Abstract
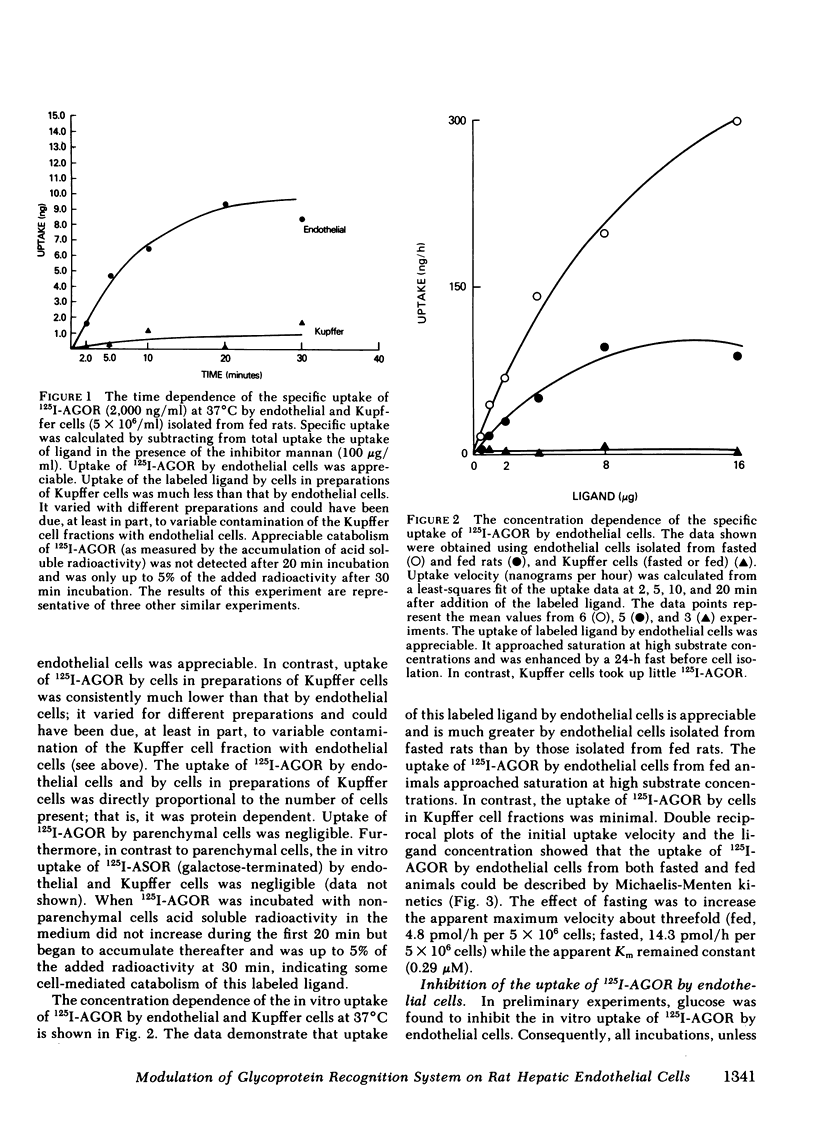
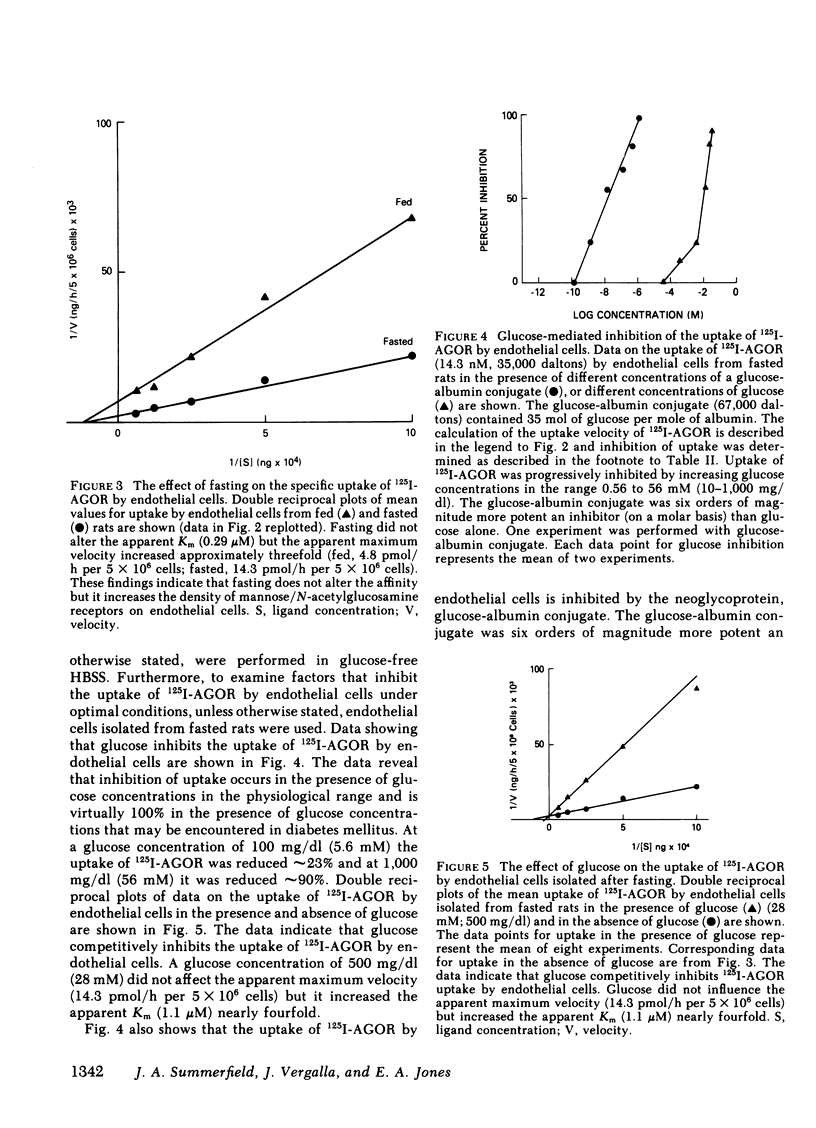
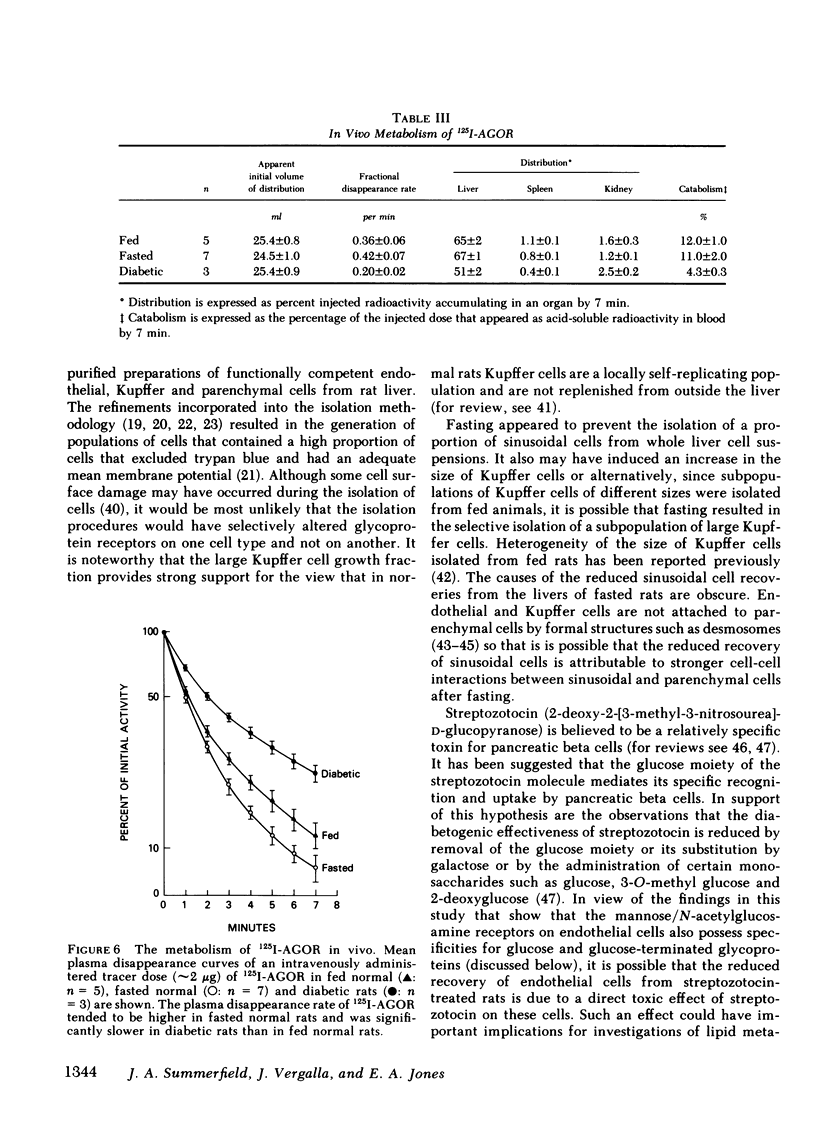
The cellular location and carbohydrate specificities of a glycoprotein recognition system on rat hepatic sinusoidal cells have been determined. Purified preparations of endothelial, Kupffer, and parenchymal cells were prepared by collagenase liver perfusion, centrifugation on Percoll gradients, and centrifugal elutriation. 125I-labeled agalactoorosomucoid, an N-acetylglucosamine-terminated glycoprotein, was selectively taken up in vitro by endothelial cells. Uptake was shown to be protein dependent, calcium ion dependent, and saturable, and could be described by Michaelis-Menten kinetics (apparent Km 0.29 μM; apparent maximum velocity 4.8 pmol/h per 5 × 106 cells). Uptake was inhibited not only by N-acetylglucosamine, mannose, and mannan but also by glucose, fructose, and a glucose-albumin conjugate. Inhibition by glucose was competitive over a wide range of concentrations and was almost 100% at a glucose concentration of 56 mM. Fasting and the induction of diabetes mellitus prior to isolation of cells was associated with 60% reductions in the recovery of endothelial cells. Uptake by cells isolated from fasted rats was enhanced (apparent maximum velocity 14.3 pmol/h per 5 × 106 cells without change in the apparent Km). These observations suggest that fasting is associated with a marked increase in the mean number of glycoprotein receptors per endothelial cell isolated from normal rats. This effect of fasting could be due to upregulation of glycoprotein receptors on endothelial cells or to the selective isolation of a subpopulation of endothelial cells from fasted animals that bears more glycoprotein receptors per cell than does another subpopulation of these cells. In addition, in vivo studies of the fate of intravenously administered 125I-agalactoorosomucoid indicated that its rate of disappearance from plasma, hepatic accumulation, and catabolism were slower in diabetic than in normal rats. The results suggest that modulation of a carbohydrate-mediated glycoprotein recognition system located on hepatic endothelial cells can be induced by glucose and glucose-conjugated proteins and by fasting and diabetes mellitus. The findings in this study suggest a mechanism for abnormal glycoprotein metabolism in diabetes mellitus.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Bell C. E., Sly W. S. Human beta-glucuronidase: in vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell. 1978 Sep;15(1):269–278. doi: 10.1016/0092-8674(78)90102-2. [DOI] [PubMed] [Google Scholar]
