18% in 60’s to 51% in the period of 2001–2006 (P<0.000). The most frequent symptom in both sides was GI bleeding (21%).
Conclusion
There was a ratio of 9:1 for neoplastic to hyperplastic polyps in our study which is more than what has been reported in Caucasians (7:1). Our data shows a shift in polyps from the left side to the right side of the colon in recent years. This data is consistent with the lack of a reduction in the incidence of colon cancer in African Americans. Screening is thus very important in AA to reduce the incidence of colon cancer.
Keywords: Colon, polyp, African American
Introduction
Colon cancer is the third common cancer in the United States. African-Americans have the highest incidence rate of colorectal cancer among different races [1]. Colon carcinogenesis is believed to be a process taking several years to be completed. A majority of colorectal cancers are expected to arise through a multistep pathway. Which include initial hyperproliferation of normal epithelial cells, formation of adenomas and finally the transition to invasive carcinomas [2, 3]. Interruption of adenoma-carcinoma sequence with colonoscopy and polypectomy reduces the incidence of CRC by as much as 90% [4].
The study of adenoma prevalence began in 1947 with examining the total colon in autopsies [5] including the autopsies surveys that have been conducted worldwide (For a complete review refer to Neugut et al. [6]). Colorectal polyps are frequent in the general population. The reported prevalence of colonic polyps varies widely between different geographical areas. It was estimated that 30% of the Western population have colonic polyps while a lower rate (10–15%) is noted in Asia and Africa [7, 8]. Results from previous studies have shown that colonic polyps are more common in men than in women and increase in frequency with increasing age in most of these studies; in some older age groups the prevalence rate exceeds 50% [9–15]
Colorectal polyps are classified histologically mainly as adenoma (neoplastic) or hyperplastic (non-neoplastic) [16]. The majority of polyps are small, non-neoplastic lesions that are found during screening or when procedures are performed for other diagnostic reasons. Surveys have generally shown that the prevalence of hyperplastic polyps varies in different population, as well as adenomas. Autopsy studies reported a 12–52% prevalence of hyperplastic polyps and a 10–40% prevalence of adenomas [17–20]. In screening colonoscopy studies, the corresponding figures were 9–34% and 24–48%, respectively [21–23].
The anatomic distribution of adenoma is shown to be different between different geographical area and races too [24, 25]. Unlike the hyperplastic polyp the adenoma are more prevalent in right side colon and are strongly associated with age [26]. The sub site distribution of colorectal adenomas removed by autopsy or colonoscopy procedures over a 20-year period was found to have a “shift to the right”, similar to that reported for colorectal carcinoma [27–30].
Whereas there is some of information relating to the epidemiology of CRC in African-Americans, much less known about adenomas in this group. The aim of this study was to determine the clinical and pathological characteristics of colon polyps in African-American population and define the risk profile of neoplastic polyps at the main hospital in the district of Colombia in last fifty years.
Material and Methods
Patients
Howard University Hospital (HUH) is located in District of Columbia (Washington DC). It was established in 1959 to serve DC area, mainly African-American population of Capital. More than 90% of HUH cases in this study are now from African-American population. Gastroenterology division at department of medicine is responsible for handling gastrointestinal (GI) diseases, including lower GI diseases and colorectal cancer screening. Cases of colorectal polyps are diagnosed through endoscopy or surgery in hospital. Then all biopsy (polyps) were sent for diagnosis in Department of Pathology. From 1959–2006 more than 150,000 pathologic reports are archived in this department. A team of health professionals were trained to review the pathologic reports (with approval from Howard University Institutional Review Boards). They reviewed all pathologic reports manually and selected the diagnosis compatible with colorectal polyps for further review. Any cases with colorectal cancer diagnosis were excluded from the study. A database in Excel was developed to store the clinical and pathological data. The selected reports were then abstracted and entered into database. Histological diagnosis, clinical and demographical data were entered into data base. Then the data were rechecked for any missing data and duplication. Year of diagnosis were categorized into five decades. Polyps location was categorized into five sites (ascending, transverse, descending, rectosigmoid and multiple/undefined location). Polyp diagnosis was categorized into six categories (tubular adenoma, tubulovillous, villous, hyperplastic polyp, mixed (hyperplastic and adenoma) and other). A further neoplastic category defined the adenomas. Different categories of symptoms or reason for referral then categorized into eight categories.
Statistical Analysis
Distribution of variables was explored by table of frequency or Mean (SD). Hypotheses were tested by Chi-2. A logistic model was then developed to assess the independent risk factors of neoplastic vs. hyperplastic polyps’ diagnosis. In this model the age, gender, anatomic site (proximal/distal) treated as independent risk factors. P value less than 0.05 considered as significant. Data analysis was performed in SPSS 15.0 (Chicago, IL).
