Abstract
Background:
Hyperuricemia has been associated with the development of high blood pressure (BP). We studied the effects of allopurinol therapy in hyperuricemic hemodialysis (HD) patients with high BP.
Materials and Methods:
This single-blind, randomized cross-over clinical study involved 55 HD patients with serum uric acid level > 6.5 (men) and > 5.5 mg/dL (women). They were randomly divided in two groups, each of which went through two phases. Group-1 in phase-1 received 100 mg/day orally of allopurinol for three months; while Group-2 was given whatever medication they received prior to the study. After two months of washing period, the groups were crossed-over. The BP levels were measured before and after HD during the eight months study period.
Results:
Fifty-three patients completed the study (33 men and 20 women, with mean age of 55.8 years). Uric acid levels decreased significantly during the12 weeks of allopurinol therapy (7.71 ± 1.53 to 5.2 ± 1.2 P < 0.005). Overall, after the 12 weeks of allopurinol therapy, systolic and diastolic BP also significantly decreased in allopurinol group, 15.8% (139 to 117, P < 0.0005) and 8.6% (81 to 74, P <.0005), respectively. There were not significant changes in body mass index, blood urea nitrogen, creatinine, albumin, cholesterol, triglyceride, hemoglobin, liver enzymes and serum electrolytes level after treatment. Patients treated with allopurinol had a significant increase in the quality of dialysis (KT/V) (P: 0.043).
Conclusions:
In HD patients, allopurinol treatment reduced BP. The results indicate a new potential therapeutic approach for controlling BP in HD patients.
Keywords: Allopurinol, end stage renal disease, high blood pressure, uric acid
INTRODUCTION
Hypertension is a very common disease affecting 25-30% of people in the world.[1] Despite a large number of safe and effective anti-hypertensive medications and useful lifestyle changes, BP control is achieved in less than 40% of patients.[2] Increased serum uric acid level has been associated with increased risk for development of hypertension (HTN)[3,4,5,6,7] and cardiovascular (CV) disorders.
Animal experiments suggest that hyperuricemia can induce high BP through activation of the renin-angiotensin system, reduction in endothelial nitric oxide levels, superoxide generation, elevated levels of systemic inflammatory mediators, induction of endothelial dysfunction and vascular smooth muscle proliferation.[8,9,10,11,12,13,14,15,16]
By inhibiting the enzyme xanthine oxidase, allopurinol can decrease serum uric acid levels. Its effect is achieved by changing plasma renin activity and nitric oxide syntheses, which improves endothelial dysfunction and prevents vasculopathy.[17,18,19,20]
Studies in humans have also shown correlation between uric acid levels with both endothelial dysfunction and elevated plasma renin activity.[21,22]
Uric acid has also been suggested as an important risk factor in the development of primary HTN in humans.[23,24] Hyperuricemia is strongly associated with increased mortality.[25,26,27]
In two cohort studies of chronic kidney disease stage-5, there were a J-shaped correlation between uric acid level and all causes of mortality.[28,29]
However, there is no data available linking uric acid levels and BP levels in patients with ESRD. The present study aimed to investigate the effect of allopurinol on lowering blood pressure in hemodialysis patients with hyperuricemia.
MATERIALS AND METHODS
This single blind, randomized crossover clinical trial study was conducted between October 2009 and August 2010. One hundred and forty two dialysis patients aged 18-88 years old were enrolled in the study. They had been dialyzed in two HD centers (Vali-e-Asr and Beheshti hospitals) in the Iranian provincial capital of Zanjan.
Inclusion criteria were high level of serum uric acid (greater than 6.5 mg/dl for men; and 5.5 mg/dL for women) and existence of HTN. The definition of hyperuricemia was based on analyses from the Atherosclerosis Risk in Communities (ARIC).[30]
Hypertension was defined as systolic BP of 140 mm Hg or higher, diastolic BP 90 mm Hg or higher; or combined factors of diagnosed with high BP and using antihypertensive medication.
