Abstract
Allergic diseases and asthma has long been hypothesized as the results of the dysregulation of type2 immune responses to environmental allergens. Recent progresses in characterizing the proinflammatory IL-17 cytokine family have added additional layer of complexity on the regulation of allergic inflammation. The delineation of IL-17-producing CD4+ T cell subset (Th17) has led to the revision of Th1/Th2 paradigm and impacts our perspectives on the basis of chronic tissue inflammation. In addition, the distinctive expression patterns and biological activities of individual IL-17 cytokine member may play different roles in the regulation of the pathogenesis of allergic diseases. Understanding the cellular source and targeting cells of IL-17 cytokine family member will provide the basis to elucidate the cellular mechanism underlying allergic inflammation and improve our therapeutic approaches for allergy.
Introduction
Allergic disorders, such as asthma and atopy, are caused by the dysregulated immune responses. Research in the past decades has revealed that allergic diseases are often resulted from an imbalance between the type 2 and type 1 branches of the immune system, which are responsible for mediating humoral immune responses and delayed hypersensitivity reactions (DTH), respectively. Breakthrough studies by Mosmann and Coffman led to the discovery of two CD4+ T cell subsets, T helper type 1 (Th1), and Th2, characterized by their distinct cytokine production profiles and effector functions. Th1 CD4+ T cells produce large amount of IFN-γ and elicit DTH responses to clear intracellular pathogens, whereas Th2 CD4+ T cells produce interleukin 4 (IL-4), IL-5, IL-13 to trigger allergic immune response and eradicate parasitic infection. Thus, the concept of Th1/Th2 paradigm has provided the basis to uncover the molecular and cellular mechanism of complex immune responses and led to the hygiene hypothesis, suggesting that dominant Th2 reaction results in allergy. Recent studies linking the discovery of IL-17 cytokine family and the analysis of IL-23 mediated immune pathogenesis previously attributed to the Th1 subsets have led to the delineation of a new effector CD4+ T cell subset that produce cytokine IL-17 (termed Th17). Severe allergic diseases are often associated with chronic inflammation characterized by the infiltration and accumulations of CD4+ T cells, neutrophils, eosinophils and mast cells. While cytokine IL-17 was shown to play an important role on the inflammatory process, the role of IL-17 cytokine members and the IL-17-producing cells during allergic inflammation is still largely unclear. In this review, we discuss recent reports regarding IL-17 cytokine family and Th17 differentiation pathways and how the IL-17-driven inflammation regulates allergic immune responses.
IL-17 cytokine family
Since the identification of IL-17A (originally named as CTLA-8) from activated T cell clones [1–3], five additional family members were subsequently uncovered and designated as IL-17A-F [4–6] (Table I). IL-17A, the prototypic family member, is a disulfide-linked homodimeric glycoprotein that possesses characteristic cystein knot structure, similar to that found in TGF-β, and nerve growth factor [7]. Among the IL-17 cytokine family, the expression and functions of IL-17A, IL-17F and IL-17E (IL-25) are better characterized. IL-17F share the greatest similarity with IL-17A (55% identity), whereas IL17E (IL-25) are the most distant (17%) [8]. Unlike other IL-17 cytokine family members located in different chromosomes, Il17a and Il17f are syntenic on mouse chromosome 1 and human chromosome 6, suggesting that the regulatory regions may exist within the IL-17A/F locus to control their expression. Indeed, the promoters and conserved noncoding sequence regions of IL-17A and IL-17F genes undergo coordinated chromatin modifications, similar to those identified in the locus of Th2 cytokine genes [9]. Both IL-17A and IL-17F were found to be produced by activated memory T cells, but other members of IL-17 cytokine family are expressed by broad range of tissues.
Table I.