Results
Adenoma history at HUH
For the period of 1959–2006, a total number of 5013 colorectal polyps were diagnosed at HUH. Fifty three percent (2650) were female. Mean age (SD) for cases was 63.2 (12.4) years, with the median of 63 years. The number of cases increases continuously with time and fifty percent (50%) of cases were diagnosed after 1999 (Figure 1). Polyps were mostly Tubular adenoma (72.6%) followed by hyperplastic polyp (10.6%) and tubulovillous (8.3%). Two hundred forty one polyps (4.8%) were mixed polyps (hyperplastic and adenoma; Table 1). The ratio of neoplastic to hyperplastic polyps were 8.
Figure 1.
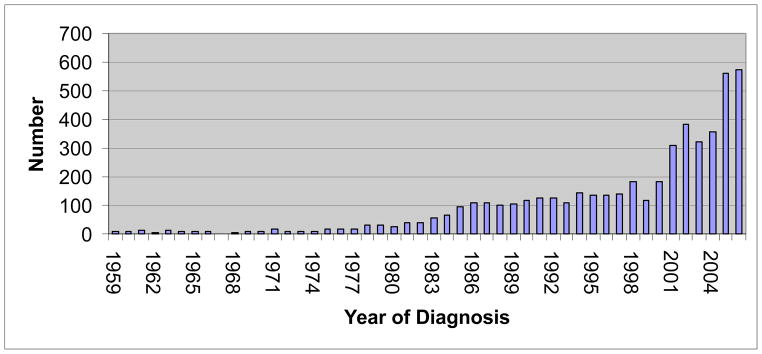
The frequency of cases by year of diagnosis
Table 1.
Histologic diagnosis and Location of Polyps
| Diagnosis | Frequency | Percent |
|---|---|---|
| Tubular Adenoma | 3641 | 72.6 |
| Tubulovillous | 414 | 8.3 |
| villous | 151 | 3.0 |
| Hyperplastic | 533 | 10.6 |
| Mixed | 241 | 4.8 |
| Others | 22 | 0.4 |
| Undefined | 11 | 0.2 |
| Total | 5013 | 100.0 |
| Location | ||
| Ascending colon | 1302 | 26.0 |
| Transverse | 504 | 10.1 |
| Descending | 1450 | 28.9 |
| Rectosigmoid | 256 | 5.1 |
| Multiple | 645 | 12.9 |
| Undefined | 856 | 17.1 |
| 5013 | 100.0 |
Polyps were mainly located in descending (28.9%) and ascending colon (26%). In 645 (12.9%) the location was multiple and in 856 (17.1%) the location was not defined well. Polyps were mostly proximal (51.4%) than distal (48.6%) to splenic flexure. The frequency of hyperplastic polyps was 5.3% in proximal colon and 23.3% in distal colon (p<0.0001)
Trend of Location and subtypes by time
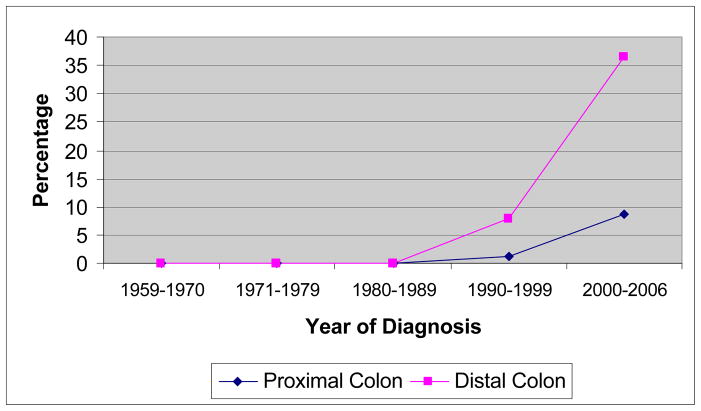
The proportion of hyperplastic polyps dramatically increased during the last fifty years. The first hyperplastic polyp cases were diagnosed in 1990’s and in period of 2000–2006 peaks at 19.2%. In this period the rate of neoplastic to hyperplastic polyps decreased to 4 (p<0.0001) (Table 2). Meanwhile the proportion of right sided polyps increased significantly from 18.2% in 1960’s to 55.9% in 1990’s and decreased in period of 2000–2006 to 50.7% (p<0.0001) (Figure 2). Trend of hyperplastic polyp is more significant in left sided polyps. It peaks to 8.6% (in 2000–2006) in proximal location and in distal colon it peaks at 36.6% at the same period (Figure 3).
Table 2.
Distribution of Hyperplastic diagnosis by year of diagnosis
| Diagnosis | ||||
|---|---|---|---|---|
| Year | Neoplastic | Hyperplastic | Mixed or others | Total |
| 1959–1970 | 90 (92.8) | 0 | 7 (7.2) | 97 (100) |
| 1971–1979 | 178(96.7) | 0 | 6(3.3) | 184(100) |
| 1980–1989 | 813(97.5) | 1(0.1) | 22(2.6) | 836(100) |
| 1990–1999 | 1266(91.0) | 50(3.6) | 75(5.4) | 1391(100) |
| 2000–2006 | 1859(74.2) | 482(19.2) | 164(6.5) | 2505(100) |
| Total | 4206(83.9) | 533(10.6) | 274(5.5) | 5013(100) |
Figure 2.
