Abstract
Background:
To determine whether uric acid levels are associated with the components of metabolic syndrome and whether uric acid is a significant factor for development of metabolic syndrome in the first-degree relatives of type 2 diabetic patients as high risk group.
Materials and Methods:
A total of 694 (182 male and 512 female, aged 30-69 years) first-degree relatives of type 2 diabetic patients during 2007-2011 were enrolled. The height, weight, waist circumference, blood pressure, fasting plasma glucose, lipid profile and uric acid concentrations were measured. Metabolic syndrome was defined by NCEP-ATP III.
Results:
Uric acid was associated with waist circumference, blood pressure, triglyceride and HDL-cholesterol level in both sexes (r = 0.1-0.3, P < 0.05). The prevalence of metabolic syndrome in the fourth quartile of uric acid (64.4% of male and 60.2% of female population) was significantly more than those in the first (25.5% of male and 31.2% of female population) and second quartiles (33.3% of male and 32.0% of female population). The mean of uric acid in people with metabolic syndrome was significantly higher than in those without (6.6 ± 1.2 mg/dL vs. 5.8 ± 1.2 mg/dL; P = 0.0001). The age-adjusted odds ratios (95% confidence interval) of uric acid for metabolic syndrome in univariate analysis were [1.60 (1.23-2.07); P = 0.008] for men and [1.61 (1.34-1.92); P = 0.0001] for women but the effect of uric acid in multivariate logistic regression was not significant.
Conclusions:
Uric acid is associated with majority of the metabolic syndrome components. People with metabolic syndrome have higher uric acid levels. However, uric acid probably is not an independent factor to predict the metabolic syndrome.
Keywords: Cardiovascular disease, insulin resistance, metabolic syndrome, obesity, type 2 diabetes
INTRODUCTION
Metabolic syndrome is a cluster of interrelated conditions characterized by dyslipidemia, hyperglycemia, high blood pressure and abdominal obesity.[1] Some studies have reported that metabolic syndrome and its components are associated with serum uric acid levels.[2,3,4,5,6] Increased serum uric acid levels are commonly correlated with glucose intolerance, hypertension and dyslipidemia.[2,7,8] It is associated with smaller low-density lipoprotein-cholesterol (LDL-cholesterol) and high-density lipoprotein-cholesterol (HDL-cholesterol) particles[9] and insulin resistance.[10,11] In a recent study, the association between elevated serum uric acid and high circulating inflammatory cytokines has been reported.[12] The studies suggest that high serum uric acid triggers sterile inflammation[12] and may indicate an early sign of atherosclerosis in asymptomatic individuals.[9] Furthermore, the metabolic syndrome increases the risk of cardiovascular disease and type 2 diabetes mellitus and is associated with insulin resistance.[13,14] However, the uric acid and metabolic syndrome may have some correlation. Almost all of the previous studies have examined the relation between uric acid and the component of metabolic syndrome in general population. However, at our best knowledge, no published data has been found about this relation in the first-degree relatives of type 2 diabetes mellitus (T2DM) patients. The first-degree relatives of T2DM patients are at risk for developing metabolic disturbances and type 2 diabetes mellitus[15,16,17] and they are a target group to identify factors that increased their metabolic disturbances. In this study, we investigated the relation between uric acid and components of metabolic syndrome in the first-degree relatives of type 2 diabetic patients.
MATERIALS AND METHODS
This cross-sectional study was performed at the Isfahan Endocrine and Metabolism Research Center, from 2007 to 2011. Our sample contained 700 people who were the first-degree relatives of type 2 diabetic patients, aged 30-69 years, selected by recruitment methods in Isfahan Diabetes Prevention Program Study, an ongoing cohort study in central Iran. Before conducting the analysis, we excluded the participants with history of gout and creatinine more than 1.2 mg/dL (n = 6) and finally 694 participants (182 males and 512 females) were enrolled.
The study was approved by Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences Medical Ethics Committee (approval number is 90009). The tenets of Declaration of Helsinki were followed. An informed consent was signed by each participant.