- Achord D. T., Brot F. E., Sly W. S. Inhibition of the rat clearance system for agalacto-orosomucoid by yeast mannans and by mannose. Biochem Biophys Res Commun. 1977 Jul 11;77(1):409–415. doi: 10.1016/s0006-291x(77)80213-1. [DOI] [PubMed] [Google Scholar]
- Achord D., Brot F., Gonzalez-Noriega A., Sly W., Stahl P. Human beta-glucuronidase. II. Fate of infused human placental beta-glucuronidase in the rat. Pediatr Res. 1977 Jul;11(7):816–822. doi: 10.1203/00006450-197707000-00008. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Baur H., Kasperek S., Pfaff E. Criteria of viability of isolated liver cells. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):827–838. doi: 10.1515/bchm2.1975.356.s1.827. [DOI] [PubMed] [Google Scholar]
- Baynes J. W., Wold F. Effect of glycosylation on the in vivo circulating half-life of ribonuclease. J Biol Chem. 1976 Oct 10;251(19):6016–6024. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. L., Henderson L. A., Thorpe S. R., Baynes J. W. The effect of alpha-mannose-terminal oligosaccharides on the survival of glycoproteins in the circulation. Rapid uptake and catabolism of bovine pancreatic ribonuclease B by nonparenchymal cells of rat liver. Arch Biochem Biophys. 1978 Jun;188(2):418–428. doi: 10.1016/s0003-9861(78)80026-5. [DOI] [PubMed] [Google Scholar]
- Bunn H. F. Nonenzymatic glycosylation of protein: relevance to diabetes. Am J Med. 1981 Feb;70(2):325–330. doi: 10.1016/0002-9343(81)90769-5. [DOI] [PubMed] [Google Scholar]
- Carson E. R., Jones E. A. Use of kinetic analysis and mathematical modeling in the study of metabolic pathways in vivo. Applications to hepatic organic anion metabolism. (First of two parts). N Engl J Med. 1979 May 3;300(18):1016–1027. doi: 10.1056/NEJM197905033001804. [DOI] [PubMed] [Google Scholar]
- EARL D. C., KORNER A. THE ISOLATION AND PROPERTIES OF CARDIAC RIBOSOMES AND POLYSOMES. Biochem J. 1965 Mar;94:721–734. doi: 10.1042/bj0940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Wilson G., Ashwell G., Stukenbrok H. An electron microscope autoradiographic study of the carbohydrate recognition systems in rat liver. I. Distribution of 125I-ligands among the liver cell types. J Cell Biol. 1979 Oct;83(1):47–64. doi: 10.1083/jcb.83.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Isolation and characterization of an avian hepatic binding protein specific for N-acetylglucosamine-terminated glycoproteins. J Biol Chem. 1977 Sep 25;252(18):6536–6543. [PubMed] [Google Scholar]