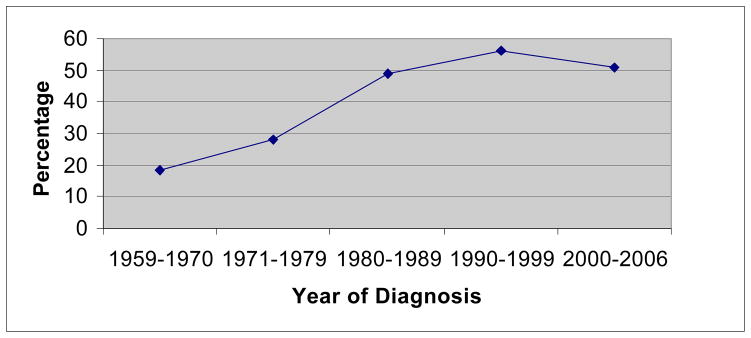
The percentage of right sided polyps by the year of diagnosis
Figure 3.
The percentage of hyperplastic diagnosis in proximal and distal colon by year of diagnosis
Polyp location and subtypes by sex and age
Cases with neoplastic polyps were older than hyperplastic polyp (p<0.001). The mean age (SD) was 64.0 (12.4) and 57.7 (10.7) years in neoplastic and hyperplastic polyp, respectively. The ratio of neoplastic to hyperplastic polyps increases steadily with age peaks to 18 in the age over 70 years (p<0.0001) (Table 3). The hyperplastic polyp was more frequent in females than male (12.6% vs. 8.4%) (p<0.0001).
Table 3.
Distribution of polyp diagnosis by age groups
| Age | Neoplastic | Hyperplastic | Mixed or others | Total |
|---|---|---|---|---|
| ≤50 | 572(76.9) | 136(18.3) | 36(4.8) | 744(100) |
| 51–60 | 1030(78.8) | 192(14.7) | 85(6.5) | 1307(100) |
| 61–70 | 1261(86.3) | 134(9.2) | 67(4.6) | 1462(100) |
| 71≤ | 1316(90.0) | 71(4.9) | 75(5.1) | 1462(100) |
| Total | 4179(84.0) | 533(10.7) | 263(5.3) | 4975(100) |
Proximal cases were older than distal significantly (p<0.0001). After stratifying for polyp diagnosis, this difference is shown to be limited to neoplastic polyps. The mean age (SD) was 64.7 (12.1) and 61.2 (12.2) in proximal and distal neoplastic polyps (p<0.0001; Table 4). The proportion of distal polyps in female is significantly higher than male (50.4% vs. 46.5%;p<0.02).
Table 4.
Age distribution in proximal and distal polyps by diagnosis
| Mean age (SD) | Proximal | Distal | p |
|---|---|---|---|
| Neoplastic | 65.1(12.1) | 62.5(12.5) | P<0.0001 |
| Hyperplastic | 59.0(11.8) | 57.4(10.3) | 0.2 |
| Other | 65.1(11.1) | 61.0(12.0) | 0.31 |
| Total | 64.8(12.1) | 61.2(12.2) | P<0.0001 |
Factor affecting the polyp diagnosis
Logistic multivariate model revealed that older age, males and proximal location increases the risk of neoplastic diagnosis significantly. This model has a sensitivity of 99.5% in predicting the risk of neoplastic diagnosis (Table 5)
Table 5.
Multivariate model for risk of neoplastic diagnosis in colon polyps
| OR | 95% CI | P | |
|---|---|---|---|
| Age (years) | 1.04 | 1.03–1.05 | 0.0001 |
| Sex (male/Female) | 1.53 | 1.24–1.88 | 0.0001 |
| Location (Proximal/Distal) | 5.07 | 4.0–6.4 | 0.0001 |
Chi-2 for Hosmer and Lemeshow test= 17.79 (p=0.02)
Symptom distribution in patients
The main reason (or symptom) for referral was defined in 3922 (78.2%) cases. Among them GI bleeding (26.6%), Screening (19.2%) and abdominal pain (3.7%) were the leading symptoms. Frequency of screening increased from 3% in 1959–1970 to 24% in 2000–2006 (p<0.001). Among the 752 cases with screening 438 (58%) had positive guiage test, and 314 (42%) had underwent other screening methods, among them 99% found after 1980. The N/H ratio is 5 in cases referred fro screening while it is 8 in other cases (p<0.001). There is no statistically significant difference between two groups of patients in sex, age and anatomic location.