Height and weight were measured using Seca stadiometer, while persons were without shoes and in light clothes.[18] Weight was rounded to the nearest 0.1 kg scale. Waist circumference was measured midway between the lower rib margin and the iliac crest using a standard tape meter.[18] Body mass index (BMI) was calculated by body weight (kg)/height (m2).[18] Resting blood pressure was measured while people were seated for 5 minutes by using a calibrated mercury sphygmomanometer (Rester, Germany) with standard techniques.[19]
In all participants, fasting plasma glucose, total cholesterol, HDL-cholesterol, triglyceride and uric acid concentrations were assessed. LDL-cholesterol was calculated using Friedewald formula when total triglyceride was less than 400 mg/dL.[20] Plasma glucose was measured by GOD-PAP method. Total cholesterol and HDL-cholesterol were measured by CHOD-PAP and triglyceride was done by GPO-PAP method. Uric acid was measured enzymatically by TOOS method using Biotecnica BT-3000 Plus Chemistry analyzer, Italy.
According to the NCEP (ATP III), modified for pre-diabetes (fasting plasma glucose ≥100 mg/dL), metabolic syndrome was identified when three of the five following criteria were present, abdominal obesity defined as waist circumference >102 cm in men and 88 cm in women; fasting glucose ≥100 mg/dL; HDL-cholesterol <40 mg/dL in men and <50 mg/dL in women; triglycerides ≥150 mg/dL; systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥85 mm Hg.[1]
Normality of data distribution was assessed with the histogram. All the variables had normal distributions, but the triglyceride. The data were presented as mean and SD (standard deviation) or median and quartiles (the 1st quartile, the 3rd quartile) for triglyceride. The means were compared with Student t-test and ANOVA test. The prevalence of metabolic syndrome was determined in males and females, according to the different quartiles of serum uric acid levels. This prevalence was compared with Chi-square test. Correlations between the uric acid and other variables were assessed by using Spearman correlation coefficient test for triglyceride and Pearson correlation coefficient test for other variables. The significance of uric acid for predicting metabolic syndrome was estimated by using age-adjusted univariate and multivariate logistic regression. We designed eight models using metabolic syndrome as dependent variables. One model was designed for univariate logistic regression analysis using uric acid alone as independent variable; six models for uric acid and each component of metabolic syndrome and the last model for uric acid and all the components of metabolic syndrome. The results were described as odds ratios (ORs) with 95% confidence intervals (CI 95%). All the analyses were performed in both men and women. Statistical analysis was done with SPSS software version 13 for Windows (SPSS, Chicago, IL), and P < 0.05 was considered to be statistically significant.
RESULTS
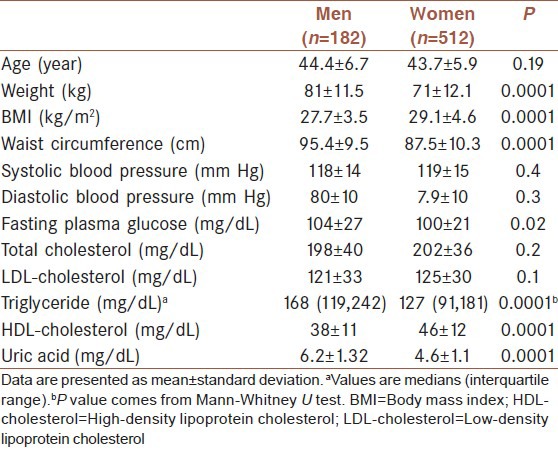
The characteristics of studied people are shown in Table 1. They were the first-degree relatives of type 2 diabetic patients, included 182 men aged 44.4 ± 6.7 years and 512 women aged 43.7 ± 5.9 years. Weight, waist circumference, triglyceride, fasting plasma glucose and uric acid were significantly higher in men than women and BMI and HDL-cholesterol were significantly higher in women. The age, systolic blood pressure, diastolic blood pressure, total cholesterol and LDL-cholesterol were not significantly different between male and female participants.
Table 1.