Exclusion criteria were malignant HTN due to Erythropoietin therapy diagnosed with cancer, alcohol consumption, noncompliance with taking prescribed medication and those patients with a known history of hypersensitivity to allopurinol or being already on allopurinol. Each participating patient gave written informed consent before enrollment in the study.
Fifty-five patients meet the inclusion criteria, hence, their BP were measured before and after each session of HD for the eight months duration of the study. To prevent any observer bias at the time of measuring BP, the nurse in charge was prevented from knowing which patients were under treatment of allopurinol.
BP was measured by a calibrated mercury sphygmomanometer in the dialysis ward, after the patients were at the sitting and relaxed position for 10 min. The mean of all weekly measurements for systolic and diastolic BP, before and after HD, was used for analysis.
During the study, the antihypertensive therapies, as well as lipid-lowering drugs such as (ACE inhibito, â blocker, Ca channel blocker and inhibiting HMG-CoA reductase) were kept constant.
In patients, who were taking antihypertensive medication, the class and dosage of medication were extracted from their records.
Patients were randomly divided into two groups, each of which went through two phases. In the first phase of the study, Group-1received 100 mg/day of allopurinol, Hakim Pharmaceutical Company of Iran, for three months; while Group-2 was given whatever medication they received prior to enrollment into the study. After three months, patients passed two months of washout period. Then in the second phase of the study, the two groups were crossed over and Group-2 was administrated allopurinol for three months, similar to Group-1 in the first phase. This was while; Group-1 was given whatever medication they received prior to enrollment into the study [Figure 1].
Figure 1.

Flow-chart of the study: Single-blind, two-period randomized cross over study
To assess the relation between serum uric acid and other paraclinical and demographic factors, the following variables were checked: Age, gender, diabetes (no, yes), serum cholesterol (Chol), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) cholesterol, hemoglobin (Hgb), serum albumin (Alb), Na, K, AST, ALT, Ca and P levels, body mass index (kg/m2) and quality of dialysis which measured by KT/V. Data and laboratory values were collected when patients got to their dry weight 6 months after the start of chronic maintenance dialysis. Each month laboratory values were checked.
To evaluate the efficacy of dialysis, KT/V was calculated. The HD protocol for all patients was 4 hours, three times per week, using polysolphane membranes from Meditechsys and Soha Companies and an average blood flow rate of 300 to 350 mL/min and with bicarbonate basis dialysate. The mean duration of dialysis was 36.21 ± 35.6 months (with maximum of 192 and minimum of one month).
Subjects were observed for any adverse events related to the use of allopurinol (including Stevens-Johnson syndrome and an increase in transaminase level), but none were found. This trial was approved by committee of ethics of research of Zanjan University of Medical Sciences (Ethics No; 19/3-3/976).
Trial Registration Number: IRCT138902043325N2
Statistical analysis
The data analysis was conducted base on intent-to-treat. All statistical analysis was performed by using the SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA).
Means of quantitative variables were compared by using student T-test between the two groups. In the case of discontinuous variables, Chi-square test was applied. Response to Allopurinol in the study groups, before and after intervention, was assessed with a paired samples T-test analysis. Paired samples T-test and independent sample T-test were used to assess carry-over effect. The repeated measures analysis of variance model was used to assess the effect of allopurinol on change of BP over time (time effect), the difference of this effect between the two groups (time*treatment interaction) and treatment effect. In the cases, which specificity test was significant, P value was corrected with Greenhouse-Geisser test (G-G3). All P values were two-tailed and a P value <.05 was considered as significant.
RESULTS
One hundred and forty two HD patients were enrolled in the study, 55 of whom met the inclusion criteria. In the first phase of the study, Group-1 (28 patients) received 100 mg/day of allopurinol for three months; while Group-2 (27 patients) was given whatever medication they received prior to enrollment into the study [Figure 1].