IL-17 cytokine family and functional effects on allergy
| Ligands | Receptors | Functions | References |
|---|---|---|---|
| IL-17A | IL-17RA | IL-6, IL-8, IL-11, Gro-α, G-CSF and GM-CSF ↑ | [38] |
| IL-17F | IL-17RA/C | MUC5AC and MUC5B ↑ | [34] |
| Airway hyper-reactivity ↑ | [16] | ||
| Neutrophilia ↑ | [33] | ||
| Severity of asthma ↑ | [16] | ||
| IL-17E | IL-17RB | IL-4, IL-5, IL-13, IgE, and eotaxin ↑ | [40,42,43,44,45,46,50] |
| Mucus secretion ↑ | [44,45] | ||
| Airway hyper-reactivity ↑ | [40,47,50] | ||
| Eosinophilia ↑ | [40,42,44,50] | ||
| Severity of asthma ↑ | [40,44,47,50] |
Studies of IL17 receptor family (IL-17RA-E) revealed additional complexities of the regulations and biological functions of IL17 cytokine family (Table I). IL-17RA, the cognate receptor for IL-17A, is ubiquitously expressed [10]. However, the biological activity of IL-17 or IL-17F is dependent on the heterodimeric complex composed of IL-17RA and IL17RC [11]. IL-17RB serves as receptor for both IL-17B and IL-17E with higher binding avidity to IL-17E [12]. Most of IL17 receptor family members exhibit broad tissue expression and often exist as alternatively spliced isoforms with no transmembrane or cytoplasmic domains, thereby acting as soluble decoy receptors. The diverse expression patterns and regulations of IL-17 cytokine family and their cognate receptors suggest that this newly identified cytokine family may possess unique immunological functions and play important roles in the maintenance of homeostasis and the progression of immune disease.
Th17 cells
The delineation of the newly defined Th17 subset has changed the perspectives of immunologist and led to the revision of Th1/Th2 paradigm [13]. The attempt to characterize the IL-17-producing T cells isolated from rheumatoid synovium or induced by microbial lipopeptides has first led to the hypothesis that IL-17A may define a new subset of Th cells functioning on local inflammatory reaction [14;15]. Studies using IL-17-deficient mice or by neutralizing IL-17A activity demonstrated that IL-17-producing cells, not Th1 cells mediate inflammatory pathology in autoimmune models [16–18]. The finding that IL-23 (p19−/−), but not IL-12 (p35−/−) mice resistant to the development of joint autoimmune inflammation were due to the lack of IL-17-producing, not Th1 T cells provide the basis for the discovery of Th17 cell lineage [19–22]. Th17 cells are now defined by their production of IL-17A, IL-17F, IL-22, and to a lesser extent, tumor necrosis factor (TNF) and IL-6 [23]. The identification of RORγt as the master transcription factor for controlling Th17 differentiation has further support the notion that IL-17-producing cells represent the additional T helper cell lineage [24]. The requirement of cytokine milieu to induce Th17 cell differentiation between humans and mice has been controversial. Recent studies suggest that TGF-β is essential for the induction of RORγt expression in both humans and mice, the addition of IL-6 plus IL-23 or IL-21 in mice, or IL-6 plus IL-1β or IL-21 in humans triggers the production of IL-17A in vitro [25–29]. Interestingly, the additional complexity of the regulation of Th17 cells exits. The combination of IL-23 with TGF-β and IL-6 was found to be important for the maintenance of inflammatory Th17 cells in vivo by downregulating IL-10 production [30], whereas the addition of IL-27 with TGF-β and IL-6 lead to the suppression of Th17-mediated inflammation by the upregulation of IL-10 and IFN-γ production [31]. The identification of Th17 subset and the analyses of their functions have led to the resolution of some inconsistencies found in the Th1/Th2 paradigim. Th17 cells represent the third branch of CD4+ Th subset and functions in the induction of tissue inflammation and host protection against extracellular pathogens. Understanding the cytokine milieu that regulates Th17 differentiation and effector function during allergic inflammation may be the key to control the pathogenesis of allergic diseases.
