Animal cells communicate in part by emitting nanosized vesicles whose molecular organization protects their contents from external degradation. Much as the space shuttle was designed as a vehicle to ferry humans and their equipment safely through the harsh environment of space, these vesicles carry various bioactive cargoes from cell to cell while affording them protection from the dangers present in biological fluids. In this issue of Molecular Therapy, Ju et al. report the isolation of such protective nanovesicles from commercially available grapes.1 They further show that following oral delivery, these vesicles trigger intestinal stem cell proliferation and exhibit therapeutic effects in an experimental model of colitis in mice. These effects appear to result from the combined action of mitogenic lipids, signaling proteins, and RNAs present in the vesicles. The findings reveal that the nanoparticles can act as biological effectors across species, from plants to animals (and presumably humans), and that they can modulate physio logical processes. Such long-distance “communication” throughout the entire digestive tract would be impossible with isolated bioactive molecules lacking their protective nanoshuttles.
Among the diverse vesicles released by animal cells,2 exosomes have recently received much attention.3 Exosomes are defined as nanovesicles that are 50–100 nm in diameter, comprise a lipid bilayer membrane and associated proteins, and contain cytosolic components such as messenger RNA and microRNA (miRNA). They originate from an intracellular compartment associated with late endosomes called multivesicular bodies.4 Exosomes mediate cell-to-cell communication by ferrying their bioactive cargo to distant cells and can facilitate changes in gene and protein expression, as well as the lipid content in the target cells. They are quite stable in biological fluids owing to a rigid membrane3 that helps prevent degradation by hydrolytic enzymes. Exosomes' physio logical importance is evidenced by their involvement in disease processes such as cancer metastasis5 and transmission of pathogenic agents in neurodegenerative diseases.6 On the other hand, exosomes derived from immunocompetent cells are rich in antigen-presenting molecules and therefore have potential as therapeutic tools for immunotherapies targeting cancer.7
Ju et al. isolated nanovesicles that they called “grape exosome-like nanoparticles” (GELNs) from crushed grapes. Exosomes have not previously been documented in plants, although vesicle-mediated plant-cell communication has drawn increasing interest.8 Multivesicular bodies have been identified in plants,9 and leaderless secreted proteins can be released in vesicles, as described recently.9 The contents of the GELNs differed from that of canonical mammalian exosomes in that they contained a low level of protein (28 identified proteins), a distinct panel of lipids, and approximately 100 miRNAs. In comparison, mammalian exosomes contain about 1,000 proteins, are rich in cholesterol but have low amounts of phosphatidic acid (PA), and vehiculate 100–300 miRNAs. However, most of the proteins (19 of 28) or associated enzymatic activities found in GELNs have previously been identified in various preparations of mammalian exosomes, suggesting that the GELNs are indeed exosome-like. These include aquaporins, enzymes of glycolytic metabolism (enolase, aldolase, glucose isomerase, triose phosphate isomerase, pyruvate kinase), phospholipase D, histones, and heat shock protein Hsc70. Ten of the proteins identified in the GELNs are homologues of leaderless proteins secreted from the plant Arabidopsis thaliana.9
The lipidic composition of the GELNs is also intriguing, differing from the classic phospholipid composition of mammalian exosomes, which are rich in sphingomyelin but also contain low levels of phosphatidylethanolamine and PA.10 By contrast, GELN lipids comprise 98% phospholipids, whereas typical plant lipids such as galactolipids (digalactosyldiacyl glycerol and monogalactosyldiacyl glycerol) account for the remaining 2%, indicating precise lipid sorting during GELN biogenesis. More remarkably, the mitogenic compound PA constitutes 50% of the phospholipid. This compound interacts with the mammalian target of rapamycin (mTOR), an intracellular molecular complex that senses nutrient levels and triggers cell growth and proliferation.11 GELNs are the first natural vesicle identified with such a high content of this mitogenic phospholipid. In addition, PA is highly fusogenic in the presence of calcium12 and could induce inter vesicular fusion, which might account for the wide range of GELN sizes observed by electron microscopy, from smaller than 100 nm to larger than 200 nm. The GELN membrane is negatively charged, as previously observed for mammalian exosomes.13
Most intriguingly, Ju and colleagues observed that oral administration of GELNs to mice led to proliferation of the intestinal epithelium. Intestinal stem cells proliferated throughout the entire intestine and in the colon. GELNs induced an increase in the number of stem cells in the intestinal crypts in a dose-dependent manner. GELNs supplied by oral administration appeared to be resistant to degradation by saliva, the acidic environment of the stomach, and the highly active proteolytic enzymes present along the intestinal tract. Whether GELNs acting on the colon display the same composition as those originally orally administered or whether they have been modified during their journey in the intestine remains to be investigated. However, the direct exposure of stem cells in culture to original GELNs triggered their proliferation and development into organoid structures. Taken together, the data demonstrate that oral administration of exosome-like nanovesicles from grapes provokes intestinal regeneration in mice.