Discussion
Polyp surveillance accounts for more that 15% of 1.7 million colonoscopies performed each year in the United States [31]. One of the major rationales underlying this surveillance is that 30–60% of patients with adenoma develop metachronous neoplasia within the first three years following index polypectomy [32, 33]. Furthermore, it is estimated that 30% to 40% of adults of ages >60 years have prevalent colorectal adenomatous polyps and individuals with a history of adenoma are at increased risk of colorectal cancer, even with routine colonoscopy exams [34, 35]. We analyzed the data of 5013 cases with polyp diagnosis in last half century from HUH. We found that more than 83% of polyps in AAs in this period were adenoma. The number of polyp cases is almost ten folded from 1980 in this hospital with a shift to right colon in our series. Our data is one of the largest colorectal polyp series have ever been analyzed that include a collection of 50 years of adenoma data from AAs serving hospital. To our knowledge there is no other paper addressing the colon polyps in AA with this large sample size. Many of cases are diagnosed through endoscopy. The chance of bias introduced in autopsy papers is little in our series. All cases were diagnosed by histological methods with highest accuracy level. As a hospital based series, we acknowledge the possible limitation of our study. These included the missing information and biases as results of referral pattern. This data may not represent an entire AA population living in the US but indicates important clues of current situation of polyp distribution in AA population.
The prevalence of colon polyps shows marked international variation. In Hawaiian Japanese, who have a high risk of colon carcinoma, the prevalence of adenoma is shown to be more than 60% [18]. In contrast, Japanese people living in Japan have a low risk of colonic polyp and malignancy. The lowest rate of polyps is reported from Iran (1.6%) [36] with one of the lowest reported rate of colonic malignancy [37]. The reported prevalence of colonic polyps varies widely due to differences in geography, in structure of the studies, and sensitivities of the test used to define prevalence [21].
In the United state it is estimated that 30–40% of age 60+ populations have colonic polyps [33]. Polyp distribution is dissimilar among different ethnic groups in the United States [38]. Though the highest rate of colon cancer is reported in African-American [1], data for colonic adenoma in this population is very scarce. Thorton et al. reviewed more 46000 colonoscopies between 2002–2003 and found that colon polyps are more proximal, bigger in size and younger in age in African-American compare to Whites [39]. The rate of neoplastic to hyper plastic polyps in our series is 8. This ratio is different across the studies. Surveys from United state [40], Spain [41], Indian [8] and Canada [42] reported a ratio of 6 or more for neoplastic to hyperplastic polyps. This ratio is near 1 as reported from Sweden [43] and Australia [44]. In England [45] and New Zealand [46] this ratio is reported to be less than one. Khan et al [42] reported a ratio of 8 for neoplastic to hyperplastic polyps very close to our study (Table 6).
Table 6.
Distribution (%) of Neoplastic and Hyperplastic polyps in different studies
| Reference | Year | No. of Polyps | Location | Neoplastic | Hyperplastic | Others |
|---|---|---|---|---|---|---|
| Autopsy | ||||||
| Eide and Stalsberg [62] | 1978 | 483 | Norway | 58 | 17 | 25 |
| Vatn and Stalsberg [19] | 1982 | 687 | Norway | 50 | 26 | 24 |
| Williams et al. [45] | 1982 | 843 | UK | 29 | 68 | 3 |
| Bombi [41] | 1988 | 122 | Spain | 73 | 2 | 25 |
| Cood et al. [7] | 1985 | 200 | Hong Kong | 62 | 31 | 7 |
| Jass et al. [46] | 1992 | 406 | New Zealand | 38 | 62 | 0 |
| Colonoscopy | ||||||
| Granqvist et al [43] | 1979 | 300 | Sweden | 37 | 39 | 24 |
| Tedesco et al. [63] | 1982 | 329 | USA | 49 | 37 | 14 |
| Isbister [44] | 1986 | 803 | Australia | 59 | 39 | 14 |
| O’Brein et al. [40] | 1990 | 5066 | USA | 67 | 11 | 22 |
| Khan et al. [42] | 2002 | 1050 | Canada | 83 | 12 | 5 |
| Tony et al. [8] | 2007 | 122 | India | 81 | 9 | 10 |
| Present study | 5013 | USA | 84 | 11 | 4 |
The difference is N/H ratio is unexplainable. Most recent studies show a higher rate of neoplastic polyps. We found a reverse time trend in our study; with higher rate of hyperplastic polyps in recent years for HUH. African-American population, especially in DC area is now experiencing dramatically changes in health status. CRC screening rate increased by 15% from 1997 to 2006 in this area [47]. More than 85% of District Colombia population were insured and had access to health cares in 2006 [48]. The number of colonoscopies in HUH has increased on a yearly basis. HUH has appointed a new GI pathologist from 2004. We indicated a 10 fold rise in screening procedure, mainly from increasing the number of screening colonoscopy in this period. An increasing of medium risk population undergone for colonoscopies together with a better diagnosis of hyperplastic polyp in HUH may explain rising number of hyperplastic polyp in recent years. Lack of robust criteria for hyperplastic polyp diagnosis, or under diagnosis of hyperplastic can predict part of observed time trend in N/H ration in this study.
Hyperplastic is usually considered as inconsequentional and is not described as part of adenoma-carcinoma pathway. Some reports currently describe large hyperplastic polyp as occurring on the right-side in association with colorectal cancer (CRC), and showing a malignant potential [49, 50]. Wynter et al., reported a two variants of hyperplastic with Kras or BRAF mutation and CIMP high with significant malignant potential [51].
