Abstract
Objectives:
To evaluate the effect of Mifepristone (25 mg) on symptomatic myoma in perimenopausal women.
Study Design:
Open label clinical trial.
Materials and Methods:
Ninety three perimenopausal women of age 35-50 years having symptomatic myoma were selected from Gynecology OPD and given 25 mg Mifepristone once daily continuously for three months. Variables as; baseline uterine size, uterine volume, myoma size, volume, their number, position, characteristics, hemoglobin and blood parameters, were taken and followed monthly for six months. Bleeding and pain scores were checked on monthly visits. Changes in above parameters were tabulated during the first three months treatment phase and then next three post-treatment phase for analysis.
Statistical Analysis:
Was done by calculating mean, standard deviation, standard error and percentage distribution of variables.
Results:
Menorrhagia was the most common symptom which led patients to report to hospital. Mean uterine volume reduced to 63.69% of baseline, Mean dominant Myoma volume reduced to 53.62% and hemoglobin level raised to 137% after complete three months of treatment. Changes persisted in next three months post-treatment follow-up, while hysterectomy was required in 10 (12.2%) cases.
Conclusion:
Three months treatment of 25 mg Mifepristone effectively controls bleeding, reduces the uterine and myoma volume and thus can avoid blood transfusion and hysterectomy in a lot of symptomatic myoma cases.
Keywords: Anti-progesterone, medical treatment, mifepristone, myoma
INTRODUCTION
Leiomyoma is the common benign tumor of uterus occurring in up to 20% of women with maximum incidence between 35-45 years of age leading to menorrhagia, pain and lump in abdomen.[1] Severity of symptoms typically depends on size, number of myomas, and tumor location. They represent one of the most frequent indications of operative procedures in woman of reproductive age. Leiomyomas are the most cited indication for more than 600,000 hysterectomies that are performed in the US annually, which is associated with morbidity, mortality and huge economic burden, estimated to be approximately $34.4 billion/year.[2]
Other treatment modes for uterine myoma includes - Myolysis, embolization of feeding arteries (invasive), while current medical management options are GnRH agonist and antagonists, selective estrogen receptor modulators (SERM), aromatase inhibitors, danazol, gestrinone, anti-progestogens and progesterone receptor modulators (SPRMS) etc., None of the drug has been approved by FDA for the treatment of myoma yet. Long term GnRH agonist treatment is problematic because of high cost and significant side effects due to hypo-estrogenic environment produced by it. Mifepristone is a synthetic steroid with both anti-progesterone and anti-glucocorticoid activities. The aim of the study was to evaluate the effect of 25 mg Mifepristone on uterine and myoma volume, hemoglobin and symptomatic improvement in view of avoiding hysterectomies and blood transfusions in perimenopausal women. In the present study, we had tried the 25 mg dose for speedy symptomatic improvement over a short period of 3 months.
MATERIALS AND METHODS
This open label clinical trial was done at department of Gynecology at U.P. RIMS and R Saifai, Etawah over a period of 2 years from July 2010 to 2012. Taking a hypothesis that the 25 mg Mifepristone daily for three months will offer at least 20% reduction in myoma volume from the baseline, with 90% power to detect the real difference and 5% significance sample size calculated was ‘82’. Non pregnant women of 35-50 years age (peri-menopausal) group, having “symptomatic myomas” (single or multiple diagnosed by pelvic ultrasonography) who wished to conserve their uterus were enrolled for the study. Excessive uterine bleeding was evidenced by passage of clots, repetitive periods lasting for more than 8 days or cycles length less than 21 days leading to anemia. Hemoglobin less than 11 gm% and hematocrit less than 30% was taken as criteria for anemia. (Mild = 11-10 gm%, moderate = 10-7 gm%, severe = less than 7 gm% and decompensated if less than 4 gm%). All women gave their written informed consent prior to inclusion and accepted the follow-up protocol of the project. Each woman received the Mifepristone 25 mg (prepared from available 200 mg tablet by chemical balance and filled in gelatin capsules) daily for the three months starting from the 3rd to 5th day of menstrual cycle from the hospital.