- Kawasaki T., Etoh R., Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit liver. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1018–1024. doi: 10.1016/0006-291x(78)91452-3. [DOI] [PubMed] [Google Scholar]
- Knook D. L., Sleyster E. C. Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp Cell Res. 1976 May;99(2):444–449. doi: 10.1016/0014-4827(76)90605-4. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Blobstein S. H., Cerami A. Structure of carbohydrate of hemoglobin AIc. J Biol Chem. 1977 May 10;252(9):2992–2997. [PubMed] [Google Scholar]
- Kominami E., Hashida S., Katunuma N. Inhibitions of degradation of rat liver aldolase and lactic dehydrogenase by N-[N-(L-3-trans-carboxyoxirane-2-carbonyl)-L-leucyl] agmatine or leupeptin in vivo. Biochem Biophys Res Commun. 1980 Apr 14;93(3):713–719. doi: 10.1016/0006-291x(80)91136-5. [DOI] [PubMed] [Google Scholar]
- Krantz M. J., Holtzman N. A., Stowell C. P., Lee Y. C. Attachment of thioglycosides to proteins: enhancement of liver membrane binding. Biochemistry. 1976 Sep 7;15(18):3963–3968. doi: 10.1021/bi00663a009. [DOI] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary R., Shaw E. Inactivation of cathepsin B1 by diazomethyl ketones. Biochem Biophys Res Commun. 1977 Dec 7;79(3):926–931. doi: 10.1016/0006-291x(77)91199-8. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Stowell C. P., Krantz M. J. 2-Imino-2-methoxyethyl 1-thioglycosides: new reagents for attaching sugars to proteins. Biochemistry. 1976 Sep 7;15(18):3956–3963. doi: 10.1021/bi00663a008. [DOI] [PubMed] [Google Scholar]
- Lentz P. E., Di Luzio N. R. Isolation of adult rat liver macrophages (Kupffer cells). Methods Enzymol. 1974;32:647–653. doi: 10.1016/0076-6879(74)32067-8. [DOI] [PubMed] [Google Scholar]
- McPhie J. L. Peroxidase activity in non-parenchymal cells isolated from rat liver: a cytochemical study. Acta Hepatogastroenterol (Stuttg) 1979 Dec;26(6):442–445. [PubMed] [Google Scholar]
- Montesano R. Junctions between sinusoidal endothelial cells in fetal rat liver. Am J Anat. 1975 Nov;144(3):387–391. doi: 10.1002/aja.1001440312. [DOI] [PubMed] [Google Scholar]
