Abstract
CTL that possess a high functional avidity are known to be optimal for the clearance of pathogens in vivo. We have shown that the amount of peptide encountered by a CD8+ CTL determines its functional avidity. Notably, in these studies non-professional APC were utilized. However, it is mature DC that are predominantly responsible for the activation of naive T cells in vivo. Whether DC also direct dose dependent differences in avidity is unknown. Here we examined the ability of mature DC presenting a high vs. low level of peptide to generate CTL of distinct avidities. In contrast to what was observed with non-professional APC, CTL generated by stimulation with mature DC were of high avidity regardless of the amount of peptide presented. This DC property may promote generation of highly effective CTL that retain plasticity, which would allow tuning of avidity in the periphery to promote optimal recognition and clearance.
Keywords: DC, CTL, functional avidity, CD8
Introduction
Mature dendritic cells (DC) are distinguished by their high expression of costimulatory molecules and secretion of cytokines. In this state, DC are potent activators of naïve T cells. Productive conjugate formation will result in naive T cell activation, proliferation, and the acquisition of effector functions. These cytotoxic T lymphocytes (CTL) then enter the periphery where they exert lytic activity and release cytokine following encounter with infected cells.
Previous studies have demonstrated that within the population of responding CTL, there are individual clones that encompass a wide spectrum of functional avidities (1). The term functional avidity refers to the number of pMHC complexes required to activate a T cell. Low avidity CTL require a high number of pMHC complexes, whereas high avidity CTL can become activated when presented with a low number of pMHC complexes. Although a diverse clonal response is generated to a given pathogen, high avidity CTL appear to be the more important effector population. Adoptive transfer studies have demonstrated that high avidity CTL are more effective then their low avidity counterparts in viral clearance and tumor eradication, e.g. (1–5).
While readily generated in vitro by stimulation with low versus high concentrations of peptide, the mechanism(s) by which high vs. low avidity CTL are generated in vivo remains unclear. Several factors have been implicated in the regulation of functional avidity, i.e. the cytokines IL-12 and IL-15 (6,7), CD8α/β expression (8–10), TCR affinity (3), and level of co-stimulatory molecules expressed by APC (11,12). Therefore, it appears that multiple signals may be important in the development/regulation of functional avidity. As mature DC are characterized by their increased expression of co-stimulatory molecules and production of IL-12, it seemed possible that mature DC may skew the responding T cell response to be higher avidity (13,14).
The work presented here directly tested the ability of mature DC to generate both high and low avidity CTL. We found that mature DC presenting a high level of pMHC were poor inducers of low avidity CTL in contrast to other APC examined. However, a single encounter with antigen-bearing DC did not permanently fix avidity in the CTL as they were capable of differentiating into a low avidity cell following encounter with a non-professional APC bearing a high level of peptide. These data indicate that regardless of pMHC level, mature DC prime a potent high avidity CTL response. Nonetheless these CTL retain plasticity that allows them to “fine tune” their peptide sensitivity as a result of subsequent antigen encounter.
Materials and Methods
Mice and peptides
C57BL/6 mice were obtained from the FCRDC (Frederick, MD). TgN(TCRP14LCMV) Rag2tm1 (15) were purchased from Taconic (Germantown, NY). All experiments in this study comply with the institutional guidelines approved by the WFU ACUC. The LCMV gp33–41 and Ova257–264 peptides were synthesized at the WFUSM CCC Protein Analysis Core Laboratory.
Generation of bone marrow-derived dendritic cells (BMDC)
The protocol used to generate BMDC was as previously reported by Pejawar et al. (16). On day 6 post-culture, 200ng of LPS was added to induce DC maturation.
Generation and maintenance of CTL lines
5×105 TgN(TCRP14LCMV) Rag2tm1 splenocytes were co-cultured with 5×106 C57BL/6 splenocytes, 2×105 EL4 cells, or 5×104-2×105 DC previously pulsed with 10−4M or 10−9M gp33–41 peptide in the presence of 10% T-stim (BD Biosciences, San Jose, CA). All stimulators were irradiated prior to culture. Cultures were maintained by weekly stimulation.
Peptide dose response curve
Functional avidity of CTL lines was measured on day 7 following the third peptide stimulation by IFNγ ELISA, as previously described (10).
