Abstract
Helicobacter pylori (H. pylori) are one of the most common bacterial infections, seen in humans, worldwide and their possible relationships to different diseases are a focus of attention nowadays. H. pylori may cause some extra intestinal manifestations some of which are dermatological conditions, including Henoch-Schönlein purpura (HSP), chronic urticaria and atopic dermatitis. We describe a 49-year-old man who presented with HSP triggered by gastric H. pylori infection. Treatment of H. Pylori infection was accompanied by prompt resolution of the gastrointestinal manifestations and purpuric rashes. These findings suggest a causative role for H. pylori in the occurrence of HSP.
Keywords: Eradication therapy, helicobacter pylori, schonlein-henoch purpura
INTRODUCTION
Henoch-Schonlein-purpura (HSP) is a systemic vasculitis, also known as a leukocytoclastic vasculitis of small vessels, resulting in skin, joint, gastrointestinal and renal involvement. HSP may affect both infants and adults, but much rarer in adults than in children. The pathogenesis of HSP remains poorly understood, but it is postulated that an unknown antigenic stimulus causes elevation of circulating IgA and that complement activation leads to necrotizing vasculitis, and a wide variety of conditions such as bacterial or viral infections, vaccinations, drugs and other environmental exposures may be responsible for the onset.[1,2,3,4] Gastrointestinal manifestations, such as colicky abdominal pain, nausea, vomiting, and occult or massive gastrointestinal bleeding, are common. It is known that HSP is generally a self-limited condition that lasts an average of four weeks. In children the prognosis is good, as HSP typically resolves rapidly and without complication. In adults, however, the prognosis of HSP and duration of the disease depends on the clinical features such as a higher rate of severe, atypical gastrointestinal problems; and delayed renal complications.[1,2,3,4] Helicobacter pylori (H. pylori) has been implicated in the pathogenesis of various extra-digestive disorders. However, a few previous reports have described an association between gastric H. pylori infection and HSP and indicated the efficacy of eradication therapy.[3,4,5,6,7] In this report, we describe an adult onset of HSP triggered by H. pylori positive gastritis and aimed to present this coexisting in terms of contributing to the literature. The patient was successfully treated by H. pylori eradication therapy.
CASE REPORT
A previously healthy 49-year-old-man was admitted to Harran University Faculty of Medicine Hospital, at Sanliurfa/Turkey in 2011, because of a one-week history of intermittent colicky abdominal pain, decreased appetite and subsequent purpura on his lower extremities. Physical examination revealed numerous erythematous macules, red-brown purpuric papules, and plaques on the legs, thighs, buttocks, and extensor surfaces of the arms [Figure 1]. On admission, his temperature was 36.9°C, pulse 76, respiration 14, and blood pressure 120/70 mmHg. Aside from mid-epigastric pain to palpation and had a previously known of vitiligo history that comprises the extremities, the remainder of the physical examination was unremarkable. Laboratory studies showed a white blood cell count of 23.070/mm3. The hemoglobin concentration was 13.07 g/dL, the platelet count was 393.200/mm3, elevated level of erythrocyte sedimentation rate, while normal results for serum creatinine level, serum IgA level. Urinalysis revealed microscopic hematuria and proteinuria (450 mg/24 hrs). Occult blood was found in the feces. Antinuclear antibody, rheumatoid factor, C3 was not detected and C3 levels was normal. Serologic tests for Human immunodeficiency virus and hepatitis virus (A, B, and C), were also negative. Upper endoscopy revealed findings of antrum predominant pangastritis, with erythema, and active peptic ulcus and showed patchy petechial hemorrhage of the duodenal bulb. Rapid urease test for H. pylori showed a positive result. Endoscopic biopsy specimens obtained from gastric mucosa revealed chronic active gastritis and multiple H. pylori organisms were detected within the lumina of antrum. The results of colonoscopy were negative. A biopsy specimen obtained from purpuric lesion showed a dermal perivascular and interstitial infiltrate rich in lymphocytes and neutrophils, as well as extravasations of erythrocytes and leukocytoclasis. Vessel walls showed endothelial swelling and necrosis with deposition of fibrinoid material [Figure 2]. The patient was treated with methylprednisolone 1 mg/kg/day for 10 days regarding to the abdominal complaints, urinalysis results, and age onset; however his complaints were not healed with this treatment. Further, the patient received a two-week treatment course consisting of lansoprazole 30 mg twice daily, amoxicillin 1000 mg twice daily, and clarithromycin 500 mg twice daily without steroid treatment. After the treatment, the abdominal manifestations dramatically subsided within three days and the purpuric skin lesions resolve within four days and by one week after starting the rash had completely resolved without further treatment. Stool test performed two months after the therapy showed a negative result, which suggested successful H. pylori eradication. The patient has had no recurrence of rash or abdominal pain, during the one year follow-up while being monitored from the outpatient clinic [Figure 3].