The baseline characteristics of the first-degree relatives of type 2 diabetic patients
The overall prevalence of metabolic syndrome in the first-degree relatives of type 2 diabetic patients in our study was 41.2% (n = 286) [n = 76 male, n = 210 female]. The prevalence of metabolic syndrome was 41.8% in men and 41% in women (P = 0.9).
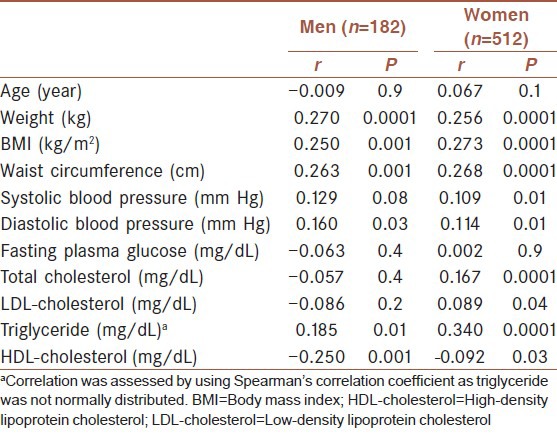
Correlation coefficient test between uric acid and other parameters are shown in Table 2. There was a correlation between waist circumference, triglyceride, BMI, weight, HDL-cholesterol, triglyceride, diastolic blood pressure and uric acid in both sexes. In women, significant correlation was also found between uric acid and systolic blood pressure. But there was no correlation between uric acid concentrations and fasting plasma glucose in both sexes; also in men, correlation was negative. We excluded diabetic cases (n = 52) and repeated Pearson correlation coefficient test between uric acid and fasting plasma glucose. The correlation became significant in women (r = 0.125, P = 0.007) and became positive but not significant in men (r = 0.103, P = 0.1).
Table 2.
Pearson correlation coefficients between uric acid and studied parameters in the first-degree relatives of type 2 diabetic patients
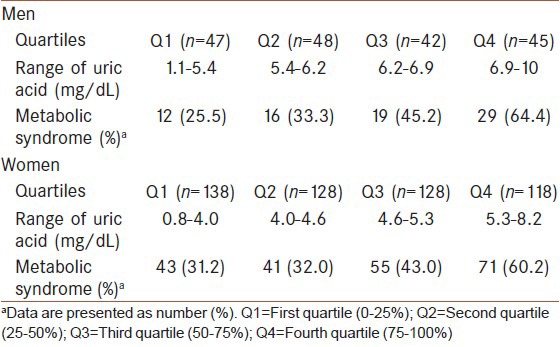
The serum uric acid was stratified by quartiles (Q) according to the gender, and the prevalence of metabolic syndrome was shown in each quartile [Table 3]. The prevalence of metabolic syndrome in the Q4 was significantly more than those in the Q1 (P = 0.0001) and Q2 (P = 0.003) in men. The prevalence of metabolic syndrome in the Q4 was significantly higher than those in the Q1 (P = 0.0001), Q2 (P = 0.0001) and Q3 (P = 0.007) in women and also the prevalence of metabolic syndrome in the Q3 was higher than in the Q1 (P = 0.04) in women.
Table 3.
Prevalence of metabolic syndrome by the quartiles of uric acid in first-degree relatives of type 2 diabetic patients
The prevalence of metabolic syndrome between other quartiles was not significantly different in these two groups. The mean of uric acid in men with metabolic syndrome was 6.6 ± 1.2 mg/dL and in those without metabolic syndrome was 5.8 ± 1.2 mg/dL (P = 0.0001). The mean of uric acid in women with metabolic syndrome was 4.9 ± 1.1 mg/dL and in those without metabolic syndrome was 4.4 ± 0.9 mg/dL (P = 0.0001).