Quantitation of Kb-Ova presentation
LPS matured BMDC and naïve C57BL/6 splenocytes were left untreated or pulsed with titrated concentrations of OVA257–264 (10−4M–10−9M) for 3 hours followed by washing. Cells were stained with FcBlock, the Ova257–264/Kb-specific antibody 25.D1.16 (a kind gift of Dr. Germain, NIH), and anti-mouse IgG Alexa-Fluor*488. Samples were acquired on a FACSCalibur and analyzed using the CellQuest Pro Software (BD Biosciences, Mountain View, CA).
TCR internalization Assay
5×105 splenocytes or LPS-matured BMDC that had been pulsed with graded concentrations of peptide were co-cultured with CFSE-labeled TgN(TCRP14LCMV) Rag2tm1 splenocytes (2×105/well) for 5 hours at 37°C. TCR surface expression was determined by staining with PE-labeled anti-TCR antibody (clone H57) followed by flow cytometric analysis.
Results
CTL generated by stimulation with mature BMDC pulsed with a high vs. low concentration of peptide exhibit similar functional avidity
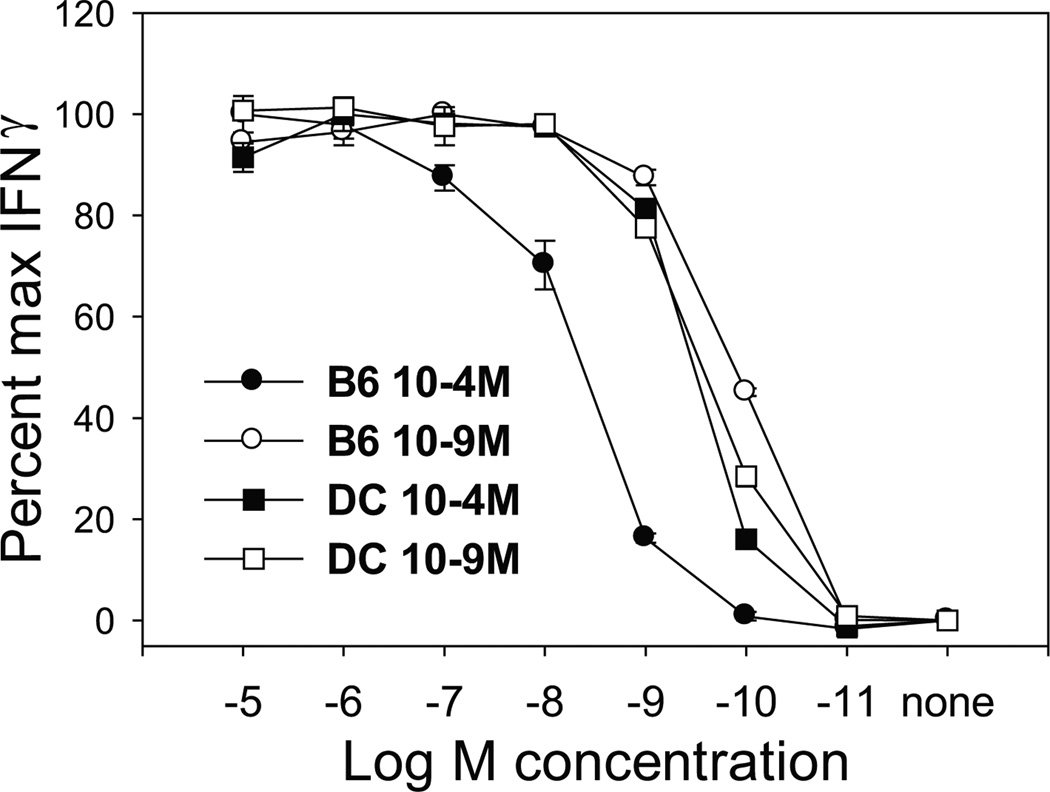
Previously published data from our lab have demonstrated that both low and high avidity effector cells can be generated from TgN(TCRP14LCMV) Rag2tm1 splenocytes, specific for the LCMV gp33–41 peptide (9,10,17). These CTL were generated by stimulation with peptide pulsed naïve splenocytes, a population with a very limited number of DC. Given that mature DC are the predominant activators of naïve T cells in vivo, we determined whether stimulation with this APC would also give rise to T cells with distinct functional avidities. TgN(TCRP14LCMV) Rag2tm1 CD8+ T cell lines were generated using our standard approach of stimulation with splenocytes or alternatively using LPS-matured BMDC, each pulsed with a high (10−4M) vs. low (10−9M) concentration of gp33–41 peptide. On day 7 post-tertiary stimulation, functional avidity was examined by assessing production of IFNγ. As previously reported, CTL stimulated with 10−4M peptide pulsed splenocytes required ~100-fold more peptide for half-maximal production of IFNγ compared to the CTL stimulated with the 10−9M peptide pulsed splenocytes (Fig. 1). This indicated that, as expected, CTL of distinct functional avidities were generated by stimulation with this predominantly non-professional APC population. However, regardless of the priming antigen concentration used, CTL stimulated with DC exhibited a similar requirement for peptide. In fact, both CTL lines exhibited a characteristically high avidity phenotype, similar to the CTL generated by stimulation with 10−9M peptide-pulsed splenocytes. This was the case at all times tested (i.e. following primary and secondary stimulation). Thus, for DC, the stimulatory peptide dose does not appear to control the quality of the T cell response generated, as both low and high concentrations of peptide led to the generation of high avidity CTL.