During phase-1, two patients in Group-1 dropped out of the study due to kidney transplantation. In the second phase, one patient in Group-1 and two patients in Group-2 died due to cardiovascular events. Thus, 53 patients (33 men and 20 women, with the mean age of 55.8 years) were included in the primary analysis [Figure 2]. This is while; twenty- seven patients in Group-1 and 23 in Group-2 completed the eight- month follow-up period of observation. Table 1 presents demographic and baseline clinical characteristics of the study population while Table 2 present baseline laboratory parameters. There was no significant difference between the two groups in different variables at each phase of the study.
Figure 2.
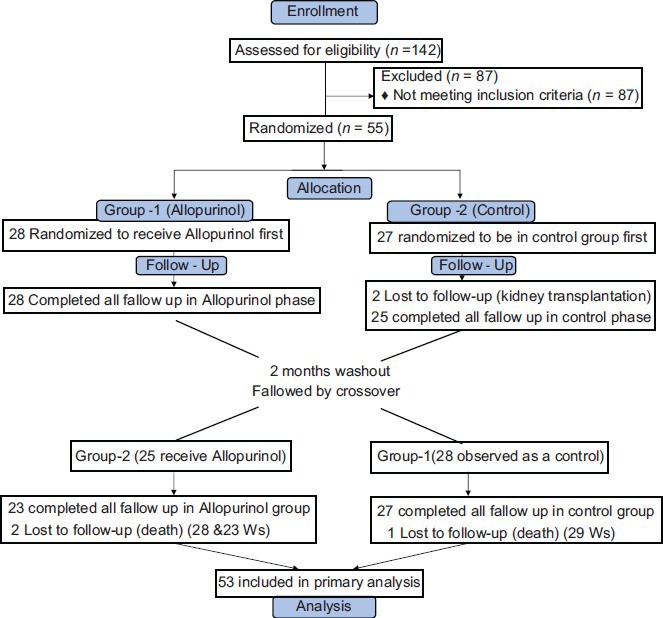
Study design
Table 1.
Baseline characteristics of patient population

Table 2.
Baseline laboratory features of patients

In the first assessment to determine the patients’ eligibility for the study, we found that the prevalence of elevated serum uric acid levels was 38.7% (55/142). 80.4% of hyperuricemic patients had HTN and 95.3% of HTN patients had elevated uric acid levels.
There was no association between age and hyperuricemia in development of HTN.
In addition, Table 1 shows the percentage of patients, who were taking antihypertensive medication: This distribution did not differ between the two groups.
Comparison of baseline BP at the beginning of the two phases of the study showed there was no carry-over effect in this crossed clinical trial [Table 3].
Table 3.
Comparison between baseline blood pressure at the beginning of two phases (testing for carry-over effect)

Pooled data analysis
After 12 weeks of allopurinol therapy, uric acid levels was reduced significantly from 7.71 ± 1.53 (5.6-11.5) to 5.2 ± 1.2 (2.6-9.1) (t: 9.24, P value < 0.005). Patients treated with allopurinol also had a significant decrease in systolic and diastolic BP.
Pooled data analysis of BP in control and allopurinol groups at baseline showed there was no significant changes in systolic and diastolic BP before and after dialysis in the control group, but BP significantly decreased in the allopurinol group over time (F: 14.707, P < 0.0005 corrected with G-G3) [Figure 3]. Overall, after 12 weeks of treatment with allopurinol, systolic BP before and after dialysis decreased from 139 and 128 to 117 (F: 14.707, P < 0.0005 corrected with G-G3) and 110 (F: 8.460, P < 0.0005 corrected with G-G3), respectively. Also, overall after 12 weeks of treatment with allopurinol diastolic BP before and after dialysis decreased from 81 and 78 to 74 (F: 5.851, P < 0.0005) and 69 (F: 8.241, P < 0.0005 corrected with G-G3), respectively, [Table 4].
Figure 3.