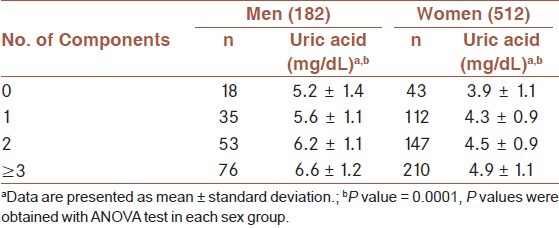
The association between uric acid and the number of metabolic syndrome components are shown in Table 4. The serum uric acid level significantly increased with increasing the numbers of metabolic syndrome in both male and female participants.
Table 4.
Mean of uric acid concentration by the number of metabolic syndrome components in the first-degree relatives of type 2 diabetic patients
In order to investigate if the uric acid level may predict the metabolic syndrome, we designed eight age-adjusted logistic regression models [Figure 1]. The odds ratios (95% confidence interval) of uric acid for metabolic syndrome in univariate analyses were [1.60 (1.23-2.07); P = 0.008] for men and [1.61 (1.34-1.92); P = 0.0001] for women. These data introduced that uric acid concentration may be a factor in the development of metabolic syndrome. The multivariate analysis showed that when uric acid was entered to analysis with each of the components of metabolic syndrome, the effect of uric acid concentration still persisted, significantly, in both sexes. On the other hand, when all the components of metabolic syndrome were used in model, the effect of uric acid was not significant in both sexes [OR, 1.44; CI 95%, (0.99-2.11); P = 0.057] in men and [OR, 1.21; CI 95% (0.90-1.63); P = 0.197] in women.
Figure 1.
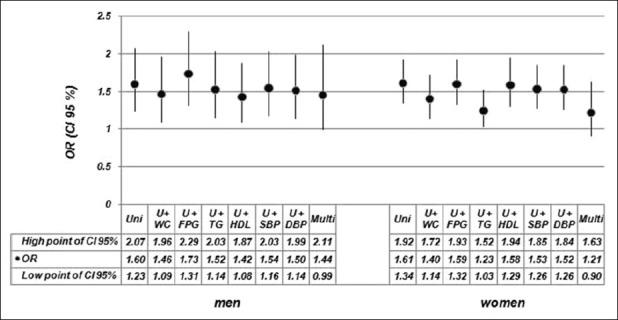
Odds ratios (ORs) and 95% confidence intervals (CI 95%) of uric acid in the development of metabolic syndrome by gender in first-degree relatives of type 2 diabetic patients. Uni: Univariate analysis with uric acid, U+WC: Analysis with uric acid and waist circumference, U+FPG: Analysis with uric acid and fasting plasma glucose, U+TG: Analysis with uric acid and triglyceride, U+HDL: Analysis with uric acid and HDL-cholesterol, U+SBP: Analysis with uric acid and systolic blood pressure, U+DBP: Analysis with uric acid and diastolic blood pressure, Multi: Multivariate analysis with uric acid and all the components of metabolic syndrome
DISCUSSION
The first-degree relatives of T2DM patients are a target group to identify factors that increased their metabolic disturbances.[15,16,17] To the best of our knowledge, this is the first study that investigates the relation between uric acid and metabolic syndrome in this group in Iranian population. We focused our analysis on the prevalence of metabolic syndrome according to the uric acid levels and the effect of uric acid on the prediction of metabolic syndrome.