Figure 1. Stimulation with mature DC resulted in high avidity CTL regardless of the level of peptide.
TgN(TCRP14LCMV) Rag2tm1 cells were stimulated weekly for three rounds with either 10−4M (●) or 10−9M (○) peptide pulsed C57BL/6 splenocytes or 10−4M (■) or 10−9M (□) peptide pulsed LPS matured BMDC. Day 7 post tertiary stimulation, CTL were co-cultured with EL4 cells previously pulsed with the indicated peptide concentration and IFNγ production determined by ELISA. Data shown are representative of 3 independently generated panels of lines.
The failure by DC to induce low avidity CTL is not the result of insufficient peptide presentation
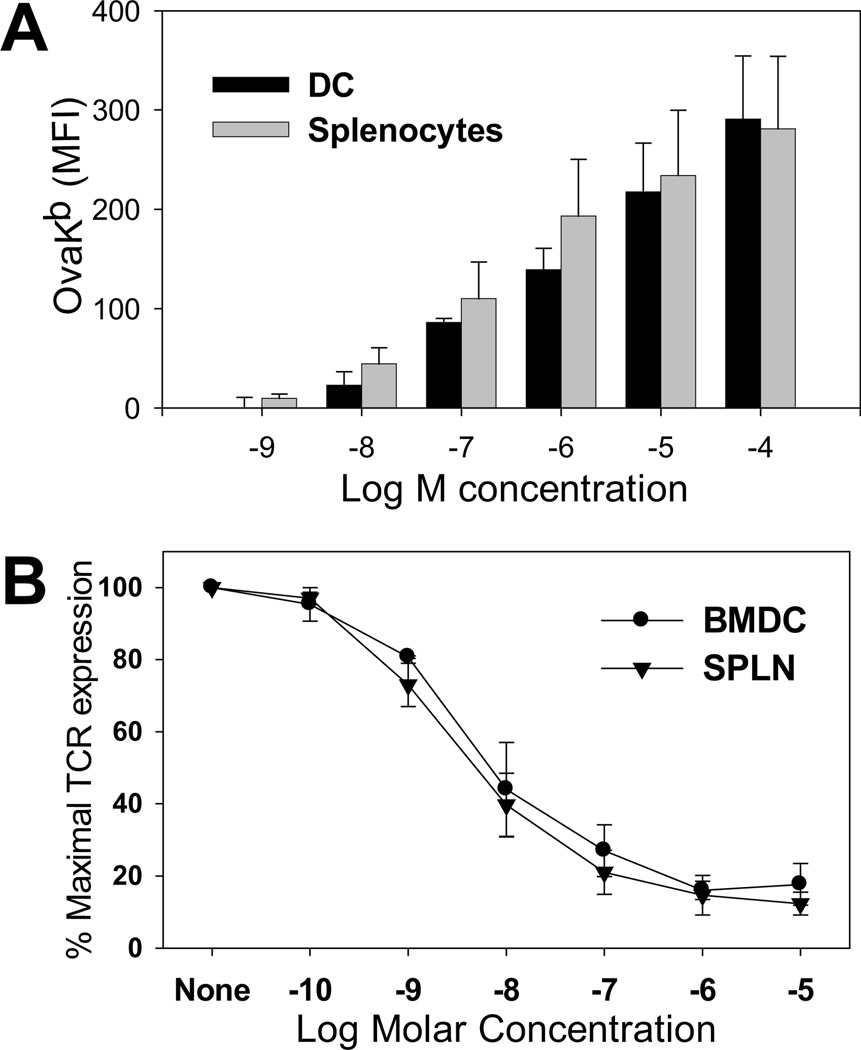
One possibility to explain the failure to generate low avidity CTL was that the number of accessible MHC binding sites on the DC was very low, thus limiting the level of peptide loading in that cell type. We used two approaches to test this possibility: 1) examining the level of exogenously loaded Ova257–263 peptide given that an Ova257–264/Kb-specific antibody is available (18) (no such reagent exists to measure presentation of gp33–41) and 2) determining the degree of TCR internalization on naïve TgN(TCRP14LCMV) Rag2tm1 cells following gp33–41 peptide encounter. For measuring Ova257–264 loading, LPS matured BMDC and C57BL/6 splenocytes were pulsed with the indicated concentration of peptide and stained with the Ova257–264/Kb-specific antibody. As shown in figure 2A, both APC exhibited similar levels of Ova257–264/Kb complexes at each antigen concentration. Using TCR internalization as a probe for the level of presented peptide, we found that co-culture with LPS-matured BMDC and splenocytes resulted in a comparable dose dependent decrease in TCR cell surface expression (Fig 2.B), consistent with TCR engagement of a similar number of pMHC molecules on the two APC. Together these data suggest mature BMDC have accessible binding sites and that there is a similar dose dependent loading of peptide onto the MHC molecules of splenocytes vs. mature BMDC. Thus it is highly unlikely that limiting pMHC would explain the failure of BMDC pulsed with the high concentration of peptide to generate lower avidity cells.