Figure 1.
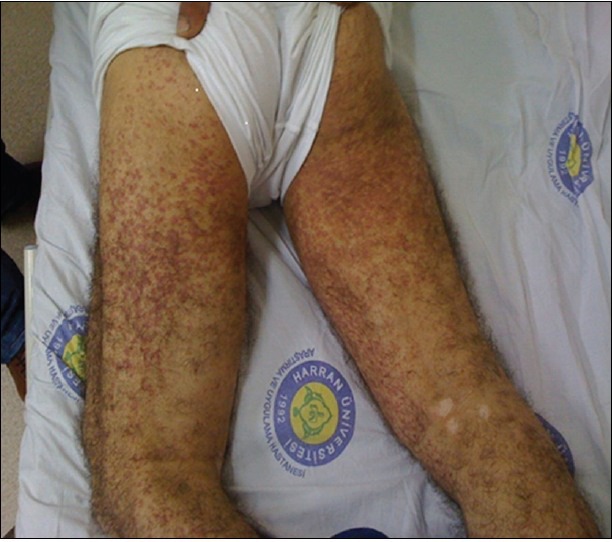
Numerous purpuric rashes on both lower extremities
Figure 2.
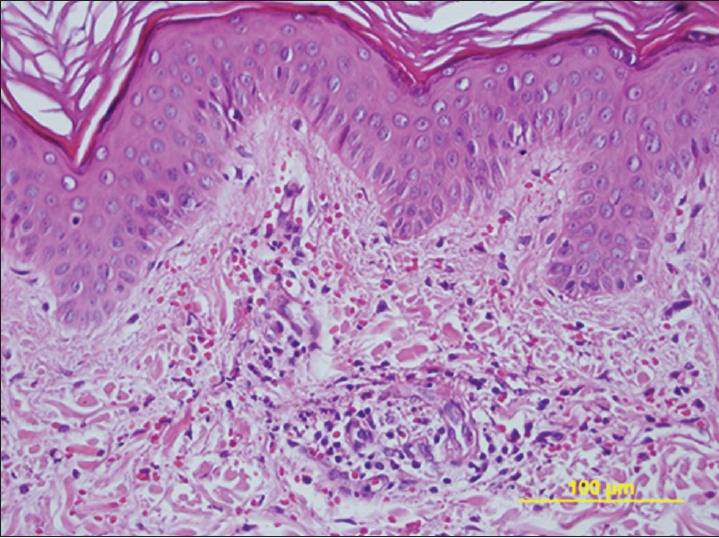
Skin biopsy specimen shows perivascular neutrophilic and lymphocytic infiltrate with leukocytoclasis and erythrocyte extravasation (H and E, ×400)
Figure 3.
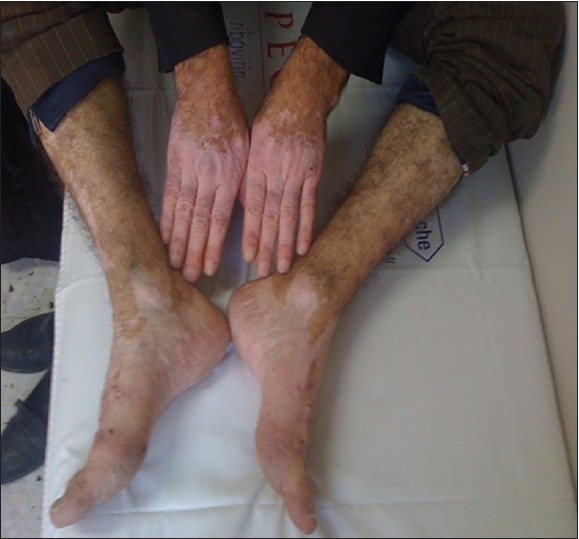
Appearance of the limbs after the H. Pylori infection therapy
DISCUSSION
This report suggests that H. pylori infection may be a precipitating factor in the development of HSP and treatment of the H. pylori infection was accompanied by rapid improvement of the HSP.
