Abstract
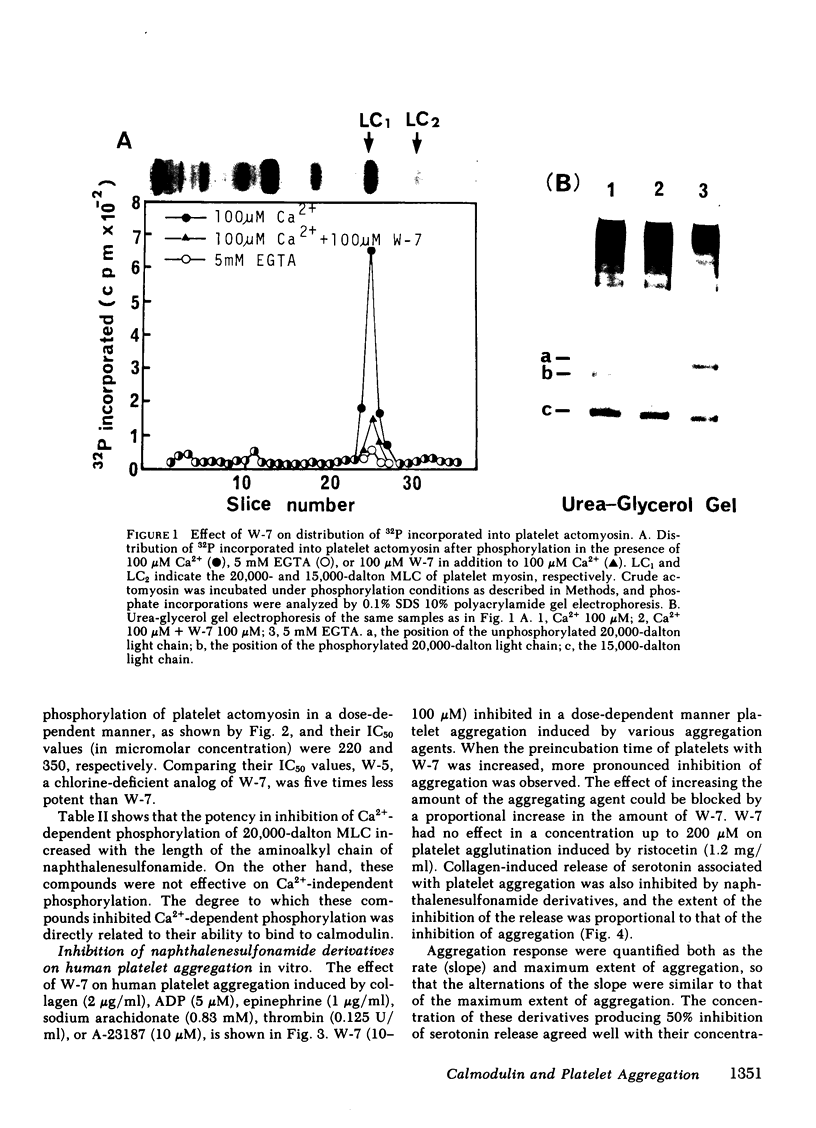
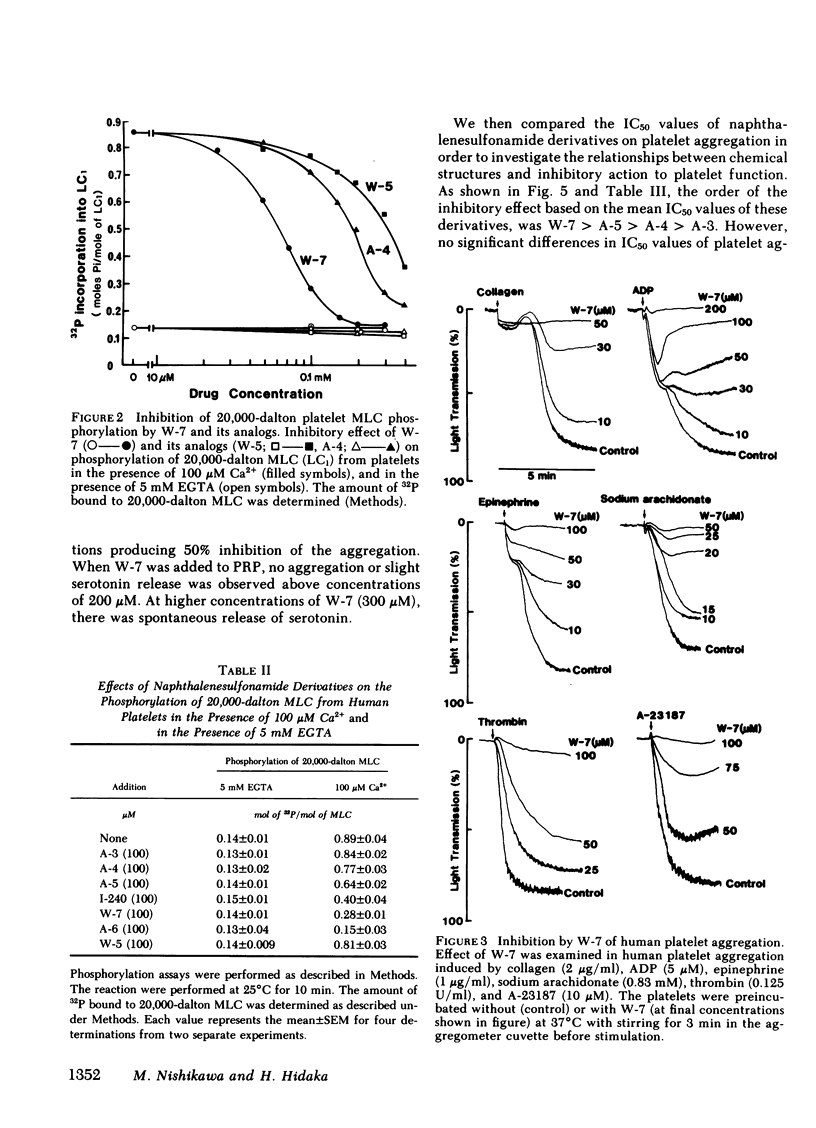
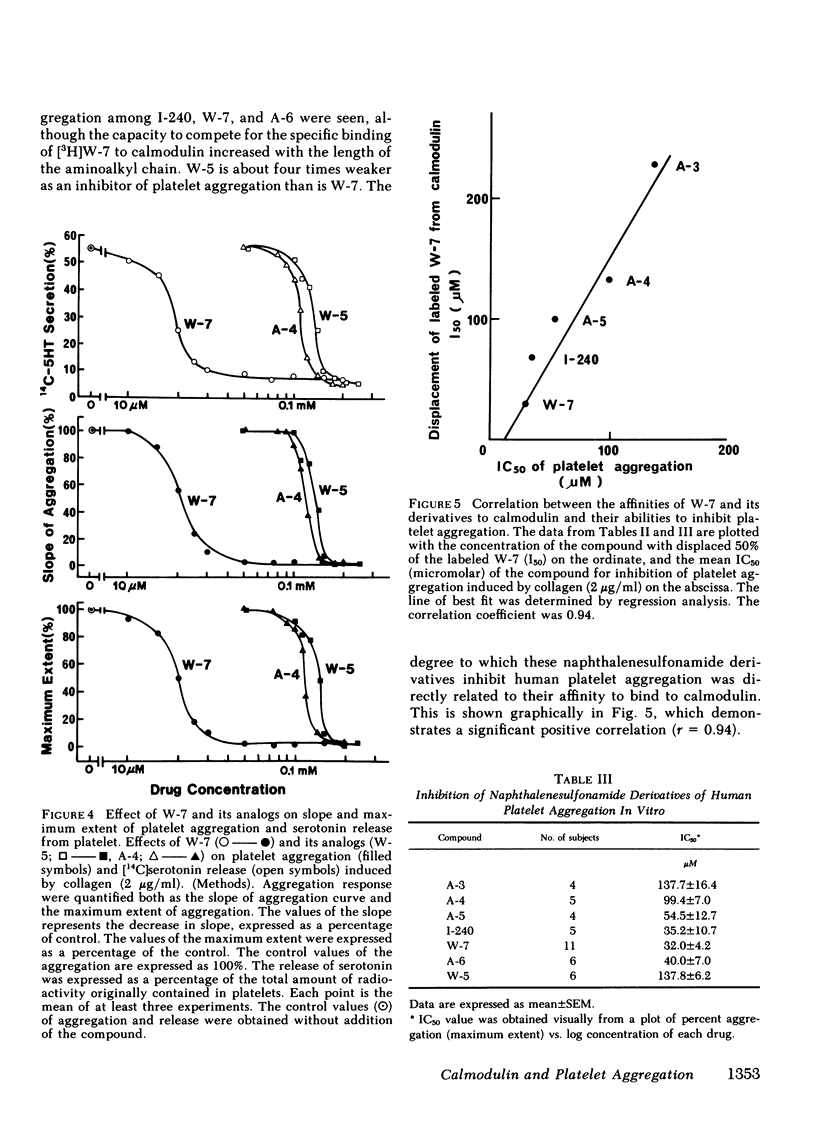
Two series of derivatives of N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide (W-7), including a dechlorinated analog of W-7 (W-5) and various aminoalkyl chain analogs of W-7 (A-3, A-4, A-5, I-240, A-6) were synthesized and their structure-activity relationships with calmodulin antagonistic actions and their potencies in inhibiting human platelet aggregation in vitro were investigated. Their binding affinities to calmodulin in the presence of 100 microM Ca2+ were dependent both on the chlorination of the naphthalene ring and on the length of aminoalkyl chain. The ability of these derivatives to inhibit Ca2+-dependent phosphorylation of 20,000-dalton myosin light chain from platelets correlated well with the magnitude of their binding affinity to calmodulin. W-7(10-100 microM) inhibited in a dose-dependent manner platelet aggregation induced by collagen (2 micrograms/ml), ADP (5 microM), epinephrine (1 microgram/ml), sodium arachidonate (0.83 mM), thrombin (0.125 U/ml), and A-23187 (10 microM). The IC50 value (concentration producing 50% inhibition of aggregation) of W-7 was lower in arachidonate- and collagen-induced aggregation than in ADP- or epinephrine-induced aggregation. A good correlation between the potency in inhibition of collagen-induced aggregation by W-7 and its derivatives and their affinities to calmodulin was obtained (r = 0.94). Thus, the inhibitory mechanism of these compounds may be due to their effect on Ca2+-calmodulin-dependent processes, such as 20,000-dalton myosin light chain phosphorylation. These data also support the hypothesis that the calmodulin-mediated system has an important role in platelet function.