IL-17A potentiates allergic inflammation by regulating innate immunity
In asthmatic patients, IL-17A expression was increased in the lungs, sputum, bronchoalveolar lavage (BAL) fluids or sera, and the severity of airway hypersensitivity in patients correlates with the level of IL-17A expression, suggesting that IL-17 cytokines play important role on driving allergic inflammation [32]. Indeed, IL-17A and/or IL-17F can orchestrate local inflammation by inducing the release of proinflammatory cytokines such as TNF-α, IL-1β, G-CSF, and IL-6, as well as chemokines CXCL1/Gro-α, CXCL2, and CXCL8/IL-8 production by human bronchial fibroblast, epithelial, and airway smooth muscle cells, as well as venous endothelial cells in vitro [33]. Furthermore, IL-17A can act in synergy with IL-6 to induce mucus proteins (MUC)5B and MUC5AC [34], or with IL-1β and TNF-α to enhance vascular endothelial growth factor expression [35]. In addition to stimulating airway structural cells, IL-17A, and IL-17F can also trigger innate effector eosinophils to release chemokine CXCL1/Gro-α, CXCL8/IL-8, and CCL4/MIP-1β. The combination of IL-17F and IL-23 can further stimulate the production of proinflammatory cytokines IL-1β and TNF-α by eosinophils [36]. The importance of IL-17A effect on driving lung inflammation has been further substantiated by the findings in animal studies. Overexpression of IL-17A or the administration of recombinant IL-17A in the lung results in the influx and accumulation of neutrophils associated with elevated level of CXCL1/Gro-α, CXCL8/IL-8, granulocyte colony-stimulating factor (G-CSF), and enhanced granulopoiesis [37;38]. Mice deficient in IL-17RA or IL-17A have marked diminished recruitment of neutrophils into the lung in response to a challenge with gram-negative pathogen or allergen [16]. Together, these studies demonstrate that IL-17A can trigger lung inflammation by stimulating innate immunity to mediate neutrophil recruitments, implicating the potential role of IL-17A on the pathogenesis of severe asthma mediated by neutrophilia.
Atopic asthma features the infiltration and accumulation of Th2 effector/memory cells, eosinphils, and mast cells, and increased IgE productions. The role of IL-17A on Th2-driven allergic immune response has been complicated. In the studies using IL-17A−/− or IL-17RA−/− mice, IL-17A was found to contribute to the induction of allergen-specific Th2 cell activation, eosinophil accumulation, and serum IgE production [16;39]. On the contrary, the administration of neutralizing anti-IL17A mAb in ovalbumin (OVA)-challenged murine asthma model in the late effector phase induced the elevated eosinophil recruitment and IL-5 productions in BAL, suggesting a regulatory role of IL-17A on the established Th2-driven allergic immune response [39]. These studies demonstrate the effect of IL-17A on the onset of lung inflammation, which facilitates the Th2-driven pathogenesis of allergic asthma. Since no evidence showed the direct effect of IL-17A on Th2 cells, the observation that endogenous IL-17A can dampen Th2-driven eosinophil recruitment and IL-5 production in the late phase of allergic immune responses remain further investigation.
IL-25 enhances allergic inflammation by regulating adaptive immunity
Distinct from other IL-17 cytokine family members, IL-25 (IL-17E) was first described as a TH2 cell-derived cytokine [40]. However, expression of IL-25 transcript was later found in mast cells activated by IgE cross-linking [41], alveolar macrophage and lung epithelial cells stimulated with allergens in mice [42]. In humans, bioactive IL-25 protein was found to be secreted by activated eosinophils and basophils [43]. Interestingly, these cells obtained from allergic patients produce more prominent amount of IL-25 after activation. These studies suggest that the primary cellular sources of IL-25 may exit in the branch of innate immunity.
In addition to low sequence homology and unique expression pattern, IL-25 also possess unique functions on evoking type 2 immune responses in animal studies [40;44;45]. Systemic administration of IL-25 protein [40;45] or overexpression of IL-25 [44;46] induces elevated TH2 cytokine and eotaxin production, which results in eosinophilia, increased serum IgE, mucus hyperplasia, and other pathological changes in many tissues. Moreover, administration of a neutralizing antibody against IL-25 in an experimental model of allergic asthma resulted in significantly reduced levels of IL-5, IL13 production, serum IgE production, the infiltration of Th2 cells and eosinophils, and prevented airway hyperresponsiveness [47]. These in vivo studies imply that IL-25 may play a pivotal role in the development of Th2-mediated allergic inflammation.