Exclusion criteria were very large myomas greater than 10 cm in size, history of hormonal treatment in last two months, history of breast cancer or other genital malignancy, pelvic inflammatory disease (PID), adnexal mass, pregnancy, suspicion of leiomyosarcoma on ultrasonography, or any contra-indication to Mifepristone itself as hypersensitivity, severe renal or hepatic disorders.
Blood samples were collected for hemoglobin, blood counts, base line liver and renal function tests, bleeding time, clotting time, along with a detailed baseline pelvic ultrasound to know the exact size and volume of uterus, number, size, volume and location of myomas and endometrial thickness at the start of treatment and then four weekly in follow-up. Three largest diameters (A, B and C) were measured in two planes in approximately perpendicular axis in all myomas. As most of myomas are spherical or ellipsoidal, therefore volume was calculated using formula 0.523 × A × B × C. In case of multiple myomas largest one (dominant) was used for volume calculations and follow-up. Viscosmi formula was used for the uterine volume, that is, 4/3 π W/2 × L/2 × T/2, where W is uterine width on transverse section at uterine fundus; L is uterine length on sagittal section from internal cervical os to fundus and T is uterine thickness measured on sagittal section between the anterior and posterior walls.[3] Women were asked to keep daily records of bleeding and symptoms as pain, pressure or any side effect on a table calendar. Symptoms were graded at every visit on a five point “Likert scale” (0 = no symptoms, 1 = light, 2 = moderate, 3 = severe and 4 = very severe).
After three months drug Mifepristone was withdrawn but cases were followed up similarly for next three months in post-treatment phase. Data were collected, tabulated and analyzed using appropriate statistical methods.
RESULTS
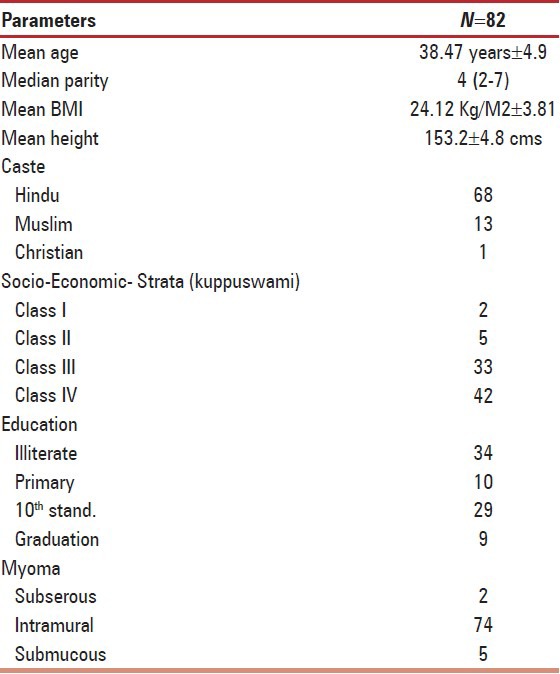
Total 93 cases were enrolled over 2 years from July 2010 to 2012 from Gynecology O.P.D. of U.P. R.I.M.S. and R. Saifai, Etawah. Eleven women left treatment (drop out rate -12%) while rest 82 completed the 3 months treatment protocol. Mean age was 38.47 ± 4.9 years. Demographic parameters are shown in Table 1. Reasons for drop out cannot be commented as they never reported back to us during the study period of 2 years.
Table 1.
Demographic parameters
Abnormal and excessive uterine bleeding (AUB) was the commonest problem reported by 77 cases (93.96%) followed by heaviness in lower abdomen in 22 (26.83%) and pain in 18 (21.95%) for which they came to hospital. Among AUB, 54.8% was menorrhagia, 26.4% poly-menorrhagia, and 12.2% reported menometrorrhagia. Amount of bleeding was not exactly found co-related with size of myoma in our study. Four (4.8%) women myomas presented with normal cycles and one (1.2%) with hypomenorrhea. Bleeding stopped within 4-5 days of start of Mifepristone and 75 women (92.68%) had complete amenorrhoea during treatment phase. Six women (7.32%) did not respond to drug and their myoma volume continued to increase progressively and had hysterectomy was done later. Dysmenorrhea, pelvic pain, heaviness backache, and urinary complaints improved a lot in first month of treatment. Symptom scores for pain showed significant change from average ‘four’ at start of treatment to ‘two’ at end of treatment. No significant changes were observed in liver enzymes or renal profile of cases.