Serrated adenomatous polyps of the colon were diagnosed as a variant of hyperplasic polyps. Hyperplasic polyps considered as innocuous and benign lesions without playing a significant role in carcinogenesis [52]. But recently some authors consider Sessile Serrated Adenoma (SSA) as aggressive hyperplastic polyp and precursor of colonic cancer [53, 54]. We found no serrated polyps in our latest series. However, we would like to reevaluate hyperplastic polyp for presence or lack of SSA.
Tubular and tubulovillous (TV) adenoma account for 87% and 10% of neoplastic polyps in our series. This rate for TV is varied across studies. It is more than 45% in Chinese [25] as a low risk population for colon cancer, about 20% in Swiss [55] and less than 5% in some Asian population [7]. TV polyps are believed to have more potential for recurrence and metachronous cancer. Agreement on diagnosis for adenoma histology subtypes is shown to be moderate or low. Kappa for TV diagnosis is reported to be 0.15 between different pathologists [56]. This high disagreement is a main source for variation between different studies for the frequency of TV in adenoma. Discrepancies between the rate of TV and colon cancer rate in different population should be studied more.
The frequency of neoplastic polyps increased with age reaching to more than 90% in ≥70. This is in agreement with other studies showing that prevalence of neoplastic polyps increases with age while prevalence of HYPERPLASTIC POLYP does not change with age [7, 19, 42]. The male gender increases the risk of neoplastic polyps to 50%. This is in agreement with other studies showing a higher chance of neoplastic polyps in males [7, 25]. The observed effect of sex and age on neoplastic polyps is independent and remained in multivariable model.
The anatomic location of polyps varies internationally. Colonoscopies survey from China [25] and India [8] indicated a frequency of left side polyps more than 60%. A study from Germany indicated a 55% of distal colon adenoma [57]. Colon rectal neoplasm is shown to be more proximal in AAs than US Caucasian [24, 38, 58]. Our study indicated a frequency of right sided polyps as much as 51%, not far from the results in North American Caucasian Jass [42, 46]. Thronton found that 57% of colon polyps are right sided in African-American [39]. Previous colonoscopy survey from 1993–1999 at HUH indicated that colon adenoma in AAs are mainly left sided [59]. Neugut et al. have reviewed different aspect of colorectal polyps. They have indicated an increase in right sided polyps from 1969 to 1990 [6]. Whereas in colonoscopy survey of 1969, less than 30% of polyps were in right side in 1990 this rate increase to 69%. We observed a 30% increase in frequency of right sided polyps in our case during last 50 years. This right sided shift increases the importance of colonoscopy as an effective CRC screening strategy in our AAs, especially in older patients.
The most attractive reason for right sided shift in colon cancer is the role of CRC screening (sigmoidoscopy) in detection of left sided adenomas. This reason may not explain the shift of polyps in our data. We observed the shift is mainly affects neoplastic polyps and not hyperplastic polyp while the percentage of hyperplastic polyps remained almost stable in right side, in left side it increased about 50% in 50 years. We propose this shift is mainly affected by the life style change; i.e. smoking, less physical activity, diet (higher animal meat and fat consumption), and environmental factors. The role of other factors such as change in bacterial flora of large intestine should be considered as well.
In this study the right sided polyps had 5 times more risk to be neoplastic compare to left side. The left sided predominance in hyperplastic polyp is observed in Japan [50], as well as India [8] and USA [42, 60]. Distal adenoma polyps is shown to increase the risk of advanced proximal neoplasm as to 7 folds [60] while there are inconclusive results for the effect of distal hyperplastic polyp on the advance proximal findings from no increase in risk to OR as much as 4 [50]. Recent meta-analysis by Otto et al indicated no increased risk of proximal neoplasia subsequent to a distal hyperplastic polyps [61].
This study is a retrospective data collection. Medical charts and pathologic reports were the main sources of our data. This sort of data has some inherent limitation. Any change in diagnosis criteria, pathologist expertise, colonoscopy policies in hospital, and catchments population may factitiously affects the trend. This limitation should be considered in interpreting the findings. Statistically significant results should be distinguished from clinical importance given the high sample size and power of study.
In conclusion, we found that neoplastic polyps in African-American population outnumber the hyperplastic polyps. The adenoma are shifting to right sided, more seen in higher risk groups for cancer, including older ages (>65) and men. This finding seeks a clear strategy for polyp prevention and removal in African-American population. Male and older ages may benefit more from colonoscopy in our population.