In our study, the overall prevalence of metabolic syndrome in the first-degree relatives of T2DM patients was 41.2% (n = 286) [n = 76 male, n = 210 female]. This prevalence is higher than that which was reported in the general population of Iran with Azizi et al (41.2% vs. 30.1%).[21] The association between a family history of diabetes and the prevalence of metabolic syndrome has been reported in previous studies.[22,23]
According to our results, the prevalence of metabolic syndrome increased with the increase in the uric acid concentration [Table 3]. The prevalence of metabolic syndrome in the fourth quartile of uric acid was significantly higher than the first and the second quartiles in both sexes. Furthermore, the uric acid concentration increased with increasing the number of metabolic syndrome components [Table 4]. Finally, the mean of uric acid is significantly higher in the individuals with metabolic syndrome than those without (6.6 ± 1.2 vs. 5.8 ± 1.2 in men and 4.9 ± 1.1 vs. 4.4 ± 0.9 in women). Similar results reported in previous studies, which had been conducted in normal populations.[3,4,6]
Metabolic syndrome is associated with insulin resistance[14] and Facchini et al., have shown that urinary uric acid clearance decreases in the setting of insulin resistance and it leads to more concentration of serum uric acid.[24]
Our findings indicate that uric acid has some correlation with the majority of metabolic syndrome components both in men and women [Table 2]. The correlation of uric acid with triglyceride and obesity indexes such as weight, waist circumference and BMI is more strong [Table 2]. In a healthy Japanese men's study, uric acid levels were correlated with abdominal obesity[25] and Matsuura et al., explained that increase in uric acid level is closely associated to obesity and the body fat distribution.[26] There are several hypotheses for this association. For example, obesity and the increase in waist circumstance are associated with insulin resistance.[27,28] Urinary uric acid clearance decreases in the setting of insulin resistance therefore, uric acid concentration increases.[24] Another hypothesis, leptin production increased in obesity and there is positive correlation between leptin and uric acid. It has been suggested that leptin could be a pathogenic role for hyperuricemia in obesity.[29]
We observed the negative correlation between HDL-cholesterol and serum uric acid concentration. Similar findings were reported in previous studies.[3,6] The negative association between HDL-cholesterol and insulin resistance was reported.[10] It is the probable mechanism for the negative relation between uric acid level and HDL-cholesterol concentration.
The other considerable result was no correlation between uric acid and fasting plasma glucose in both sexes [Table 2]. Similar findings were observed in Brazilian population and Japanese men.[3,6] However, the correlation in Japanese men became weakly positive when the diabetic men were excluded.[3] In our study when we excluded diabetes persons from the analysis, the correlation in women became significant and in men became positive but not significant. Data about the uric acid levels in T2DM has inconsistent results.[8,30,31] Some studies reported that the levels of uric acid decreased as glucose levels increased to diabetic levels but in the setting of insulin resistance and impaired glucose tolerance is high.[8,30,31]
We found that the likelihood of metabolic syndrome increases approximately 60% by 1 SD increment in uric acid levels if the uric acid enters in the model alone in logistic regression analysis [OR = 1.60 and 1.61 in men and women, respectively] [Figure 1].
However, when all the components of metabolic syndrome were entered to the multivariate analysis, the effect of uric acid for prediction of metabolic syndrome was not statistically significant in both sexes [Figure 1]. By entering each component of metabolic syndrome into the logistic regression model with uric acid, we found that the odds ratio of uric acid for predicting metabolic syndrome reduced modest in all models (except for fasting plasma glucose in men); however, this association still was significant [Figure 1].
Similar results were reported in multivariate analysis in Korean men study.[5] There is a study in Turkish population that showed uric acid levels were a determinant of metabolic syndrome. However, in Turkish study, a significant OR [OR, 1.35; CI 95%, (1.01-1.81)] was observed in the total sample. It was not significant when men and women were analyzed, separately. It was [OR, 1.15; CI 95%, (0.76-1.74)] in males and [OR, 1.51; CI 95%, (0.99-2.31)] in females. On the other hand, not all the components of metabolic syndrome had been entered in model for analysis and they had modified the criteria of metabolic syndrome and had not used the original ATP III criteria.[4]
Korean and Turkish studies were population-based studies while our study population was first-degree relatives of type 2 diabetes and they might have some characteristics that make them different from normal population. It may be responsible for some differences that were observed in our study.
In present study, uric acid level had correlation with the majority of metabolic syndrome components. In the univariate analysis, uric acid had potency for predicting metabolic syndrome. However, it lost this effect while controlling all the components of metabolic syndrome as the confounding variables in multiple logistic regressions. It seems that uric acid probably is not an independent factor to predict the metabolic syndrome in the first-degree relative of T2DM. However, the further investigations are required.