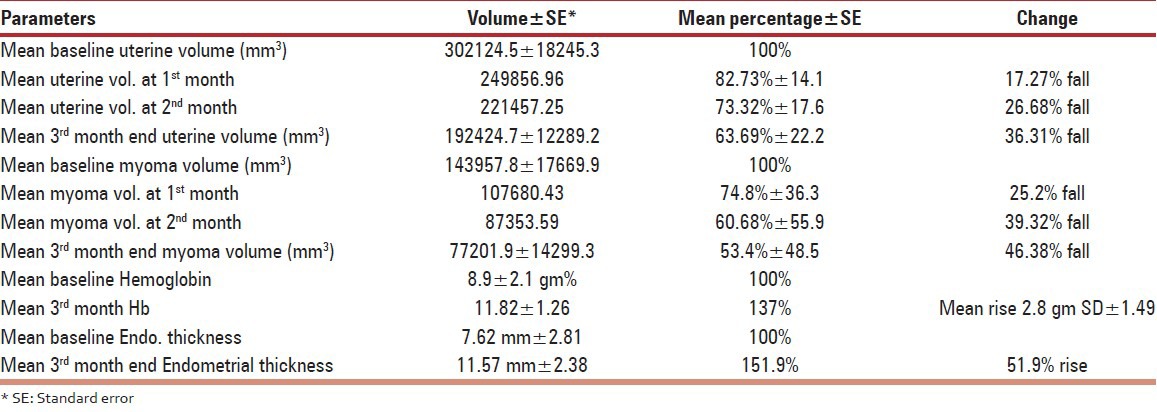
Median number of leiomyomas at baseline sonography was two (range: 1-5) and it was two (range: 1-4) at the end of 3 months. Mean uterine volume decreased to 63.69% (SD ± 22.2), while mean volume of dominant leiomyoma decreased to 53.62% (SD ± 48.5) after three months of complete treatment. Intra-mural and subserous responded well, while in out of five submucous myomas, two pedunculated ones prolapsed out of cervix and required polypectomy and one was non - responder and one had hysterectomy for recurrence. Mifepristone myoma volume decreased to a greater extent compared to uterine volume. Rate of fall in myoma volume was steep initially and flattened in later half of treatment phase [Figure 1].
Figure 1.
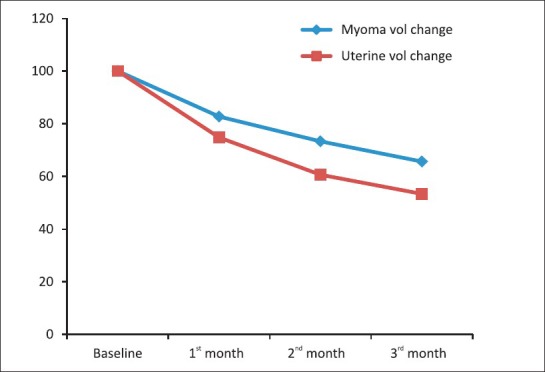
Uterine and Myoma volume change in treatment phase
Hemoglobin counts improved significantly 2.8 gram + SD 1.49, from mean 8.9 gram% at start. Base line endometrial thickness (ET) at start of treatment was 7.6 + 2.8 which progressively increased in all ‘82’ cases during the treatment phase with mean 51.9% rise over three months [Table 2]. Only in two cases ET crossed the 20 mm mark, after which endometrial biopsy was done and simple endometrial hyperplasia was diagnosed in both the cases. No specific side effect of the drug was noted except headache (12%) in first month and hot flush (3.65%) in second month. Liver transaminases (AST, ALT) were found raised in 26 (31.7%) women but none have crossed double of the normal value.