Change of blood pressure in two study groups over time
Table 4.
Is there effect of time the same for both groups (time * treatment interaction) and is there any treatment effect (between 2 group)? (Pooled analysis for treatment effect)

The effect of time was not the same for the two groups (time* treatment). As it is shown in Figure 3, trend of BP in allopurinol group is significantly decrescendo, but it is crescendo in the control group. There were no significant changes in intradialytic weight gain, nPNA, BUN, Cr, Alb, Chol, TG, Hgb, liver enzymes and serum electrolytes after treatment period. Patients treated with allopurinol also had a significant increase in kt/v (t: 2.1, P value: 0.043). There was a significant treatment effect with allopurinol in BP of the intervention group [Table 4].
Parallel analysis for each phase
Parallel analysis was also conducted separately for each phase of the study. In first Phase, before and after dialysis, systolic BP were 134.6 and 122.1, which after treatment with allopurinol decreased to 119.8(P < 0.0005) and 111.4 (P <.0005) l. Before and after dialysis, diastolic BP were 78.5 and 75.7, which dropped to 74.6 (P < 0.0005) and 70.7 (P < 0.0005) after treatment. In second Phase, before and after dialysis, systolic BP were 145.6 and 136, which decreased to 113.9(P < 0.0005) and 109.5 (P <.0005) after treatment with allopurinol. Before and after dialysis, diastolic BP was 85.6 and 81.3, which dropped to 73.6 (P < 0.0005) and 68.6 (P < 0.0005) after treatment. This trend was not the same between the two groups. In phase-1 of the allopurinol group, systolic BP before and after dialysis decreased from 134 and 122 to 119 (F: 4.553, P < 0.0005) and 111(F: 2.481, P = 0.025), respectively. However, in the control group, systolic BP increased from 138 and 130 to 153 (F: 2.858, P = 0.001) and 133 (F: 1.160, P = 0.333), respectively. Treatment effect in phase-1 and phase-2 statistically were statistically significant.
DISCUSSION
In our study, we investigated the effect of hyperuricemia on blood pressure of HD patients. Hypertension is caused by multifactorial etiology. Family or genetic background, diet, sodium intake, physical activity and stress level are among the many factors considered as contributing to the development of high BP. Hypertension is also associated with CV diseases such as coronary heart disease, peripheral vascular disease, stroke and renal disease.[31,32,33]
Uric acid is considered as a strong predictor of hypertension and BP progression.[3,4,5,6,7,34,35]
There is no universally accepted definition for hyperuricemia. In one study, patients who had a serum uric acid >5.5 mg/dL had an 89% positive predictive value for essential HTN, whereas a serum uric acid level of less than <5.0 mg/dL had a 96% negative predictive value for essential HTN.[36]
In our study, we investigated the effect of hyperuricemia on blood pressure of HD patients. We defined hyperuricemia as serum uric acid of >6.5 mg/dL in men and >5.5 mg/dL in women.
Epidemiologic studies support possible role of uric acid on development of hypertension and cardiovascular events
Hyperuricemia has been reported in 25% of untreated HTN patients,[37] in 50% of those taking diuretics and in 75% of subjects with malignant hypertension.[38] It has been reported that 50-70% of hyperuricemic patients had HTN.[39] In our study, we found 80.4% of hyperuricemic patients had HTN and 95.3% of HTN patients had elevated uric acid levels.