Acknowledgments
This work was supported by Grant #CA102681, funded by the National Cancer Institute, NIH, GCRC and Marcia Johnson award from Howard University.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Lipkin M, Higgins P. Biological markers of cell proliferation and differentiation in human gastrointestinal diseases. Adv Cancer Res. 1988;50:1–24. doi: 10.1016/s0065-230x(08)60433-9. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 4.Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, Waye JD, Bond J, Schapiro M, Stewart ET, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The national polyp study workgroup. N Engl J Med. 1993;328:901–906. doi: 10.1056/NEJM199304013281301. [DOI] [PubMed] [Google Scholar]
- 5.Helwig EB. The evolution of adenomas of the large intestine and their relation to carcinoma. Gynecol Obstet. 1947;84:36–49. [PubMed] [Google Scholar]
- 6.Neugut AI, Jacobson JS, De Vivo I. Epidemiology of colorectal adenomatous polyps. Cancer Epidemiol Biomarkers Prev. 1993;2:159–176. [PubMed] [Google Scholar]
- 7.Coode PE, Chan KW, Chan YT. Polyps and diverticula of the large intestine: A necropsy survey in hong kong. Gut. 1985;26:1045–1048. doi: 10.1136/gut.26.10.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tony J, Harish K, Ramachandran TM, Sunilkumar K, Thomas V. Profile of colonic polyps in a southern indian population. Indian J Gastroenterol. 2007;26:127–129. [PubMed] [Google Scholar]
- 9.Weston AP, Campbell DR. Diminutive colonic polyps: Histopathology, spatial distribution, concomitant significant lesions, and treatment complications. Am J Gastroenterol. 1995;90:24–28. [PubMed] [Google Scholar]
- 10.Villavicencio RT, Rex DK. Colonic adenomas: Prevalence and incidence rates, growth rates, and miss rates at colonoscopy. Semin Gastrointest Dis. 2000;11:185–193. [PubMed] [Google Scholar]
- 11.Patel K, Hoffman NE. The anatomical distribution of colorectal polyps at colonoscopy. J Clin Gastroenterol. 2001;33:222–225. doi: 10.1097/00004836-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Olsen HW, Lawrence WA, Snook CW, Mutch WM. Review of recurrent polyps and cancer in 500 patients with initial colonoscopy for polyps. Dis Colon Rectum. 1988;31:222–227. doi: 10.1007/BF02552551. [DOI] [PubMed] [Google Scholar]
- 13.Lieberman DA, Smith FW. Frequency of isolated proximal colonic polyps among patients referred for colonoscopy. Arch Intern Med. 1988;148:473–475. [PubMed] [Google Scholar]
- 14.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Prevalence of polyps greater than 9 mm in a consortium of diverse clinical practice settings in the united states. Clin Gastroenterol Hepatol. 2005;3:798–805. doi: 10.1016/s1542-3565(05)00405-2. [DOI] [PubMed] [Google Scholar]
- 15.Hoff G, Vatn M. Epidemiology of polyps in the rectum and sigmoid colon. Endoscopic evaluation of size and localization of polyps. Scand J Gastroenterol. 1985;20:356–360. doi: 10.3109/00365528509091664. [DOI] [PubMed] [Google Scholar]
- 16.Colucci PM, Yale SH, Rall CJ. Colorectal polyps. Clin Med Res. 2003;1:261–262. doi: 10.3121/cmr.1.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato E, Ouchi A, Sasano N, Ishidate T. Polyps and diverticulosis of large bowel in autopsy population of akita prefecture, compared with miyagi. High risk for colorectal cancer in japan. Cancer. 1976;37:1316–1321. doi: 10.1002/1097-0142(197603)37:3<1316::aid-cncr2820370312>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Stemmermann GN, Yatani R. Diverticulosis and polyps of the large intestine. A necropsy study of hawaii japanese. Cancer. 1973;31:1260–1270. doi: 10.1002/1097-0142(197305)31:5<1260::aid-cncr2820310535>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 19.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in oslo: An autopsy study. Cancer. 1982;49:819–825. doi: 10.1002/1097-0142(19820215)49:4<819::aid-cncr2820490435>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 20.Paspatis GA, Papanikolaou N, Zois E, Michalodimitrakis E. Prevalence of polyps and diverticulosis of the large bowel in the cretan population. An autopsy study. Int J Colorectal Dis. 2001;16:257–261. doi: 10.1007/s003840100304. [DOI] [PubMed] [Google Scholar]
- 21.Johnson DA, Gurney MS, Volpe RJ, Jones DM, VanNess MM, Chobanian SJ, Avalos JC, Buck JL, Kooyman G, Cattau EL., Jr A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. Am J Gastroenterol. 1990;85:969–974. [PubMed] [Google Scholar]
- 22.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans affairs cooperative study group 380. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 23.Rex DK, Lehman GA, Ulbright TM, Smith JJ, Pound DC, Hawes RH, Helper DJ, Wiersema MJ, Langefeld CD, Li W. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: Influence of age, gender, and family history. Am J Gastroenterol. 1993;88:825–831. [PubMed] [Google Scholar]