Figure 2. DC and C57BL/6 splenocytes present similar levels of peptide following pulsing.
(A) LPS matured BMDC or C56BL/6 splenocytes were pulsed with graded concentrations of OVA257–264 followed by staining with an antibody that recognizes the Ova257–264/Kb complex. Data shown are the average of 4 independent experiments. (B) LPS matured BMDC or splenocytes pulsed with titrated concentrations of gp33–41 peptide were incubated with CFSE-labeled TgN(TCRP14LCMV) Rag2tm1 splenocytes for 5 hours. TCR expression levels were quantified using an anti-TCR antibody. Data are the average of 3 independent experiments.
High and low avidity CTL can be generated using an equivalent number of non-BMDC stimulators
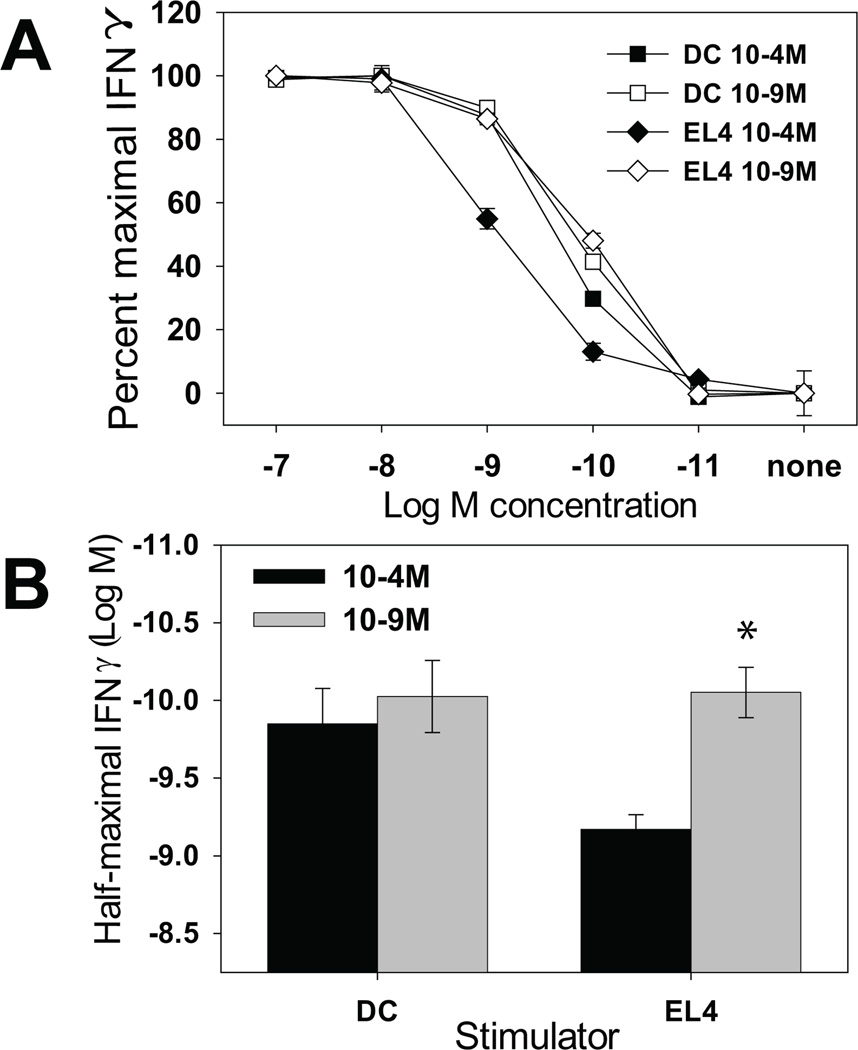
Another potential explanation for the inability of BMDC to modulate T cell avidity is that different numbers of splenocytes and BMDC were used to stimulate the TgN(TCRP14LCMV) Rag2tm1 cells (5×106 vs. 5×104). Initially we attempted to generate CTL lines by increasing the number of DC to 5×106 or decreasing the number of splenocytes to 5×104. However this resulted in either a high level of T cell death (with the BMDC) or poor recruitment of cells into the activated population (with the splenocytes). Therefore we turned to EL4 cells, a thymoma cell line, as a non-DC stimulator. TgN(TCRP14LCMV) Rag2tm1 cells were stimulated with 2×105 EL4 cells or mature BMDC. CTL generated with BMDC again exhibited no difference in their peptide sensitivity. In contrast, EL4 cells presenting the high vs. low concentration of antigen resulted in the generation of low vs. high avidity CTL, respectively. The amount of peptide required for ½ maximal IFN-γ production from multiple CTL lines generated in this fashion is shown in figure 3B. The generation of both high and low avidity CTL using the matched number of EL4 cells and BMDC as APC suggested that the lower BMDC number was not responsible for the failure of DC to generate low avidity cells.
Figure 3. EL4 cells can generate CTL with distinct functional avidities.