Table 2.
Clinical outcomes after Mifepristone treatment
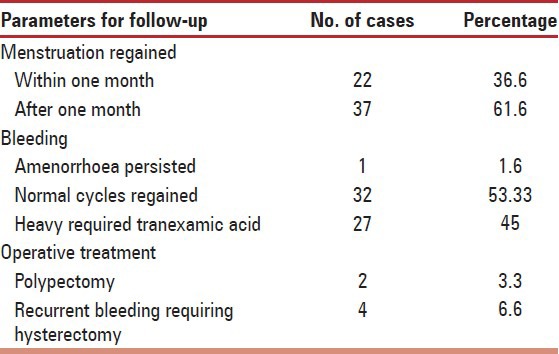
Post-treatment follow-up could be completed only in ‘60’ women as some stopped coming to hospital after drug was withdrawn. Menstruation was regained in mean duration of 34.72 (SD ± 17.48), days. One case persisted with amenorrhoea and only four required hysterectomy for recurrence of heavy bleeding episodes [Table 3]. Initial cycles in post treatment phase were slightly heavier in 45% (27/60) women which responded to hemostatic tranexamic acid. Incidence of hysterectomy was 12.1% (10/82) in the study. In two cases pedunculated submucous myoma prolapsed into vagina at 2nd and 3rd month respectively, in whom polypectomy solved the problem. Blood transfusion was given in only five very severely anemic decompensated (Hb < 5 gm%) cases to improve their general condition.
Table 3.
Post treatment follow-up (n = 60)
DISCUSSION
Fibroid being a tumor of hyper-estrogenic environment, therefore medical treatment that lowers estrogen levels as GnRH agonists (Lupride) and antagonists (Cetrorelix), Danazol, Gestrinone, Cabergoline, reduces aromatase activity (Letrozole) or modifies estrogen response (SERM-Raloxifene) are effective in reducing the size of fibroid and improve symptoms in most of cases.[4] Current studies support that growth of myoma in humans is progesterone dependent also and therefore antiprogestins (Mifepristone) and selective progesterone receptor modulators (SPRMs-Asoprisnil) can be effective in treatment. Hormonal treatment reduces size, improves hemoglobin by controlling bleeding and renders surgery unnecessary as patient reaches menopause, because fibroid being a hormone depended tumor stops to grow after menopause. Mifepristone has both antiprogesterone and antiglucocorticoid properties in dose dependent manner.
Clinical trials using 5-50 mg doses of Mifepristone were conducted for varying periods between 3 to 12 months but exact dose and the duration are yet to be determined. Eisinger, et al.,[5] reported fall of 48% in mean uterine volume while amenorrhoea in 61% only after 6 months of 10 mg mifepristone. Another study by Kettle et al., reported amenorrhoea in 40-70% over one year at 5-10 mg dose, while 100 mg led to 100% amenorrhoea.[6] AUB is the main reason of worry in women as it affects their daily routines, work efficiency and health status, therefore mostly opt for hysterectomy as one time management in developing countries. With higher doses speedy and better control of bleeding is achieved, this improves the general condition of women and hemoglobin levels, relieves anxiety and provides women a sense of well being and affectivity of treatment but produce hot flushes and other anti-glucocorticoid side-effects. Murphy et al.[7] had a comparative study of 5 mg, 25 mg and 50 mg dosage and suggested 25 mg to be the most effective dose to cause clinically significant decrease in leiomyoma volume. We chose 25 mg daily to achieve rapid symptomatic improvement (improved compliance with low drop out rate) in short period of time (3 months) with minimal side effects.
Mechanism of reduced bleeding and myoma size is likely to be due to structural, functional and micro vascular effects of Mifepristone on the endometrium and uterine musculature in dose and duration dependent manner. In our study 25 mg Mifepristone reduced uterine size to 63.69% of baseline (−36.4% decline) while Bagaria et al.,[8] had 26.6% reduction with 10 mg over 3 months, and Eisinger et al., 11% with ultra low 2.5 mg over 6 months [Table 4].[9]
Table 4.