- 24.Johnson H, Jr, Margolis I, Wise L. Site-specific distribution of large-bowel adenomatous polyps. Emphasis on ethnic differences. Dis Colon Rectum. 1988;31:258–260. doi: 10.1007/BF02554356. [DOI] [PubMed] [Google Scholar]
- 25.Liu HH, Wu MC, Peng Y, Wu MS. Prevalence of advanced colonic polyps in asymptomatic chinese. World J Gastroenterol. 2005;11:4731–4734. doi: 10.3748/wjg.v11.i30.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rex DK, Khan AM, Shah P, Newton J, Cummings OW. Screening colonoscopy in asymptomatic average-risk african americans. Gastrointest Endosc. 2000;51:524–527. doi: 10.1016/s0016-5107(00)70283-5. [DOI] [PubMed] [Google Scholar]
- 27.Cress RD, Morris C, Ellison GL, Goodman MT. Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992–2001. Cancer. 2006;107:1142–1152. doi: 10.1002/cncr.22011. [DOI] [PubMed] [Google Scholar]
- 28.Cucino C, Buchner AM, Sonnenberg A. Continued rightward shift of colorectal cancer. Dis Colon Rectum. 2002;45:1035–1040. doi: 10.1007/s10350-004-6356-0. [DOI] [PubMed] [Google Scholar]
- 29.Shavers VL. Racial/ethnic variation in the anatomic subsite location of in situ and invasive cancers of the colon. J Natl Med Assoc. 2007;99:733–748. [PMC free article] [PubMed] [Google Scholar]
- 30.Wu X, Chen VW, Martin J, Roffers S, Groves FD, Correa CN, Hamilton-Byrd E, Jemal A. Subsite-specific colorectal cancer incidence rates and stage distributions among asians and pacific islanders in the united states, 1995 to 1999. Cancer Epidemiol Biomarkers Prev. 2004;13:1215–1222. [PubMed] [Google Scholar]
- 31.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the united states: Results from a national consortium. Gastrointest Endosc. 2005;62:875–883. doi: 10.1016/j.gie.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Amonkar MM, Hunt TL, Zhou Z, Jin X. Surveillance patterns and polyp recurrence following diagnosis and excision of colorectal polyps in a medicare population. Cancer Epidemiol Biomarkers Prev. 2005;14:417–421. doi: 10.1158/1055-9965.EPI-04-0342. [DOI] [PubMed] [Google Scholar]
- 33.Yood MU, Oliveria S, Boyer JG, Wells K, Stang P, Johnson CC. Colon polyp recurrence in a managed care population. Arch Intern Med. 2003;163:422–426. doi: 10.1001/archinte.163.4.422. [DOI] [PubMed] [Google Scholar]
- 34.Loeve F, Boer R, Zauber AG, Van Ballegooijen M, Van Oortmarssen GJ, Winawer SJ, Habbema JD. National polyp study data: Evidence for regression of adenomas. Int J Cancer. 2004;111:633–639. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 35.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’Brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, Bond JH, Brooks D, Byers T, Hyman N, Kirk L, Thorson A, Simmang C, Johnson D, Rex DK. Guidelines for colonoscopy surveillance after polypectomy: A consensus update by the us multi-society task force on colorectal cancer and the american cancer society. CA Cancer J Clin. 2006;56:143–159. doi: 10.3322/canjclin.56.3.143. quiz 184-145. [DOI] [PubMed] [Google Scholar]
- 36.Haghighi P, Nasr K, Mohallatee EA, Ghassemi H, Sadri S, Nabizadeh I, Sheikholeslami MH, Mostafavi N. Colorectal polyps and carcinoma in southern iran. Cancer. 1977;39:274–278. doi: 10.1002/1097-0142(197701)39:1<274::aid-cncr2820390142>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Ansari R, Mahdavinia M, Sadjadi A, Nouraie M, Kamangar F, Bishehsari F, Fakheri H, Semnani S, Arshi S, Zahedi MJ, Darvish-Moghadam S, Mansour-Ghanaei F, Mosavi A, Malekzadeh R. Incidence and age distribution of colorectal cancer in iran: Results of a population-based cancer registry. Cancer Lett. 2006;240:143–147. doi: 10.1016/j.canlet.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Francois F, Park J, Bini EJ. Colon pathology detected after a positive screening flexible sigmoidoscopy: A prospective study in an ethnically diverse cohort. Am J Gastroenterol. 2006;101:823–830. doi: 10.1111/j.1572-0241.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 39.Thornton JG, Morris AM, Thornton JD, Flowers CR, McCashland TM. Racial variation in colorectal polyp and tumor location. J Natl Med Assoc. 2007;99:723–728. [PMC free article] [PubMed] [Google Scholar]
- 40.O’Brien MJ, Winawer SJ, Zauber AG, Gottlieb LS, Sternberg SS, Diaz B, Dickersin GR, Ewing S, Geller S, Kasimian D, et al. The national polyp study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990;98:371–379. [PubMed] [Google Scholar]
- 41.Bombi JA. Polyps of the colon in barcelona, spain. An autopsy study. Cancer. 1988;61:1472–1476. doi: 10.1002/1097-0142(19880401)61:7<1472::aid-cncr2820610734>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 42.Khan A, Shrier I, Gordon PH. The changed histologic paradigm of colorectal polyps. Surg Endosc. 2002;16:436–440. doi: 10.1007/s00464-001-8207-6. [DOI] [PubMed] [Google Scholar]