Full text
PDF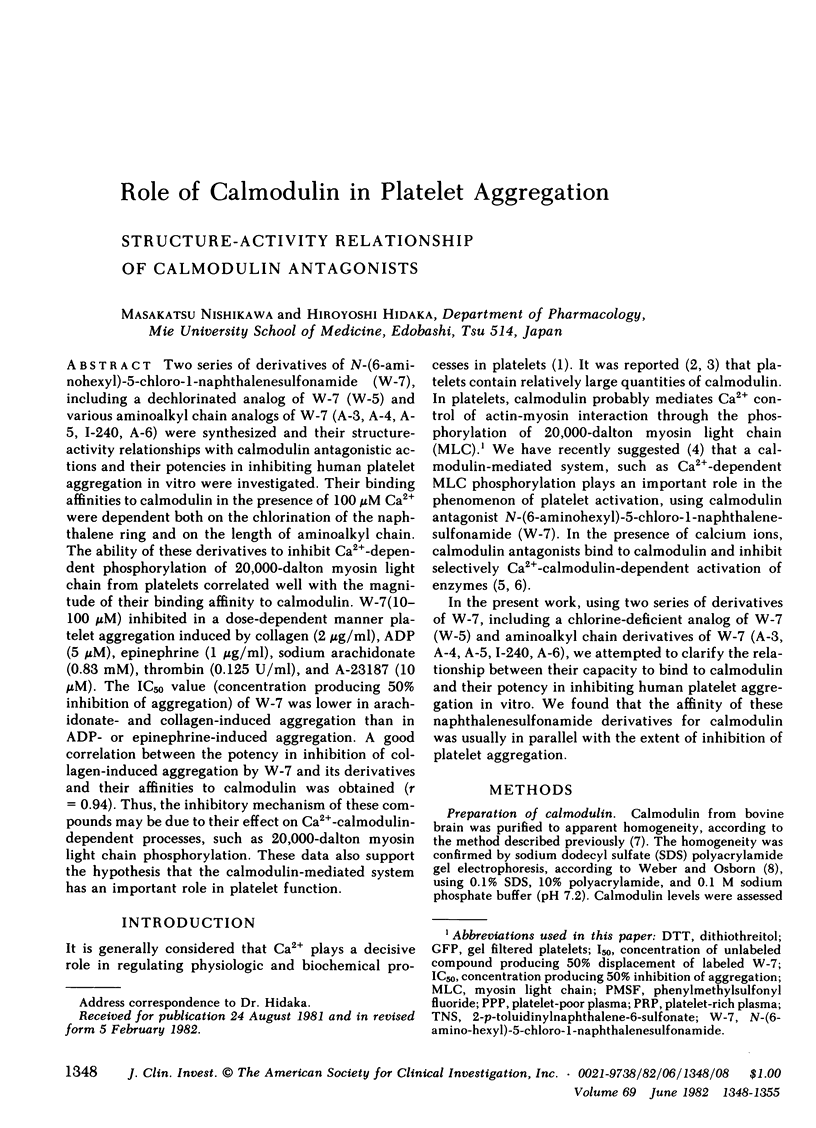




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A., Barylko B. The role of myosin phosphorylation in regulating actin-myosin interaction in human blood platelets. Thromb Haemost. 1978 Oct 31;40(2):241–244. [PubMed] [Google Scholar]
- Adelstein R. S., Conti M. A. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature. 1975 Aug 14;256(5518):597–598. doi: 10.1038/256597a0. [DOI] [PubMed] [Google Scholar]
- BORN G. V. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962 Jun 9;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Hartshorne D. J. A Ca2+-and modulator-dependent myosin light chain kinase from non-muscle cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1352–1359. doi: 10.1016/0006-291x(78)91152-x. [DOI] [PubMed] [Google Scholar]
- Daniel J. L., Molish I. R., Holmsen H. Myosin phosphorylation in intact platelets. J Biol Chem. 1981 Jul 25;256(14):7510–7514. [PubMed] [Google Scholar]
- Detwiler T. C., Charo I. F., Feinman R. D. Evidence that calcium regulates platelet function. Thromb Haemost. 1978 Oct 31;40(2):207–211. [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Adelstein R. S. Human platelet myosin light chain kinase requires the calcium-binding protein calmodulin for activity. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1653–1657. doi: 10.1073/pnas.76.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Asano M., Iwadare S., Matsumoto I., Totsuka T., Aoki N. A novel vascular relaxing agent, N-(6--aminohexyl)-5-chloro-1-naphthalensulfonamide which affects vascular smooth muscle actomyosin. J Pharmacol Exp Ther. 1978 Oct;207(1):8–15. [PubMed] [Google Scholar]
- Hidaka H., Naka M., Yamaki T. Effect of novel specific myosin light chain kinase inhibitors on Ca2+-activated Mg2+-ATPase of chicken gizzard actomyosin. Biochem Biophys Res Commun. 1979 Oct 12;90(3):694–699. doi: 10.1016/0006-291x(79)91883-7. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Yamaki T., Asano M., Totsuka T. Involvement of calcium in cyclic nucleotide metabolism in human vascular smooth muscle. Blood Vessels. 1978;15(1-3):55–64. doi: 10.1159/000158153. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Yamaki T., Naka M., Tanaka T., Hayashi H., Kobayashi R. Calcium-regulated modulator protein interacting agents inhibit smooth muscle calcium-stimulated protein kinase and ATPase. Mol Pharmacol. 1980 Jan;17(1):66–72. [PubMed] [Google Scholar]