GELNs must be internalized by cells to induce their proliferation because there is no specific PA receptor on the plasma membrane, and analysis of the protein content of the vesicles did not reveal any specific cell-binding ligand. Ju et al. showed that the target cells displayed macro pinocytotic activity that could account for GELN uptake. This process does not exclude direct fusion of GELNs with the cell membrane owing to the fusogenic properties of PA.
The authors next investigated how these exosome-like nanovesicles triggered proliferation of the intestinal stem cells. They observed nuclearization of β-catenin, suggesting activation of the Wnt/β-catenin signaling pathway. Masking of PA on the GELNs with propranolol inhibited cell growth at 6 hours after treatment by more than 50%. Thus, both PA-mediated and Wnt-mediated signaling contributes to stem cell proliferation, suggesting interplay between these two pathways. Consistent with these findings, the expression of a number of Wnt-regulated genes was increased in the GELN-treated stem cells. The study thus sheds some light on the molecular signaling pathways involved in the “signalosome” activity of vesicles upon interaction with target cells.2
Because intestinal stem cells regulate tissue homeostasis, Ju et al. asked whether GELNs could prevent a widespread human intestinal disease such as colitis. This pathology was reproduced in mice by exposing them to dextran sodium sulfate (DSS) present in their drinking water. DSS administration causes severe tissue damage, such as shortening and inflam mation of the colon and loss of body weight. Continuous oral administration of the exosome-like nanovesicles (GELNs) over several weeks led to a striking reduction of several manifestations of DSS-induced colitis. The authors show that GELN administration prevented the colitis-associated reduction of both intestinal length and villus height, and, importantly, treated mice lived twice as long as untreated mice.
It would be of interest to test whether these observations could be extended to other intestinal pathologies or to other animal models. In addition, these findings raise the likelihood that we will detect the presence of bioactive exosome-like nanovesicles in other commercially available fruits and plants. In this study, 2 mg of GELNs were delivered to mice daily over a 3-week period. Translated, a human weighing 70 kg would require approximately 150 g of these vesicles. The translat ability of such a treatment is thus far difficult to estimate because the efficiency and feasibility of large-scale production of GELNs remain to be determined. However, grapes are readily available. A further caveat is that overstimulation of the Wnt/phospholipase D (which generates PA) pathway (Figure 1) might promote uncontrolled cell proliferation, particularly in treating a chronic disease,14 although this phenomenon might be counterbalanced by the presence of other lipids in GELNs, such as the fatty-acid content,15 which is not reported in this work. Further studies of GELN fatty acid composition should provide additional insights into their mechanism(s) of action.
Figure 1.
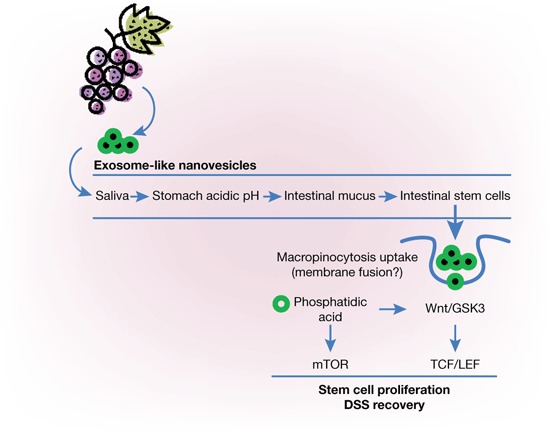
From grapes to exosome-like nanovesicles. Regular grapes contain nanovesicles that can be isolated after crushing by ultracentrifugation. When orally administrated to mice, grape exosome-like nanoparticles (GELNs) can pass through the digestive tract and trigger recovery of chemically induced colitis. GELNs enter intestinal stem cells by macropinocytosis and stimulate their proliferation both by activation of the Wnt/β-catenin/GSK3 pathway and by the mitogenic lipid phosphatidic acid, whose effect might involve the mammalian target of rapamycin (mTOR) complex. DSS, dextran sodium sulfate; LEF, lymphoid enhancer factor; TCF, T-cell factor.