Dose duration dependence of Mifepristone by past studies
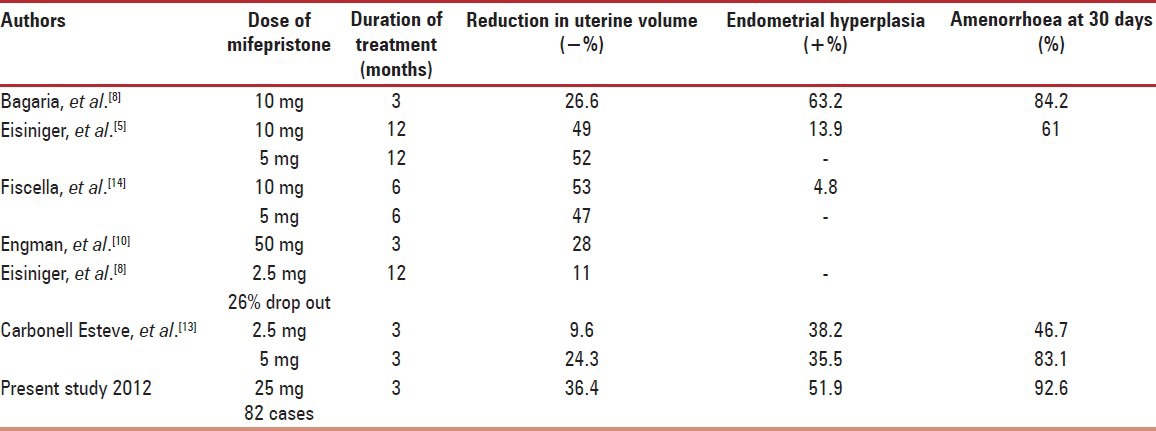
Mean myoma volume reduced by 46% with 25 mg dose in our study which is quiet better than other studies as, Engman et al.,[10] 28% decline with 50 mg and Kettle et al.,[6] 49% decline with 100 mg in three months. More number of receptors is there in leiomyoma compared to rest of normal myometrium therefore more steady fall is seen in it. Submucosal myomas prolapsed through cervix as uterine volume decreased and simple polypectomy served the purpose.
Endometrial hyperplasia is the notable adverse effect of the drug Mifepristone.[11] Long term use of high dose of anti-progesterone may promote an unopposed estrogen milieu leading to endometrial hyperplasia.[12] Keeping the duration short can avoid atypical endometrial hyperplasia and chances of malignant changes. On prolonging the treatment prevalence of amenorrhoea drops due to resumption of menstruation, which leads to regression of endometrial thickness, but chances of atypia have to be checked by endometrial biopsy. The entire women baseline ET was less than 10 mm and after three months only in two cases it became double of it, without any atypia in present study. Hot flush, headache, nausea, fatigue, malaise and rise in liver transaminase enzymes; AST and ALT are reported in past studies.[13] This short term treatment was well tolerated; although large studies are needed to add safety information, about endometrial and breast proliferation and long term follow-up after stopping treatment. In a young reproductive age female medical therapy results may not be as good, as myoma may re-grow after discontinuation of treatment. Typically best candidates are peri-menopausal women with anemia and those who want to avoid surgery. It can be used preoperative cases to reduce size and buildup hemoglobin level to have better surgical outcome.
Woman's compliance and acceptance of this medical management protocol is clear from the low drop out (11/82) and 73% post-treatment follow-up rate (60/82). Acceptance is high because it is really cost effective (no hospital admission, preoperative tests, blood transfusion, surgical and medicinal charges). Having a hysterectomy not only causes a surgical trauma but poses a great mental stress to the women.