- Hidaka H., Yamaki T., Totsuka T., Asano M. Selective inhibitors of Ca2+-binding modulator of phosphodiesterase produce vascular relaxation and inhibit actin-myosin interaction. Mol Pharmacol. 1979 Jan;15(1):49–59. [PubMed] [Google Scholar]
- Kanamori M., Naka M., Asano M., Hidaka H. Effects of N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide and other calmodulin antagonists (calmodulin interacting agents) on calcium-induced contraction of rabbit aortic strips. J Pharmacol Exp Ther. 1981 May;217(2):494–499. [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Reimers H. J., Mustard J. F., Packham M. A. Sodium arachidonate can induce platelet shape change and aggregation which are independent of the release reaction. Science. 1976 Jun 4;192(4243):1011–1012. doi: 10.1126/science.1273582. [DOI] [PubMed] [Google Scholar]
- Kobayashi R., Tawata M., Hidaka H. Ca2+ regulated modulator protein interacting agents: inhibition of Ca2+-Mg2+-ATPase of human erythrocyte ghost. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1037–1045. doi: 10.1016/0006-291x(79)91513-4. [DOI] [PubMed] [Google Scholar]
- Lebowitz E. A., Cooke R. Contractile properties of actomyosin from human blood platelets. J Biol Chem. 1978 Aug 10;253(15):5443–5447. [PubMed] [Google Scholar]
- Muszbek L., Kuznicki J., Szabó T., Drabikowski W. Troponin C like protein of blood platelets. FEBS Lett. 1977 Aug 15;80(2):308–312. doi: 10.1016/0014-5793(77)80464-x. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Tanaka T., Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980 Oct 30;287(5785):863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoake J. A., Song S. Y., Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Distribution and developmental changes of the enzyme and its protein activator in mammalian tissues and cells. Biochim Biophys Acta. 1974 Apr 25;341(2):402–411. doi: 10.1016/0005-2744(74)90233-2. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Hidaka H. Hydrophobic regions function in calmodulin-enzyme(s) interactions. J Biol Chem. 1980 Dec 10;255(23):11078–11080. [PubMed] [Google Scholar]
- Tangen O., Berman H. J., Marfey P. Gel filtration. A new technique for separation of blood platelets from plasma. Thromb Diath Haemorrh. 1971 Jun 30;25(2):268–278. [PubMed] [Google Scholar]
- Wang J. H., Waisman D. M. Calmodulin and its role in the second-messenger system. Curr Top Cell Regul. 1979;15:47–107. doi: 10.1016/b978-0-12-152815-7.50006-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]
- Wolff D. J., Brostrom C. O. Properties and functions of the calcium-dependent regulator protein. Adv Cyclic Nucleotide Res. 1979;11:27–88. [PubMed] [Google Scholar]