The function of IL-25 on type2 immunity, which play protective role in defense against parasitic infection was further elucidated by recent studies in animal models using helminth infection. In the absence of IL-25, mice infected with Trichuris muris, the gastrointestinal parasite, failed to develop a lymphocyte dependent protective type2 immunity to expel chronic parasitic infection [48]. In the other study, IL-25 was found to trigger the non-B/non-T, c-kit+ cells for the rapid clearance of N. brasiliensis acute infection [49]. Using allergen-induced allergic animal models, one study showed that administration of recombinant IL-25 proteins can induce acute lung inflammation mediated by the unidentified IL-5-producing non-B/non-T cells [45], whereas the other demonstrated that enforced expression of IL-25 in lung resulted in the amplification of allergic inflammation driven by CD4+T cells and STAT6 signaling pathway [50]. These findings suggest that depending on experimental models, IL-25 can enhance type2 immune responses by regulating CD4+ T cells or non-B/non-T, c-kit+ cells.
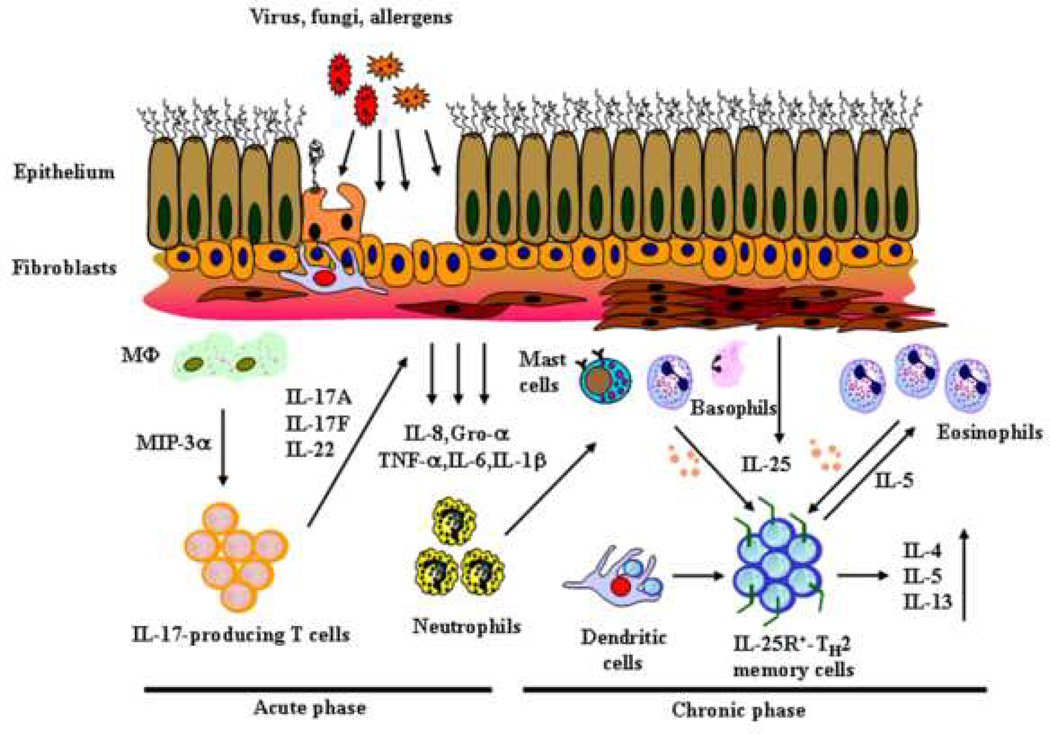
The finding that IL-25 receptor (IL-25R or IL-17BR) is highly expressed on CD4+ Th2 memory cells in humans has provided direct evidence that IL-25 can function directly on CD4+ T cells to mediate enhanced type2 immune response [43;51]. Indeed, IL-25 costimulates the proliferation of the TH2 memory cells, and enhances their TH2 polarization and cytokine productions, in particular IL-5, by upregulating the gene expression of the transcription factors, GATA-3, c-MAF, and junB in an IL-4 independent manner [43]. In a parallel study in mouse, IL-25 treatment during T cell differentiation can enhance Th2 cytokine production, and inhibit IFN-γ production, indicative of the Th2 polarizing function [42]. Together, these results suggest that IL-25 may amplify allergic immune response by inducing Th2 differentiation and the local expansion and augmented effector functions of Th2 memory/effector cells. On the contrary to the T cell derived proinflammatory cytokine, IL-17A/F, which regulates the innate effectors or structural cells during the onset of allergic inflammation, IL-25 (IL-17E) produced by innate effectors, such as eosinophils, and basophils may exert a critical role in maintaining the functional capacity and homeostatic maintenance of IL-25R-expressing allergen-specific Th2 memory cells, thus propagating a positive feed back loop between innate effectors and adaptive immunity leading to the amplification of allergic inflammation. (Fig.1)
Fig. 1.