- Mordes J. P., Rossini A. A. Animal models of diabetes. Am J Med. 1981 Feb;70(2):353–360. doi: 10.1016/0002-9343(81)90772-5. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seglen P. O., Seljelid R. Mass isolation and culture of rat kupffer cells. J Exp Med. 1975 Jan 1;141(1):1–10. doi: 10.1084/jem.141.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opheim D. J., Touster O. alpha-D-Mannosidase from rat liver lysosomes. Methods Enzymol. 1978;50:494–500. doi: 10.1016/0076-6879(78)50051-7. [DOI] [PubMed] [Google Scholar]
- Pertoft H., Laurent T. C., Lås T., Kågedal L. Density gradients prepared from colloidal silica particles coated by polyvinylpyrrolidone (Percoll). Anal Biochem. 1978 Jul 15;88(1):271–282. doi: 10.1016/0003-2697(78)90419-0. [DOI] [PubMed] [Google Scholar]
- Pertoft H., Rubin K., Kjellén L., Laurent T. C., Klingeborn B. The viability of cells grown or centrifuged in a new density gradient medium, Percoll(TM). Exp Cell Res. 1977 Dec;110(2):449–457. doi: 10.1016/0014-4827(77)90311-1. [DOI] [PubMed] [Google Scholar]
- Pizzo S. V., Lehrman M. A., Imber M. J., Guthrow C. E. The clearance of glycoproteins in diabetic mice. Biochem Biophys Res Commun. 1981 Jul 30;101(2):704–708. doi: 10.1016/0006-291x(81)91315-2. [DOI] [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Sainsbury G. M., Stubbs M., Hems R., Krebs H. A. Loss of cell constituents from hepatocytes on centrifugation. Biochem J. 1979 Jun 15;180(3):685–688. doi: 10.1042/bj1800685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M., Liao J., Kabat E. A., Tanabe T., Ashwell G. The binding site of rabbit hepatic lectin. J Biol Chem. 1979 May 10;254(9):3170–3174. [PubMed] [Google Scholar]
- Schlesinger P. H., Doebber T. W., Mandell B. F., White R., DeSchryver C., Rodman J. S., Miller M. J., Stahl P. Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with beta-glucuronidase, N-acetyl-beta-D-glucosaminidase, ribonuclease B and agalacto-orosomucoid. Biochem J. 1978 Oct 15;176(1):103–109. doi: 10.1042/bj1760103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger P. H., Rodman J. S., Doebber T. W., Stahl P. D., Lee Y. C., Stowell C. P., Kuhlenschmidt T. B. The role of extra-hepatic tissues in the receptor-mediated plasma clearance of glycoproteins terminated by mannose or N-acetylglucosamine. Biochem J. 1980 Nov 15;192(2):597–606. doi: 10.1042/bj1920597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger P., Rodman J. S., Frey M., Lang S., Stahl P. Clearance of lysosomal hydrolases following intravenous infusion. The role of liver in the clearance of beta-glucuronidase and N-acetyl-beta-D-glucosaminidase. Arch Biochem Biophys. 1976 Dec;177(2):606–614. doi: 10.1016/0003-9861(76)90472-0. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer C. J., Clarenburg R. Unique distribution of glycoprotein receptors on parenchymal and sinusoidal cells of rat liver. J Biol Chem. 1979 Jun 10;254(11):4457–4461. [PubMed] [Google Scholar]