TgN(TCRP14LCMV) Rag2tm1 cells were stimulated weekly with 10−4M or 10−9M peptide pulsed LPS matured BMDC or EL4 cells. The functional avidity of the resulting CTL lines was determined as in figure 1. Data shown are (A) representative peptide dose-response curves from the lines and (B) the average amount of peptide required to obtain ½ maximal IFNγ production from 4 independently generated lines. *=p<0.05
T cells initially stimulated with DC are capable of modulating their functional avidity following stimulation with nonprofessional APC
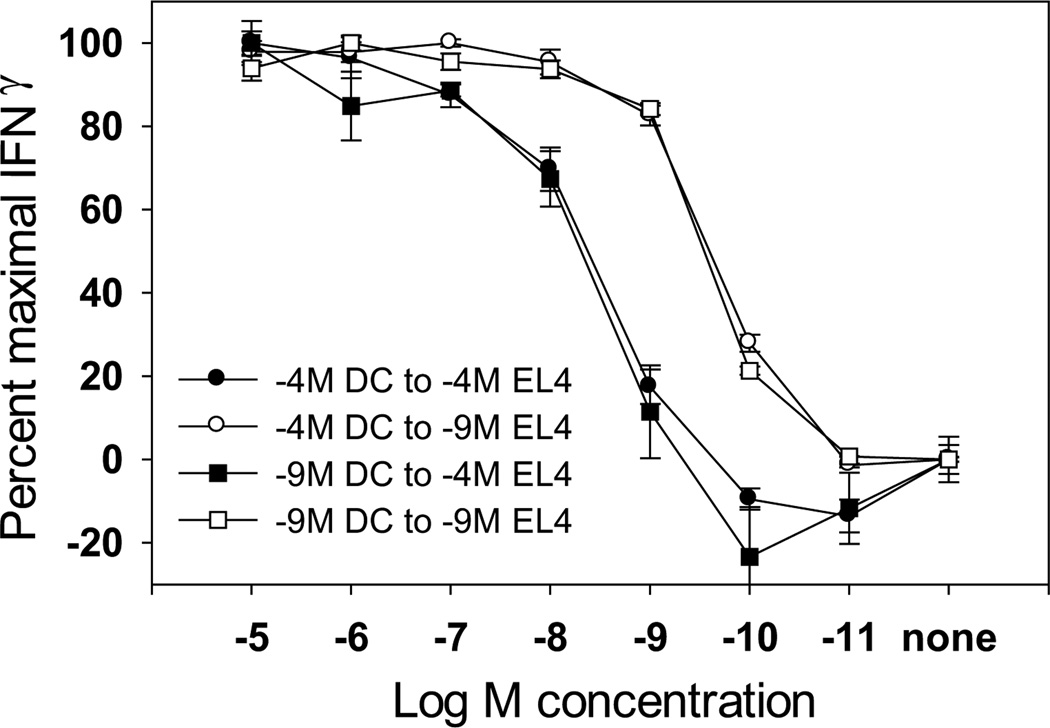
It is known that CTL clones with distinct functional avidities are generated in vivo during an immune response (1) and that naïve T cells are activated by DC. While these data may appear at odds with the findings presented here, in the context of a viral infection CTL would encounter antigen presented by various cell types, including DC in the lymphoid tissue and non-professional APC in the periphery, i.e. epithelial cells. We hypothesized that subsequent interactions with non-professional APC presenting a high level of peptide could induce the T cells to modulate their functional avidity. To address this possibility, we sought to determine if naïve T cells stimulated with mature DC exhibited a “fixed” peptide sensitivity or if following encounter with a non-professional APC, the responding CTL could “fine tune” their peptide sensitivity according to the stimulatory concentration of peptide. Naïve TgN(TCRP14LCMV) Rag2tm1 cells were stimulated with mature DC previously pulsed with the high or low concentration of peptide. Post-primary stimulation, the 10−4M and 10−9M-stimulated CTL were divided and restimulated for two additional rounds with EL4 cells pulsed with either a high or low concentration of peptide. The functional avidity of the various cultures was then examined (Fig. 4). In support of the proposed hypothesis, differences in functional avidity could be detected when CTL initially stimulated with BMDC were restimulated with EL4 cells presenting a high vs. low concentration of peptide. CTL stimulated with 10−4 M-pulsed EL4 cells required a higher concentration of peptide for their half-maximal production of IFN-γ compared to the CTL stimulated with 10−9M-pulsed EL4 cells. Therefore CTL initially activated by DC appear to maintain a window of plasticity that allows “tuning” of their peptide sensitivity following encounter with a non-professional APC.