- 43.Granqvist S, Gabrielsson N, Sundelin P. Diminutive colonic polyps--clinical significance and management. Endoscopy. 1979;11:36–42. doi: 10.1055/s-0028-1098322. [DOI] [PubMed] [Google Scholar]
- 44.Isbister WH. Colorectal polyps: An endoscopic experience. Aust N Z J Surg. 1986;56:717–722. [PubMed] [Google Scholar]
- 45.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: A necropsy study in liverpool. Gut. 1982;23:835–842. doi: 10.1136/gut.23.10.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in new zealand. Gut. 1992;33:1508–1514. doi: 10.1136/gut.33.11.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Centers for disease control and prevention (cdc) Behavioral risk factor surveillance system survey data. Atlanta, georgia: U.S. Department of health and human services, centers for disease control and prevention; 2006. [Google Scholar]
- 48.DeNavas-Walt C, Proctor Bernadette D, Smith Jessica U.S. Census Bureau. Income, Poverty, and Health Insurance Coverage in the United States: 2006. U.S. Government Printing Office; Washington, DC: 2007. Current Population Reports, P60–233. [Google Scholar]
- 49.Hyman NH, Anderson P, Blasyk H. Hyperplastic polyposis and the risk of colorectal cancer. Dis Colon Rectum. 2004;47:2101–2104. doi: 10.1007/s10350-004-0709-6. [DOI] [PubMed] [Google Scholar]
- 50.Yano T, Sano Y, Iwasaki J, Fu KI, Yoshino T, Kato S, Mera K, Ochiai A, Fujii T, Yoshida S. Distribution and prevalence of colorectal hyperplastic polyps using magnifying pan-mucosal chromoendoscopy and its relationship with synchronous colorectal cancer: Prospective study. J Gastroenterol Hepatol. 2005;20:1572–1577. doi: 10.1111/j.1440-1746.2005.03970.x. [DOI] [PubMed] [Google Scholar]
- 51.Wynter CV, Walsh MD, Higuchi T, Leggett BA, Young J, Jass JR. Methylation patterns define two types of hyperplastic polyp associated with colorectal cancer. Gut. 2004;53:573–580. doi: 10.1136/gut.2003.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jass JR. Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Clin Pract Oncol. 2005;2:398–405. doi: 10.1038/ncponc0248. [DOI] [PubMed] [Google Scholar]
- 53.Sandmeier D, Seelentag W, Bouzourene H. Serrated polyps of the colorectum: Is sessile serrated adenoma distinguishable from hyperplastic polyp in a daily practice? Virchows Arch. 2007;450:613–618. doi: 10.1007/s00428-007-0413-8. [DOI] [PubMed] [Google Scholar]
- 54.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine: A morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005;124:380–391. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- 55.Schoepfer A, Marbet UA. Colonoscopic findings of symptomatic patients aged 50 to 80 years suggest that work-up of tumour suspicious symptoms hardly reduces cancer-induced mortality. Swiss Med Wkly. 2005;135:679–683. doi: 10.4414/smw.2005.11033. [DOI] [PubMed] [Google Scholar]
- 56.Costantini M, Sciallero S, Giannini A, Gatteschi B, Rinaldi P, Lanzanova G, Bonelli L, Casetti T, Bertinelli E, Giuliani O, Castiglione G, Mantellini P, Naldoni C, Bruzzi P. Interobserver agreement in the histologic diagnosis of colorectal polyps. The experience of the multicenter adenoma colorectal study (smac) J Clin Epidemiol. 2003;56:209–214. doi: 10.1016/s0895-4356(02)00587-5. [DOI] [PubMed] [Google Scholar]
- 57.Wegener M, Borsch G, Schmidt G. Colorectal adenomas. Distribution, incidence of malignant transformation, and rate of recurrence. Dis Colon Rectum. 1986;29:383–387. doi: 10.1007/BF02555053. [DOI] [PubMed] [Google Scholar]
- 58.Ozick LA, Jacob L, Donelson SS, Agarwal SK. Freeman hyperplastic polyp: Distribution of adenomatous polyps in african-americans. Am J Gastroenterol. 1995;90:758–760. [PubMed] [Google Scholar]
- 59.Odelowo OO, Hoque M, Begum R, Islam KK, Smoot DT. Colonoscopy for colorectal cancer screening in african americans. J Assoc Acad Minor Phys. 2002;13:66–68. [PubMed] [Google Scholar]
- 60.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169–174. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 61.Lin OS, Gerson LB, Soon MS, Schembre DB, Kozarek RA. Risk of proximal colon neoplasia with distal hyperplastic polyps: A meta-analysis. Arch Intern Med. 2005;165:382–390. doi: 10.1001/archinte.165.4.382. [DOI] [PubMed] [Google Scholar]
- 62.Eide TJ, Stalsberg H. Polyps of the large intestine in northern norway. Cancer. 1978;42:2839–2848. doi: 10.1002/1097-0142(197812)42:6<2839::aid-cncr2820420645>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 63.Tedesco FJ, Hendrix JC, Pickens CA, Brady PG, Mills LR. Diminutive polyps: Histopathology, spatial distribution, and clinical significance. Gastrointest Endosc. 1982;28:1–5. doi: 10.1016/s0016-5107(82)72954-2. [DOI] [PubMed] [Google Scholar]