Conclusion
Tissue inflammation is often one of the characteristic features of allergic diseases. Studies of IL-17 cytokine family and Th17 cells have advanced our understanding of cellular mechanisms underlying allergic inflammation. Th17 cells can mediate tissue inflammation by the induction of chemokines and proinflammatory cytokines in the structural cells, thereby supporting neutrophil recruitment and survival. However, the introduction of this third branch of adaptive immunity also raises new questions as to how Th17 cells and Th2 cells cooperate in the pathogenesis of allergic diseases, such as asthma. Severe asthma caused by neutrophilia can be further classified into the eosinophilic or noneosinophilic asthmatics, suggesting that the heterogeneity in the pathology of asthma may be the results of the interplay between these two T cell subsets. Moreover, recent studies support a hypothesis that a reciprocal relationship between regulatory T cells and Th17 differentiation pathway may exist [27], adding further complexity to the immune regulation during allergic inflammation. Thus, the approaches to design curative therapy for chronic allergic diseases in a phase-specific manner may require not only the understanding of the factors that drive the various T helper subsets, but also their temporal sequence and potential interaction in the induction of immunopathology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 2.Yao Z, Maraskovsky E, Spriggs MK, Cohen JI, Armitage RJ, Alderson MR. Herpesvirus saimiri open reading frame 14, a protein encoded by T lymphotropic herpesvirus, binds to MHC class II molecules and stimulates T cell proliferation. J. Immunol. 1996;156:3260–3266. [PubMed] [Google Scholar]
- 3.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, it-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das MB, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI, Gurney AL. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J. Biol. Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 5.Starnes T, Robertson MJ, Sledge G, Kelich S, Nakshatri H, Broxmeyer HE, Hromas R. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J. Immunol. 2001;167:4137–4140. doi: 10.4049/jimmunol.167.8.4137. [DOI] [PubMed] [Google Scholar]
- 6.Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, Dowd P, Gurney AL, Wood WI. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc. Natl. Acad. Sci. U.S.A. 2000;97:773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, Maruoka M, Mao W, Foster J, Kelley RF, Pan G, Gurney AL, de Vos AM, Starovasnik MA. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 9.Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin 17 (IL-17)-IL 17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. 2007;282:5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- 10.Yao Z, Spriggs MK, Derry JM, Strockbine L, Park LS, VandenBos T, Zappone JD, Painter SL, Armitage RJ. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- 11.Toy D, Kugler D, Wolfson M, Vanden BT, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 12.Tian E, Sawyer JR, Largaespada DA, Jenkins NA, Copeland NG, Shaughnessy JD., Jr Evi27 encodes a novel membrane protein with homology to the IL17 receptor. Oncogene. 2000;19:2098–2109. doi: 10.1038/sj.onc.1203577. [DOI] [PubMed] [Google Scholar]
- 13. Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 2007;13:139–145. doi: 10.1038/nm1551. • This excellent review provides historical perspectives about the discovery of Th17 cell lineages.
- 14. Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J. Immunol. 1999;162:1246–1251. • This paper proposed that IL-17 producing T cells may be a distinct effector T cell lineage different from Th1 and Th2 cells.
- 15.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 16. Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. • This study provides the direct in vivo evidence using IL-17A deficient mice that IL-17A play important role in inducing pulmonary cell infiltrations, OVA-specific IgE production and Th2 cytokine production in the OVA/Alum-induced allergy model.
- 17.Bush KA, Farmer KM, Walker JS, Kirkham BW. Reduction of joint inflammation and bone erosion in rat adjuvant arthritis by treatment with interleukin-17 receptor IgG1 Fc fusion protein. Arthritis Rheum. 2002;46:802–805. doi: 10.1002/art.10173. [DOI] [PubMed] [Google Scholar]
- 18.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 19.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 20. Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. •• This report and [19] demonstrate that a distinct IL-17-producing CD4 effector cell lineage, but not Th1 cells, were potently pathogenic in an adoptive transfer model of EAE.