Figure 4. CTL initially stimulated with mature DC can subsequently modulate their functional avidity following encounter with nonprofessional APC.
Naïve TgN(TCRP14LCMV) Rag2tm1 cells were stimulated with mature BMDC pulsed with high or low amounts of peptide. Post primary stimulation, the CTL were divided and restimulated twice with EL4 cells pulsed with 10−4M or 10−9M peptide. Post-tertiary stimulation, the functional avidity of the CTL lines was determined. The data shown are representative of 4 independently generated panels of lines.
Discussion
Although the property of functional avidity is well documented, much remains to be learned with regard to the mechanism(s) that regulate this T cell property. In addition, the extrinsic factors that direct how high and low avidity CTL arise in vivo remain unclear. Dendritic cells are considered to be the most potent APC for the activation of naïve T cells due to their increased expression of costimulatory molecules and cytokines, i.e. IL-12 (reviewed in (13,14)). Interestingly, high costimulatory molecule expression and IL-12 have been shown to promote higher avidity CTL responses (7,11,12). Therefore, we sought to determine whether mature DC presenting different concentrations of peptide were capable of generating high vs. low avidity CTL, or if the signals provided by mature DC would inhibit the emergence of T cells with distinct functional avidities.
Our data demonstrated that the CD8+ T cells generated by stimulation with mature BMDC did not exhibit dose-dependent differences in peptide sensitivities; rather the responding CTL were of high avidity regardless of pMHC levels. This is in stark contrast to the differences in functional avidity observed following stimulation with peptide pulsed non-professional APC. The inability of mature DC to induce low avidity cells was also observed using splenic DC (data not shown).