The relation between uric acid level and HTN decreases with higher patient age and duration of HTN,[40] suggesting that uric acid may be more of a significant factor in younger patients with early-onset of HTN. In a recent Framingham Heart Study, serum uric acid level was found to be an independent predictor of HTN incidence and BP progression over as few as four years in young and middle age adults who had never been diagnosed with HTN.[41]
In our study, there was no association between age and hyperuricemia in development of HTN. Feig and Johnson[42] have demonstrated a significant correlation between elevated uric acid levels and BP in children and adolescents. The Moscow Children's Hypertension Study found hyperuricemia in 9.5% of children with normal BP, 49% of children with borderline HTN and 73% of children with moderate and severe HTN.[43] A Hungarian Children's Health Study followed 17,624 children for 13 years and found that significant risk factors for the development of HTN were elevated heart rate, early sexual maturity and hyperuricemia.[44]
Other studies have shown a linear relationship between serum uric acid and systolic BP in both white and black individuals.[45,46] However one study, involving patients in whom hypertension had developed after the age of 60, has shown that uric acid did not predict the development of HTN.[46]
In addition, high uric acid levels have been associated with organ damage in hypertensive patients.[47,48] There are also studies related to an association between serum uric acid and subsequent CV events, left ventricular hypertrophy and risk for cardiac death in the general population.[49,50,51,52]
In our study, we did not investigate the association between uric acid level and CV events and mortality, since survival analysis needs larger sample size and more follow-up.
Dose allopurinol has an effect on high BP
Allopurinol treatment can reduce BP in hyperuricemic adolescents with newly diagnosed hypertension.[53,54,55] Mehmet Kanbay and colleagues investigated the effect of allopurinol on blood pressure, creatinine clearence and proteinuria in patients with normal renal functions. They found that hyperuricemia increases blood pressure and decreases GFR.[53]
In our study, we also found that treatment of hyperuricemia with allopurinol reduced serum uric acid levels and controlled BP.
Finally, since daily protein intake of the patients may influence the uric acid levels, normalized protein nitrogen appearance (nPNA), which reflects the daily protein intake in HD patients were considered to be calculated. We found that before and after HD there was not any statistical significant difference in terms of nPNA between these two groups, Table 5.
Table 5.
Effect nPNA on the uric acid levels

SUMMARY
Several clinical and laboratory studies have shown that uric acid level might be an important factor in the development of hypertension in humans. Our study has found that reducing hyperuricemia decreases BP, while decreasing KT/V in HD patients. Uric acid is a pro-inflammatory factor,[55] so reducing uric acid to normal level may have anti-inflammatory effect. This may be the reason for improvement of KT/V in our study population. Hence, management of hyperuricemia may prevent HTN and consequently reduce CV events.
To our knowledge, this is the first study in the literature that shows the benefits of allopurinol therapy on BP in hyperuricemic patients who are under HD treatment.
For further confirmation of the findings, we suggest studies with larger sample size. We also recommend studies to be conducted to access the effect of early treatment with allopurinol on patients with normal BP, as an important part of a prevention regime for any kind of vascular events in ESRD patients.
Limitation of this study
One of the main limitations of this study was avoidance of placebo.
Also, it is important to note that the results of our study may be confined by the concurrent use of antihypertensive drugs by patients. However, we agree that our sample size was too small to allow us to make concrete conclusion.
ACKNOWLEDGMENTS
Iran's Zanjan University of Medical Sciences supported this work. The authors also wish to thank Dr. Fatemeh Mirzamohamadi M.D. for assisting in data analysis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Riaz k. Hypertension. [Last accessed on 2012 Jan 27]. Available from: http://www.emedicine.medscape.com/article/241381-overview .
- 2.Sarafidis PA, Bakris GL. State of hypertension management in the United States: Confluence of risk factors and the prevalence of resistant hypertension. J Clin Hypertens (Greenwich) 2008;10:130–9. doi: 10.1111/j.1751-7176.2008.07309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ventura H, Piña IL, Lavie CJ. Hypertension and Antihypertensive Therapy in Hispanics and Mexican Americans Living in the United States. Postgrad Med. 2011;123:46–57. doi: 10.3810/pgm.2011.11.2494. [DOI] [PubMed] [Google Scholar]
- 4.Nainggolan L. Uric acid a “probable cause” of CVD. Hypertention. [Last accessed on 2012 Apr 30]. Availablr from: http://www.theheart.org/article/1391079.do .