- 21. Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. •• This study reported inhibitory effects of IFN-γ and IL-4 on the development of IL-17-producing effectors. It also demonstrated that STAT1, T-bet, STAT4 and STAT6 were dispensable for development of IL-17-producing effectors in vitro, establishing an independent developmental lineage for Th17.
- 22. Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. •• This report provided independent confirmation of a distinct developmental lineage for Th17, extending key observations in vivo.
- 23.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. •• This report demonstrates that the generation of Th17 cell lineage requires a transcription factor RORgammat.
- 25.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 28.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 29.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 31.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 32.Kolls JK, Kanaly ST, Ramsay AJ. Interleukin-17: an emerging role in lung inflammation. Am. J. Respir. Cell Mol. Biol. 2003;28:9–11. doi: 10.1165/rcmb.2002-0255PS. [DOI] [PubMed] [Google Scholar]
- 33.Linden A, Laan M, Anderson GP. Neutrophils, interleukin-17A and lung disease. Eur. Respir. J. 2005;25:159–172. doi: 10.1183/09031936.04.00032904. [DOI] [PubMed] [Google Scholar]
- 34. Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J. Biol. Chem. 2003;278:17036–17043. doi: 10.1074/jbc.M210429200. • This report provides an underlying molecular mechanism in vitro by which IL-17A triggers an inflammatory response in airway.
- 35.Honorati MC, Cattini L, Facchini A. IL-17, IL-1beta and TNF-alpha stimulate VEGF production by dedifferentiated chondrocytes. Osteoarthritis. Cartilage. 2004;12:683–691. doi: 10.1016/j.joca.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Cheung PF, Wong CK, Lam CW. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J. Immunol. 2008;180:5625–5635. doi: 10.4049/jimmunol.180.8.5625. [DOI] [PubMed] [Google Scholar]
- 37.Schwarzenberger P, La R, V, Miller A, Ye P, Huang W, Zieske A, Nelson S, Bagby GJ, Stoltz D, Mynatt RL, Spriggs M, Kolls JK. IL-17 stimulates granulopoiesis in mice: use of an alternate, novel gene therapy-derived method for in vivo evaluation of cytokines. J. Immunol. 1998;161:6383–6389. [PubMed] [Google Scholar]
- 38.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 39.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. • This study reports that the distinct function of IL-25 for inducing enhanced type2 immune response is mediated by targeting accessory cells that are MHC classIIhigh, CD11cdull, and lineage−.
- 41.Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–3596. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 42. Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. •• This study reports that IL-25 can mediate the expression of NFATc1 and JunB transcription factors, which in turns, results in increased levels of initial IL-4 production, upregulation of GATA-3 expression in naïve CD4+ T cells, thereby enhanced TH2 cell differentiation.
- 43. Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, Yao Z, Ying S, Huston DP, Liu YJ. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. •• This study demonstrates that eosinphils, basophils, and epithelial cells are the potential cellular sources of human IL-25, and IL-25R-expressing Th2 memory cells are the target cells. The study propose a collaborative cross talk mediated by IL-25 between innate eosinphils and adaptive Th2 memory cells can propagate positive feed back loops, thus leading to the amplification of allergic inflammation
- 44.Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, Sun J, DeRose ML, Stolina M, Chang D, Van GY, Clarkin K, Nguyen HQ, Yu YB, Jing S, Senaldi G, Elliott G, Medlock ES. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 45.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 46.Pan G, French D, Mao W, Maruoka M, Risser P, Lee J, Foster J, Aggarwal S, Nicholes K, Guillet S, Schow P, Gurney AL. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J. Immunol. 2001;167:6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 47.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 48.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, Tokuhisa T, Iwamoto I, Nakajima H. IL-25 enhances allergic airway inflammation by amplifying a T(H)2 cell-dependent pathway in mice. J. Allergy Clin. Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. • This report demonstrates that the effect of IL-25 on amplifying Th2 immune response is CD4+ T cell dependent using the allergen challenged animal model.
- 51. Wang YH, Ito T, Wang YH, Homey B, Watanabe N, Martin R, Barnes CJ, McIntyre BW, Gilliet M, Kumar R, Yao Z, Liu YJ. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. • This study demonstrates that the circulated human Th2 memory cells express high level of IL-25R transcript.