In addition to differences in peptide sensitivity, we have previously found that CD8 is expressed differentially between high and low avidity TgN(TCRP14LCMV) Rag2tm1 CTL, with low avidity lines exhibiting either lower absolute CD8αβ levels or lower β:α ratios, the latter suggesting higher expression of CD8αα homodimers (8–10,19). Consistent with the failure of DC to induce CTL of low avidity, cells stimulated with mature DC pulsed with a high vs. low concentration of peptide both exhibited high levels of CD8 (data not shown). Of note, the increased CD8 expression on high avidity CTL generated by stimulation with 10−4M pulsed DC did not appear to solely account for the high avidity phenotype. Removing the contribution of CD8 (by the addition of blocking antibody) in the lines generated by stimulation with high peptide pulsed DC vs. splenocytes did not result in similar peptide sensitivities (data not shown). Thus while increased CD8 expression likely contributes to high avidity in the DC generated line, it does not seem sufficient to explain it. In summary, our findings show that while the level of pMHC on non-professional APC drives the generation of high vs. low avidity CTL, DC selectively promote generation of CTL with high avidity regardless of stimulatory pMHC levels.
Given the reported role for costimulatory molecules and IL-12 in promoting high avidity CTL (7), we tested whether the presence of these DC-derived signals might explain the selective generation of high avidity cells in this model. However, BMDC from CD80/86, ICAM-1, IL-15, or p35 deficient animals all failed to generate CTL with differences in avidity (data not shown). Therefore, none of these molecules independently was responsible for the selective generation of high avidity cells by DC.
It has recently been shown that the IS formed by naïve T cells following encounter with DC differs from the iconical bulls-eye pattern formed following interaction with B cells. T cells conjugated to DC preferentially form multifocal IS (for review see (20)). Such changes have the potential to significantly impact the nature of the signal delivered, either quantitatively or qualitatively. Additionally, the kinetics of TCR signaling within T cells appears to be different upon conjugation with a professional vs. non-professional APC. Delon et al showed that naïve T cells in conjugate with DC fluxed Ca+2 with increased kinetics compared to resting B cells (21). Additionally, with regard to the length of time T cells are in conjugate with the APC, previous studies have shown that contacts between mature DC are more transient and dynamic compared to those with resting B cells (22). It is tempting to speculate that these transient contacts fail to result in the signal strength that may be required for the induction of low avidity, or the additional signaling through co-stimulatory molecules or cytokine receptors produces a unique signaling pattern protecting the cells from the consequences of encountering high levels of pMHC such as death or differentiation into low avidity CTL.
The results from our study shed light on a previously published report from Bullock et al. (23). In that study DC pulsed with a high vs. low concentration of peptide were used to immunize mice. No differences in avidity were detected in populations generated by immunization with DC pulsed with high versus low peptide levels. However, whether the failure to generate cells of disparate avidity was the result of in vitro vs. in vivo differences or in the nature of the APC was unknown. Our data support the latter. The ability of DC to promote the generation of high avidity cells even in the presence of high levels of antigen could be of significant benefit as the level of antigen presented by DC may not reflect that which would be encountered in the periphery. Such divergence in antigen levels could result from cell-type specific differences in permissivity to infection. The preferential generation of high avidity cells by DC would promote an optimally effective population as the default. In this scenario, if the level of presented peptide were significantly higher in the periphery, effectors would retain the capacity to down-modulate their avidity to protect from apoptosis (24). Such a system would promote optimal recognition and thus clearance by CTL.
ACKNOWLEDGEMENTS
We thank Dr. Ron Germain for provision of the 25.D1.16 hybridoma. We appreciate the helpful comments of Dr. Sharad Sharma and Dr. Marlena Wescott.
Abbreviations used
- BMDC
bone marrow derived dendritic cells
- DC
dendritic cells
- GM-CSF
granulocyte monocyte colony stimulating factor
- pMHC
peptide MHC
- IS
immunological synapse
Footnotes
This work was supported by National Institutes of Health grant R01 AI43591 to M.A.A-M. C.J.K was supported by a National Research Training Award: Grant AI07401.