- 5.Lohr J. Part II: Clinical challenges and renal considerations in managing gout. Renal Urol News Apr 01, 2012. [Last accessed on 16 Oct 2012]. Available from: http://www.renalandurologynews.com/gout/topic/7022 .
- 6.O’Keefe JH, Carter MD, Lavie CJ. Primary and Secondary Prevention of Cardiovascular Diseases: A Practical Evidence-Based Approach. Mayo Clin Proc. 2009;84:741–57. doi: 10.4065/84.8.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper-DeHoff RM, Wen S, Beitelshees AL, Zineh I, Gums JG, Turner ST, et al. Impact of abdominal obesity on incidence of adverse metabolic effects associated with antihypertencive medications. Hypertension. 2010;55:61–8. doi: 10.1161/HYPERTENSIONAHA.109.139592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez-Lozada LG, Tapia E, Santamaría J, Avila-Casado C, Soto V, Nepomuceno T, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67:237–47. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 9.Gaffo AL, Saag KG. Drug treatment of yyperuricemia to prevent cardiovascular outcomes: Are we there yet? Am J Cardiovasc Drugs. 2012;12:1–6. doi: 10.2165/11594580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity-induced hypertension. Hypertens Res. 2010;33:386–93. doi: 10.1038/hr.2010.9. [DOI] [PubMed] [Google Scholar]
- 11.Dubois Xu Yi Chun, Hertig A, Baugey E. The tubulr expression of mesenchymal markers includes extracellular matrix proteins Nephrol. Dial Transplant. 2012;27(Suppl 2):ii427–49. [Google Scholar]
- 12.Goicoechea M, García de Vinuesa S, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, et al. Effect of Allopurinol in Chronic Kidney Disease Progression and Cardiovascular Risk. Clin J Am Soc Nephrol. 2010;5:1388–93. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reslan O, Khalil R. Molecular and Vascular Targets in the Pathogenesis and Management of the Hypertension Associated with Preeclampsia. Cardiovasc Hematol Agents Med Chem. 2010;8:204–26. doi: 10.2174/187152510792481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glushakova O, Kosugi T, Roncal C, Mu W, Heinig M, Cirillo P, et al. Fructose Induces the Inflammatory Molecule ICAM-1 in Endothelial Cells. J Am Soc Nephrol. 2008;19:1712–20. doi: 10.1681/ASN.2007121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasalic D, Marinkovic N, Feher-Turkovic L. Uric acid as one of the important factors in multifactorial disorders – facts and controversies. Biochemia Med. 2012;22:63–75. doi: 10.11613/bm.2012.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neogi T. Asymptomatic Hyperuricemia: Perhaps Not So Benign.? J Rheumatol. 2008;35:734–7. [PubMed] [Google Scholar]
- 17.López-Novoa JM, Rodríguez-Peña AB, Ortiz A, Martínez-Salgado C, López Hernández FJ. Etiopathology of chronic tubular, glomerular and renovascular nephropathies: Clinical implications. J Transl Med. 2011;9:13. doi: 10.1186/1479-5876-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feig DI, Soletsky B, Johnson RJ. Effect of Allopurinol on Blood Pressure of Adolescents with Newly Diagnosed Essential Hypertension: A randomized trial. JAMA. 2008;300:924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toma I, Kang J, Meer E, Pet-Peterdi J. Uric acid triggers renin release via a macula dens dependent pathway. JAMA. 2008;300:924–32. [Google Scholar]
- 20.Balakumar P, Sharma R, Kalia, Singh M. Hyperuricemia: Is it a risk factor for Vascular Endothelial Dysfunction and Associated Cardiovascular Disorders? Curr Hypertens Rev. 2009;5:1. [Google Scholar]