- Steer C. J., Kusiak J. W., Brady R. O., Jones E. A. Selective hepatic uptake of human beta-hexosaminidase A by a specific glycoprotein recognition system on sinusoidal cells. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2774–2778. doi: 10.1073/pnas.76.6.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. The existence of a second route for the transfer of certain glycoproteins from the circulation into the liver. Biochem Biophys Res Commun. 1976 Feb 9;68(3):988–993. doi: 10.1016/0006-291x(76)91243-2. [DOI] [PubMed] [Google Scholar]
- Vierling J. M., Steer C. J., Bundy B. M., Strober W., Jones E. A., Hague N. E., Nelson D. L. Studies of complement receptors on cytotoxic effector cells in human peripheral blood. Cell Immunol. 1978 Feb;35(2):403–413. doi: 10.1016/0008-8749(78)90159-4. [DOI] [PubMed] [Google Scholar]
- Widmann J. J., Cotran R. S., Fahimi H. D. Mononuclear phagocytes (Kupffer cells) and endothelial cells. Identification of two functional cell types in rat liver sinusoids by endogenous peroxidase activity. J Cell Biol. 1972 Jan;52(1):159–170. doi: 10.1083/jcb.52.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelhake J. L., Nicolson G. L. Aglycosylantibody. Effects of exoglycosidase treatments on autochthonous antibody survival time in the circulation. J Biol Chem. 1976 Feb 25;251(4):1074–1080. [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Preparation of plasma-membrane subfractions from isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):415–422. doi: 10.1042/bj1640415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J Ultrastruct Res. 1970 Apr;31(1):125–150. doi: 10.1016/s0022-5320(70)90150-4. [DOI] [PubMed] [Google Scholar]
- Wisse E. Observations on the fine structure and peroxidase cytochemistry of normal rat liver Kupffer cells. J Ultrastruct Res. 1974 Mar;46(3):393–426. doi: 10.1016/s0022-5320(74)90064-1. [DOI] [PubMed] [Google Scholar]
- Yee A. G., Revel J. P. Endothelial cell junctions. J Cell Biol. 1975 Jul;66(1):200–204. doi: 10.1083/jcb.66.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahlten R. N., Hagler H. K., Nejtek M. E., Day C. J. Morphological characterization of Kupffer and endothelial cells of rat liver isolated by counterflow elutriation. Gastroenterology. 1978 Jul;75(1):80–87. [PubMed] [Google Scholar]