Our study has several strengths and limitations. A strong point includes the large sample size consisting of the first-degree relatives of type 2 diabetes patients. The limitations include, first, this study had a cross-sectional design, which cannot elucidate causal relationships between uric acid and metabolic syndrome. Second, the participants were 30-69 years old and results may not refer to the other age groups and finally this study was not a multicenter study.
In conclusion, the serum uric acid levels are correlated with metabolic syndrome components. The concentration of uric acid in the first-degree relative of T2DM with metabolic syndrome is higher than those without. However, in the presence of metabolic syndrome components, uric acid does not have power for prediction of metabolic syndrome in this group.
ACKNOWLEDGMENTS
We appreciate Mr. Majid Abyar for his technical computer assistance and thank all the first-degree relatives of T2DM patients who participated in this study.
Footnotes
Source of Support: Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 2.Conen D, Wietlisbach V, Bovet P, Shamlaye C, Riesen W, Paccaud F, et al. Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a developing country. BMC Public Health. 2004;4:9. doi: 10.1186/1471-2458-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oda E, Kawai R, Sukumaran V, Watanabe K. Uric acid is positively associated with metabolic syndrome but negatively associated with diabetes in Japanese men. Intern Med. 2009;48:1785–91. doi: 10.2169/internalmedicine.48.2426. [DOI] [PubMed] [Google Scholar]
- 4.Onat A, Uyarel H, Hergenç G, Karabulut A, Albayrak S, Sari I, et al. Serum uric acid is a determinant of metabolic syndrome in a population-based study. Am J Hypertens. 2006;19:1055–62. doi: 10.1016/j.amjhyper.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Rho YH, Woo JH, Choi SJ, Lee YH, Ji JD, Song GG. Association between serum uric acid and the Adult Treatment Panel III-defined metabolic syndrome: Results from a single hospital database. Metabolism. 2008;57:71–6. doi: 10.1016/j.metabol.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues SL, Baldo MP, Capingana P, Magalhães P, Dantas EM, Molina Mdel C, et al. Gender distribution of serum uric acid and cardiovascular risk factors: Population based study. Arq Bras Cardiol. 2012;98:13–21. doi: 10.1590/s0066-782x2011005000116. [DOI] [PubMed] [Google Scholar]
- 7.Hwu CM, Lin KH. Uric acid and the development of hypertension. Med Sci Monit. 2010;16:RA224–30. [PubMed] [Google Scholar]
- 8.Yuan HJ, Yang XG, Shi XY, Tian R, Zhao ZG. Association of serum uric acid with different levels of glucose and related factors. Chin Med J (Engl) 2011;124:1443–8. [PubMed] [Google Scholar]
- 9.Vekic J, Jelic-Ivanovic Z, Spasojevic-Kalimanovska V, Memon L, Zeljkovic A, Bogavac-Stanojevic N, et al. High serum uric acid and low-grade inflammation are associated with smaller LDL and HDL particles. Atherosclerosis. 2009;203:236–42. doi: 10.1016/j.atherosclerosis.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 10.Bonora E, Capaldo B, Perin PC, Del Prato S, De Mattia G, Frittitta L, et al. Hyperinsulinemia and insulin resistance are independently associated with plasma lipids, uric acid and blood pressure in non-diabetic subjects. The GISIR database. Nutr Metab Cardiovasc Dis. 2008;18:624–31. doi: 10.1016/j.numecd.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Vuorinen-Markkola H, Yki-Järvinen H. Hyperuricemia and insulin resistance. J Clin Endocrinol Metab. 1994;78:25–9. doi: 10.1210/jcem.78.1.8288709. [DOI] [PubMed] [Google Scholar]