- 21.Kanbay M, Ilker Yilmaz M, Sonmez A, Turgut F, Saglam M, Cakir E, et al. Serum uric acid level and endothelial dysfunction in patients with nondiabetic chronic Kidney Disease. Am J Nephrol. 2011;33:298–304. doi: 10.1159/000324847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin M, Yang F, Yang I, Yin Y, Luo JJ, Wang H, et al. Uric Acid, Hyperuricemia and Vascular Diseases. Front Biosci. 2012;17:656–69. doi: 10.2741/3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berbari A. The role of uric acid in hypertention, cardiovascular events, and chronic kidney disease. Eur Soc Hypertens Sci Newsl. 2010;11:49. [Google Scholar]
- 24.Viazzi F, Leoncini G, Ratto E, Falqui V, Parodi A, Conti N, et al. Mild Hyperuricemia and Subclinical renal damage in untreated primary hypertension. Am J Hypertens. 2007;20:1276–82. doi: 10.1016/j.amjhyper.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Lin WY, Liu CS, Li TC, Lin T, Chen W, Chen CC, et al. In addition to insulin resistance and obesity, hyperuricemia is strongly associated with metabolic syndrome using different definitions in Chinese populations: A population-based study (Taichung Community Health Study) Ann Rheum Dis. 2008;67:432–33. doi: 10.1136/ard.2007.073601. [DOI] [PubMed] [Google Scholar]
- 26.Charnow J. High uric acid raises CKD Risk, Study Finds. Renal Urol News. 2012. [Last accessed on June 01 2012]. Available from: http://www.renalandurologynews.com/chronic-kidney-disease/topic/6999 .
- 27.Giacomo Zoppini, Giovanni Targher, Michel Chonchol, Vittorio Ortalda, Cataldo Abaterusso, Isabella Pichiri, Carlo Negri, Enzo Bonora. Serum Uric Acid Levels and Incident Chronic Kidney Disease in patients with type 2 Diabetes and Preserved Kidney Function. Diabetes Care. 2012;35:99–104. doi: 10.2337/dc11-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heimbach E, Bowden R, Griggs J, Beaujean A, Doyle E, Doyle R. The Effects Lowering Uric acid Levels Using Allopurinol on Components Metabolic Syndrome. Cardiol Res. 2012;3:80–6. doi: 10.4021/cr168w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madero M, Sarnak M, Wang X, Greene T, Beck GJ, Kusek JW, et al. Uric Acid and Long-term Outcomes in CKD. Am J Kidney Dis. 2009;53:796–803. doi: 10.1053/j.ajkd.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson RJ, Rideout BA. Uric acid and diet-insights into the epidemic of cardiovascular disease. N Engl J Med. 2004;350:1071–3. doi: 10.1056/NEJMp048015. [DOI] [PubMed] [Google Scholar]
- 31.Hypertensive Vascular Disease. 18 ed. Chapter 247. New York: Copyright© The McGraw-Hill Companies; 2012. HARRISON's Principles of Internal Medicine. [Google Scholar]
- 32.Kim SY, De Vera MA, Choi HK. Gout and mortality. Clin Exp Rheumatol. 2008;26(Suppl 51):S115–9. [PubMed] [Google Scholar]
- 33.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang T, Chu CH, Bai CH, You SL, Chou YC, Hwang LC, et al. Uric acid concentration as a risk marker for blood pressure progression and incident hypertension: A Chinese cohort study. Metabolism. 2012;61:1747–55. doi: 10.1016/j.metabol.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Yang T, Chu CH, Bai CH, You SL, Chou YC, Chou WY, et al. Uric acid level as a risk marker for metabolic syndrome: A Chinese cohort study. Atherosclerosis. 2012;220:525–31. doi: 10.1016/j.atherosclerosis.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–52. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Firestein GS, Budd RC, Harris ED, McInnes IB, Ruddy S, John S. 8th ed. Philadelphia: Copyright© W.B. Saunders Company; 2008. Kelley's Textbook of Rheumatology. [Google Scholar]