The lipid components of GELN membranes evidently play a key role in their bioactivity. This concept has already been reported in previous work by Ju et al., who showed that mammalian exosomes loaded with membrane self-penetrating lipids such as polyphenols (curcumin) can protect mice from brain disease.16,17 However, the efficiency of exosomes or exosome-like nanoparticles results from an optimal combination of lipids, proteins, and RNAs originating from a specific sorting within the parental cell. Bioengineering of plants in order to trigger optimal molecular sorting to obtain more efficient “therapeutic” nanovesicles is therefore a tantalizing prospect suggested by this landmark proof-of-concept study.
References
- Ju S, Mu J, Dokland T, Zhuang X, Wang Q, Jiang H.et al. (2013Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis Mol Ther 211345–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelbrunn M., and, Sanchez-Madrid F. Intercellular communication: diverse structures for exchange of genetic information. Nat Rev Mol Cell Biol. 2012;13:328–335.. doi: 10.1038/nrm3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M, Subra C, Silvente-Poirot S., and, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–1182.. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Bobrie A, Colombo M, Raposo G., and, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668.. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E.et al. (2012Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration Cell 1511542–1556.. [DOI] [PubMed] [Google Scholar]
- Russo I, Bubacco L., and, Greggio E. Exosomes-associated neurodegeneration and progression of Parkinson's disease. Am J Neurodegener Dis. 2012;1:217–225.. [PMC free article] [PubMed] [Google Scholar]
- Viaud S, Ploix S, Lapierre V, Thery C, Commere PH, Tramalloni D.et al. (2012Updated technology to produce highly immunogenic dendritic cell–derived exosomes of clinical grade: a critical role of interferon J Immunother 3465–75.. [DOI] [PubMed] [Google Scholar]
- Regente M, Pinedo M, Elizalde M., and, de la Canal M. Apoplastic exosome-like vesicles: a new way of protein secretion in plants. Plant Signal Behav. 2012;7:544–546.. doi: 10.4161/psb.19675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Wang J, Stierhof YD, Robinson DG., and, Jiang L. Unconventional protein secretion. Trends Plant Sci. 2012;17:606–615.. doi: 10.1016/j.tplants.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF.et al. (2004Mast cell- and dendritic cell–derived exosomes display a specific lipid composition and an unusual membrane organization Biochem J 380161–171.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiczer BM, Thomas G. Phospholipase D. mTORC1: nutrients are what bring them together. Sci Signal. 2012;5:pe13.. doi: 10.1126/scisignal.2003019. [DOI] [PubMed] [Google Scholar]
- Blackwood RA, Smolen JE, Transue A, Hessler RJ, Harsh DM, Brower RC.et al. (1997Phospholipase D activity facilitates Ca2+-induced aggregation and fusion of complex liposomes Am J Physiol 272C1279–C1285.. [DOI] [PubMed] [Google Scholar]
- Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M.et al. (2010Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins J Lipid Res 512105–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW., and, Min do S. Positive feedback regulation between phospholipase D and Wnt signaling promotes Wnt-driven anchorage-independent growth of colorectal cancer cells. PLoS ONE. 2012;5:e12109. doi: 10.1371/journal.pone.0012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K, Han C, Dai Y, Shen M., and, Wu T. Omega-3 polyunsaturated fatty acids inhibit hepatocellular carcinoma cell growth through blocking beta-catenin and cyclooxygenase-2. Mol Cancer Ther. 2009;8:3046–3055. doi: 10.1158/1535-7163.MCT-09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC.et al. (2011Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain Mol Ther 191769–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C.et al. (2010A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes Mol Ther 181606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]