CONCLUSION
Most important and useful effect of Mifepristone found to be the control of bleeding leading to improvement in hemoglobin levels and general condition. Study supports that 25 mg Mifepristone daily for three months is effective in alleviating hysterectomy in 87.8% women.[14]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Salhan Sudha. 1st ed. New Delhi: Jaypee; 2011. Text book of gynaecology; pp. 320–1. [Google Scholar]
- 2.Cardozo ER, Segars JH, Banks BJ, Henne MB, Stregman BJ. The estimated annual cost of uterine myoma in United Sates. Am J Obstet Gynecol. 2012;206:211.e1–9. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salle B, Sergeant P, Awada A, Bied-Damon V, Gaucherand P, Boisson C, et al. Transvaginal ultrasound vascular and morphological changes in uteri exposed to diethylstilbestrol in utero. Hum Reprod. 1996;11:2531–6. doi: 10.1093/oxfordjournals.humrep.a019153. [DOI] [PubMed] [Google Scholar]
- 4.Sabry M, Hendy AA. Innovative oral treatments of uterine leiomyoma. Obstet Gynecol Int. 2012;2012:943635. doi: 10.1155/2012/943635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisinger SH, Bonfiglio T, Fiscella K, Meldrum S, Guzick DS. Twelve month safety and efficacy of low dose mifepristone for uterine myomas. J Minim Invasive Gynecol. 2005;12:227–33. doi: 10.1016/j.jmig.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Kettel LM, Murphy AA, Morales AJ, Yen SS. Clinical efficacy of the antiprogesterone RU486 in the treatment of endometriosis and uterine fibroids. Hum Reprod. 1994;9:116–20. doi: 10.1093/humrep/9.suppl_1.116. [DOI] [PubMed] [Google Scholar]
- 7.Murphy AA, Morales AJ, Kettel LM, Yen SS. Regression of uterine leiomyomata to the antiprogesterone RU486-dose-response effect. Fertil Steril. 1995;64:187–90. [PubMed] [Google Scholar]
- 8.Bagaria M, Suneja A, Vaid NB, Gulaeria K, Mishra K. Low dose mifepristone in treatment of uterine myoma: A randomized double blind placebo controlled clinical trial. Aust N Z J Obstet Gynaecol. 2009;49:77–83. doi: 10.1111/j.1479-828X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 9.Eisinger SH, Fiscella J, Bonfiglio T, Meldrum S, Fiscella K. Open-label study of ultra low-dose mifepristone for the treatment of uterine leiomyomata. Eur J Obstet Gynecol Reprod Biol. 2009;146:215–8. doi: 10.1016/j.ejogrb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Engman M, Granberg S, Williams AR, Meng CX, Lalitkumar PG, Gemzell DK. Mifepristone for treatment of uterine leiomyoma-A prospective randomized placebo cotrolled trial. Hum Reprod. 2009;24:1870–9. doi: 10.1093/humrep/dep100. [DOI] [PubMed] [Google Scholar]
- 11.Newfield RS, Spitz IM, Isacson C, New MI. Long-term mifepristone (RU486) therapy resulting in massive benign endometrial hyperplasia. Clin Endocrinol (Oxf) 2001;54:399–404. doi: 10.1046/j.1365-2265.2001.01026.x. [DOI] [PubMed] [Google Scholar]
- 12.Steinauer J, Pritts EA, Jackson R, Jacoby AF. Systematic review of mifepristone for the treatment of uterine leiomyomata. Obstet Gynecol. 2004;103:1331–6. doi: 10.1097/01.AOG.0000127622.63269.8b. [DOI] [PubMed] [Google Scholar]
- 13.Carbonell Esteve JL, Riveron AM, Cano M, Ortiz AI, Valle A, Texido CS. Mifepristone 2.5mg versus 5 mg daily in treatment of leiomyoma before surgery. Int J Women's Health. 2012;4:75–84. doi: 10.2147/IJWH.S28103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiscella K, Eisinger SH, Meldrum S, Feng C, Fisher SG, Guzick DS. Effect of mifepristone for symptomatic leiomyomata on quality of life and uterine size: A randomized controlled trial. Obstet Gynecol. 2006;108:1381–7. doi: 10.1097/01.AOG.0000243776.23391.7b. [DOI] [PubMed] [Google Scholar]