H. pylori infection may lead extradigestive consequences directly or indirectly, in various ways. A marked local and a chronic systemic immune response are triggered by H. pylori infection.[8] This bacterium has been associated with certain extra-digestive dermatological conditions, including chronic urticaria, rosacea, HSP, Sweet syndrome, systemic sclerosis, and atopic dermatitis.[9] Reinauer et al, described the first case of HSP associated with gastric H. Pylori infection in 1995. The symptoms of HSP disappeared after H. Pylori eradication therapy, and then recurred 10 months later due to reinfection with H. Pylori. These clinical manifestations faded again after the second eradication treatment.[3] Since then, some similar case reports have been described.[4,5,6,7] More recently, Novak et al, found serum antibody to H. pylori in 10 of 11 adults with HSP compared with 11 of 20 adult controls.[10]
In general, gastrointestinal symptoms, such as colicky abdominal pain, frequently occur in patients with H. pylori infection as well as in patients with HSP.[11] Although the association of H. pylori infection with HSP may be underestimated, because endoscopic examination is not systematically performed in patients with HSP. Patients with HSP are treated supportively or symptomatically because of the self-limited nature, and it is evident that not all HSP patients need early steroid treatment, and treatment should be targeted at patients who have a high risk of renal involvement or severe extra renal symptoms. These risk factors are age over six years, severe abdominal pain and renal symptoms at onset. However, the effect of steroids on the prevention or treatment of renal complications has been controversial, and recurrences could be seen.[12] In our case H. pylori infection was confirmed in the gastric mucosa by rapid urease test and study of endoscopic biopsy specimens, and the skin lesions were resolved promptly following the treatment of H. pylori infection, without the corticosteroid treatment. Regarding this successful H. pylori eradication treatment and no recurrence was shown within a one year follow-up, the possibly diagnosis was considered HSP associated with H. pylori infection.
In conclusion, H. pylori may be an etiological factor in pathogenesis of HSP and upper endoscopy and H. pylori examination should be considered to confirm the presence of gastric H. pylori infection. Also, HSP patients should be considered to evaluate the usefulness of H. pylori eradication therapy, especially with serious gastrointestinal manifestations. To address this study, further studies must focus on this relationship.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lawee D. Atypical clinical course of Henoch-Schonlein purpura. Can Fam Physician. 2008;54:1117–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Saulsbury FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2010;22:598–602. doi: 10.1097/BOR.0b013e32833af608. [DOI] [PubMed] [Google Scholar]
- 3.Reinauer S, Megahed M, Goerz G, Ruzicka T, Borchard F, Susanto F, et al. Schönlein-Henoch purpura associated with gastric Helicobacter pylori infection. J Am Acad Dermatol. 1995;33:876–9. doi: 10.1016/0190-9622(95)90426-3. [DOI] [PubMed] [Google Scholar]
- 4.Hoshino C. Adult onset Schönlein-Henoch purpura associated with Helicobacter pylori infection. Intern Med. 2009;48:847–51. doi: 10.2169/internalmedicine.48.1718. [DOI] [PubMed] [Google Scholar]
- 5.Machet L, Vaillant L, Machet MC, Büchler M, Lorette G. Schönlein-Henoch purpura associated with gastric Helicobacter pylori infection. Dermatology. 1997;194:86. doi: 10.1159/000246068. [DOI] [PubMed] [Google Scholar]
- 6.Mozrzymas R, d’Amore ES, Montini G, Guariso G. Schönlein-Henoch vasculitis and chronic Helicobacter pylori associated gastritis and duodenal ulcer: A case report. Pediatr Med Chir. 1997;19:467–8. [PubMed] [Google Scholar]
- 7.Cecchi R, Torelli E. Schönlein-Henoch purpura in association with duodenal ulcer and gastric Helicobacter pylori infection. J Dermatol. 1998;25:482–4. doi: 10.1111/j.1346-8138.1998.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 8.Aydogan T, Ulas T, Selcoki Y, Alkan R, Yılmaz OC, Yalcın KS, et al. Effects of Helicobacter pylori eradication on proteinuria: A prospective study. Wien Klin Wochenschr. 2012;124:241–4. doi: 10.1007/s00508-012-0150-0. [DOI] [PubMed] [Google Scholar]
- 9.Abdel Hafez HZ, Mahran AM, Hofny EM, Attallah DA, Sayed DS, Rashed H. Alopecia areata is not associated with Helicobacter pylori. Indian J Dermatol. 2009;54:17–9. doi: 10.4103/0019-5154.48979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak J, Szekanecz Z, Sebesi J, Takáts A, Demeter P, Bene L, et al. Elevated levels of anti-Helicobacter pylori antibodies in Henoch–Schonlein purpura. Autoimmunity. 2003;36:307–11. doi: 10.1080/08916930232000114535. [DOI] [PubMed] [Google Scholar]
- 11.Fiedorek SC, Casteel HB, Pumphrey CL, Evans DJ, Jr, Evans DG, Klein PD, et al. The role of Helicobacter pylori in recurrent, functional abdominal pain in children. Am J Gastroenterol. 1992;87:347–9. [PubMed] [Google Scholar]
- 12.Ronkainen J, Koskimies O, Ala-Houhala M, Antikainen M, Merenmies J, Rajantie J, et al. Early prednisone therapy in Henoch-Schonlein purpura: A randomized, double blind, placebo-controlled trial. J Pediatr. 2006;149:241–7. doi: 10.1016/j.jpeds.2006.03.024. [DOI] [PubMed] [Google Scholar]