- 12.Lyngdoh T, Marques-Vidal P, Paccaud F, Preisig M, Waeber G, Bochud M, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One. 2011;6:e19901. doi: 10.1371/journal.pone.0019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 14.Zavaroni I, Bonini L, Fantuzzi M, Dall’Aglio E, Passeri M, Reaven GM. Hyperinsulinaemia, obesity, and syndrome X. J Intern Med. 1994;235:51–6. doi: 10.1111/j.1365-2796.1994.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 15.Forsblom CM, Eriksson JG, Ekstrand AV, Teppo AM, Taskinen MR, Groop LC. Insulin resistance and abnormal albumin excretion in non-diabetic first-degree relatives of patients with NIDDM. Diabetologia. 1995;38:363–9. doi: 10.1007/BF00400643. [DOI] [PubMed] [Google Scholar]
- 16.Vaag A, Lehtovirta M, Thye-Rönn P, Groop L European Group of Insulin Resistance. Metabolic impact of a family history of Type 2 diabetes. Results from a European multicentre study (EGIR) Diabet Med. 2001;18:533–40. doi: 10.1046/j.1464-5491.2001.00496.x. [DOI] [PubMed] [Google Scholar]
- 17.Florez H, Ryder E, Campos G, Fernandez V, Morales LM, Valbuena H, et al. Women relatives of Hispanic patients with type 2 diabetes are more prone to exhibit metabolic disturbances. Invest Clin. 1999;40:127–42. [PubMed] [Google Scholar]
- 18.Norton K, Whittingham N, Carter L, Kerr D, Gore CJ, Marfell-Jones M. Measurement techniques in anthropometry. In: Norton K, Olds T, editors. Anthropometrica: A Text-book of Body Measurement for Sports and Health Courses. Sydney: University of New South Wales; 1996. pp. 25–75. [Google Scholar]
- 19.O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 21.Azizi F, Salehi P, Etemadi A, Zahedi-Asl S. Prevalence of metabolic syndrome in an urban population: Tehran Lipid and Glucose Study. Diabetes Res Clin Pract. 2003;61:29–37. doi: 10.1016/s0168-8227(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 22.Siewert S, Filipuzzi S, Codazzi L, Gonzalez I, Ojeda MS. Impact of metabolic syndrome risk factors in first-degree relatives of type 2 diabetic patients. Rev Diabet Stud. 2007;4:177–84. doi: 10.1900/RDS.2007.4.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meis SB, Schuster D, Gaillard T, Osei K. Metabolic syndrome in nondiabetic, obese, first-degree relatives of African American patients with type 2 diabetes: African American triglycerides-HDL-C and insulin resistance paradox. Ethn Dis. 2006;16:830–6. [PubMed] [Google Scholar]
- 24.Facchini F, Chen YD, Hollenbeck CB, Reaven GM. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. JAMA. 1991;266:3008–11. [PubMed] [Google Scholar]
- 25.Takahashi S, Yamamoto T, Tsutsumi Z, Moriwaki Y, Yamakita J, Higashino K. Close correlation between visceral fat accumulation and uric acid metabolism in healthy men. Metabolism. 1997;46:1162–5. doi: 10.1016/s0026-0495(97)90210-9. [DOI] [PubMed] [Google Scholar]
- 26.Matsuura F, Yamashita S, Nakamura T, Nishida M, Nozaki S, Funahashi T, et al. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: Visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism. 1998;47:929–33. doi: 10.1016/s0026-0495(98)90346-8. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita S, Nakamura T, Shimomura I, Nishida M, Yoshida S, Kotani K, et al. Insulin resistance and body fat distribution. Diabetes Care. 1996;19:287–91. doi: 10.2337/diacare.19.3.287. [DOI] [PubMed] [Google Scholar]
- 28.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–81. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fruehwald-Schultes B, Peters A, Kern W, Beyer J, Pfützner A. Serum leptin is associated with serum uric acid concentrations in humans. Metabolism. 1999;48:677–80. doi: 10.1016/s0026-0495(99)90163-4. [DOI] [PubMed] [Google Scholar]
- 30.Bandaru P, Shankar A. Association between Serum Uric Acid Levels and Diabetes Mellitus. Int J Endocrinol 2011. 2011:604715. doi: 10.1155/2011/604715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai XL, Han XY, Ji LN. Association between serum uric acid and different states of glucose metabolism and glomerular filtration rate. Chin Med J (Engl) 2010;123:3118–22. [PubMed] [Google Scholar]