Reference List
- 1.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc.Natl.Acad.Sci.USA. 1996;93:4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallimore A, Dumrese T, Hengartner H, Zinkernagel RM, Rammensee HG. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J.Exp.Med. 1998;187:1647–1657. doi: 10.1084/jem.187.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yee C, Savage PA, Lee PP, Davis MM, Greenberg PD. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J.Immunol. 1999;162:2227–2234. [PubMed] [Google Scholar]
- 4.Zeh HJ, Perry-Lalley D, III, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J.Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 5.Sedlik C, Dadaglio G, Saron MF, Deriaud E, Rojas M, Casal SI, Leclerc C. In vivo induction of a high-avidity, high-frequency cytotoxic T-lymphocyte response is associated with antiviral protective immunity. J.Virol. 2000;74:5769–5775. doi: 10.1128/jvi.74.13.5769-5775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15R alpha-mediated avidity maturation of memory CD8+ T cells. Proc.Natl.Acad.Sci.USA. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu SW, Koski GK, Faries M, Bedrosian I, Mick R, Maeurer M, Cheever MA, Cohen PA, Czerniecki BJ. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J.Immunol. 2003;171:2251–2261. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 8.Renard V, Romero P, Vivier E, Malissen B, Luescher IF. CD8 beta increases CD8 coreceptor function and participation in TCR- ligand binding. J.Exp.Med. 1996;184:2439–2444. doi: 10.1084/jem.184.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cawthon AG, Lu H, Alexander-Miller MA. Peptide requirement for CTL activation reflects the sensitivity to CD3 engagement: Correlation with CD8αβ versus CD8αα expression. J.Immunol. 2001;167:2577–2584. doi: 10.4049/jimmunol.167.5.2577. [DOI] [PubMed] [Google Scholar]
- 10.Kroger CJ, Alexander-Miller MA. Dose-dependent modulation of CD8 and functional avidity as a result of peptide encounter. Immunology. 2007;122:167–178. doi: 10.1111/j.1365-2567.2007.02622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh S, Hodge JW, Ahlers JD, Burke DS, Schlom J, Berzofsky JA. Selective induction of high avidity CTL by altering the balance of signals from APC. J.Immunol. 2003;170:2523–2530. doi: 10.4049/jimmunol.170.5.2523. [DOI] [PubMed] [Google Scholar]
- 12.Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J.Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu.Rev.Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 15.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 16.Pejawar SS, Parks GD, Alexander-Miller MA. Abortive versus productive viral infection of dendritic cells with a paramyxovirus results in differential upregulation of select costimulatory molecules. J.Virol. 2005;79:7544–7557. doi: 10.1128/JVI.79.12.7544-7557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroger CJ, Alexander-Miller MA. Cutting edge: CD8+ T cell clones possess the potential to differentiate into both high- and low-avidity effector cells. J Immunol. 2007;179:748–751. doi: 10.4049/jimmunol.179.2.748. [DOI] [PubMed] [Google Scholar]
- 18.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide- MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 19.Cawthon AG, Alexander-Miller MA. Optimal colocalization of TCR and CD8 as a novel mechanism for the control of functional avidity. J.Immunol. 2002;169:3492–3498. doi: 10.4049/jimmunol.169.7.3492. [DOI] [PubMed] [Google Scholar]
- 20.Dustin ML, Tseng SY, Varma R, Campi G. T cell-dendritic cell immunological synapses. Curr.Opin.Immunol. 2006;18:512–516. doi: 10.1016/j.coi.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Delon J, Bercovici N, Raposo G, Liblau R, Trautmann A. Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J.Exp.Med. 1998;188:1473–1484. doi: 10.1084/jem.188.8.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunzer M, Weishaupt C, Hillmer A, Basoglu Y, Friedl P, Dittmar KE, Kolanus W, Varga G, Grabbe S. A spectrum of biophysical interaction modes between T cells and different antigen-presenting cells during priming in 3-D collagen and in vivo. Blood. 2004;104:2801–2809. doi: 10.1182/blood-2004-03-1193. [DOI] [PubMed] [Google Scholar]
- 23.Bullock TNJ, Mullins DW, Engelhard VH. Antigen density presented by dendritic cells in vivo differentially affects the number and avidity of primary, memory, and recall CD8+ T cells. J.Immunol. 2003;170:1822–1829. doi: 10.4049/jimmunol.170.4.1822. [DOI] [PubMed] [Google Scholar]
- 24.Alexander-Miller MA, Leggatt GR, Sarin A, Berzofsky JA. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J.Exp.Med. 1996;184:485–492. doi: 10.1084/jem.184.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]