- 38.Khan M, Mashori G, Memon K. Pakistan: Journal of Liaquat University of Medical and Health Sciences (JLUMHS) Jamshoro; 2008. Safety of Losartan in Hypertensive Patients with Thiazide Induced Hyperuricemia; pp. 163–7. [Google Scholar]
- 39.Ouppatham S, Bancha S, Choovichian P. The relationship of hyperuricemia and blood pressure in the Thai army population. J Postgrad Med. 2008;54:259–62. doi: 10.4103/0022-3859.43509. [DOI] [PubMed] [Google Scholar]
- 40.Mercuro G, Vitale C, Cerquetani E, Zoncu S, Deidda M, Fini M, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol. 2004;94:932–5. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 41.Sundström J, Sullivan L, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension. 2005;45:28–33. doi: 10.1161/01.HYP.0000150784.92944.9a. [DOI] [PubMed] [Google Scholar]
- 42.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–52. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramsay LE. Hyperuricemia in hypertension: Role of alcohol. Br Med J. 1979;1:653–4. doi: 10.1136/bmj.1.6164.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sánchez-Fructuoso AI, Torralbo A, Arroyo M, Luque M, Ruilope LM, Santos JL, et al. Occult lead intoxication as a cause of hypertension and renal failure. Nephrol Dial Transplant. 1996;11:1775–80. [PubMed] [Google Scholar]
- 45.Alper AB, Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: The Bogalusa Heart Study. Hypertension. 2005;45:34–8. doi: 10.1161/01.HYP.0000150783.79172.bb. [DOI] [PubMed] [Google Scholar]
- 46.Klein R, Klein BE, Cornoni JC, Maready J, Cassel JC, Tyroler HA. Serum uric acid. Its relationship to coronary heart disease risk factors and cardiovascular disease, Evans County, Georgia. Arch Intern Med. 1973;132:401–10. doi: 10.1001/archinte.132.3.401. [DOI] [PubMed] [Google Scholar]
- 47.Galvan AQ, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol. 1995;268:E1–5. doi: 10.1152/ajpendo.1995.268.1.E1. [DOI] [PubMed] [Google Scholar]
- 48.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47:51–9. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Short RA, Johnson RJ, Tuttle KR. Uric acid, microalbuminuria and cardiovascular events in high-risk patients. Am J Nephrol. 2005;25:36–44. doi: 10.1159/000084073. [DOI] [PubMed] [Google Scholar]
- 50.Mellen PB, Bleyer AJ, Erlinger TP, Evans GW, Nieto FJ, Wagenknecht LE, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: The Atherosclerosis Risk in Communities study. Hypertension. 2006;48:1037–42. doi: 10.1161/01.HYP.0000249768.26560.66. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Tubulo-interstitial damage and progression of renal failure. Kidney Int Suppl. 2005;99:S82–6. doi: 10.1111/j.1523-1755.2005.09915.x. [DOI] [PubMed] [Google Scholar]
- 52.Viazzi F, Parodi D, Leoncini G, Parodi A, Falqui V, Ratto E, et al. Serum uric acid and target organ damage in primary hypertension. Hypertension. 2005;45:991–6. doi: 10.1161/01.HYP.0000161184.10873.ea. [DOI] [PubMed] [Google Scholar]
- 53.Kanbay M, Ozkara A, Selcoki Y, Isik B, Turgut F, Bavbek N, et al. Effect of treatment of hyperuricemia with allopurinol on blood pressure, creatinine clearence, and proteinuria in patients with normal renal functions. Int Urol Nephrol. 2007;39:1227–33. doi: 10.1007/s11255-007-9253-3. [DOI] [PubMed] [Google Scholar]
- 54.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension. JAMA. 2008;300:924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohno I. Relationship between hyperuricemia and chronic kidney disease. Nucleosides Nucleotides Nucleic Acids. 2011;30:1039–44. doi: 10.1080/15257770.2011.611484. [DOI] [PubMed] [Google Scholar]


