Abstract
Background:
Non-ionizing radiofrequency radiation has been increasingly used in industry, commerce, medicine and especially in mobile phone technology and has become a matter of serious concern in present time.
Objective:
The present study was designed to investigate the possible deoxyribonucleic acid (DNA) damaging effects of low-level microwave radiation in brain of Fischer rats.
Materials and Methods:
Experiments were performed on male Fischer rats exposed to microwave radiation for 30 days at three different frequencies: 900, 1800 and 2450 MHz. Animals were divided into 4 groups: Group I (Sham exposed): Animals not exposed to microwave radiation but kept under same conditions as that of other groups, Group II: Animals exposed to microwave radiation at frequency 900 MHz at specific absorption rate (SAR) 5.953 × 10−4 W/kg, Group III: Animals exposed to 1800 MHz at SAR 5.835 × 10−4 W/kg and Group IV: Animals exposed to 2450 MHz at SAR 6.672 × 10−4 W/kg. At the end of the exposure period animals were sacrificed immediately and DNA damage in brain tissue was assessed using alkaline comet assay.
Results:
In the present study, we demonstrated DNA damaging effects of low level microwave radiation in brain.
Conclusion:
We concluded that low SAR microwave radiation exposure at these frequencies may induce DNA strand breaks in brain tissue.
Keywords: Brain, comet assay, deoxyribonucleic acid damage, microwave radiation
INTRODUCTION
The electromagnetic field generated from the extremely low frequencies (30-300 Hz) to radiofrequency and microwaves (100 kHz-300 GHz) is usually recognized as non-ionizing radiation. Microwave radiation is a part of non-ionizing electromagnetic radiation present in the environment and this radiation has become a threat to human health since the introduction of wireless communication system and its increased usage.[1,2] The environmental issues surrounding mobile phones are proving to be a sizeable challenge. The potential adverse effects of these non-ionizing radiations may depend on their accumulation over a long period of time. Public concerns about possible hazardous effects of exposure to these frequencies are increasing in our society due to increased usage of devices such as mobile phones, TV, Wi-Fi, etc.
Effect of microwave radiation exposure on induction of deoxyribonucleic acid (DNA) damage is a subject of keen interest. Studies reporting in vivo and in vitro genotoxic effects of microwave radiation exposure are contradictory and intriguing.[3,4] Vijayalaxmi et al.,[5] have reported no evidence for induction of DNA single-strand breaks in human blood lymphocytes exposed in vitro to pulsed-wave 2450 MHz radiofrequency radiation for 4 h. McNamee et al.,[6] have not found evidence of genotoxicity in human blood cell cultures after 24 h and exposure of 1.9 GHz. In addition, some studies using experimental animals have also given contradictory reports on genotoxic effects of microwave radiation.[7,8]
Although, there are some studies that have reported DNA damaging effect of microwave exposure at high level, i.e., up to 1-2 W/kg of specific absorption rate (SAR), but to the best of our knowledge, there is no study until date reporting the effect of microwave radiation exposure at very low-level with various frequencies. In the present study, we tried to bridge this gap by using three frequencies at very low-level of microwave exposure in male Fischer rats. The Comet assay is a sensitive technique which has widely been used to detect biological effects of various environmental or occupational substances on DNA in humans and animals.[9,10,11,12] Hence, this technique is highly useful in evaluating the extent of DNA damage at primary level even after a duration of 30 days exposure to microwave radiation.
In view of the above, the present study was designed to assess the possible DNA damaging effects of chronic and low level microwave radiation exposure at different frequencies viz. 900, 1800 and 2450 MHz in brain tissue of male Fischer rats. For this purpose, the highly sensitive alkaline comet assay method was used to detect primary DNA damage.
MATERIALS AND METHODS
Microwave exposure setup and dosimetry
The gigahertz transverse electromagnetic (GTEM) cell, GTE10 is used for the microwave exposure and has been designed with the help of Center for Applied Research in Electronics (Microwave Laboratory), Indian Institute of Technology, New Delhi and Amitech Electronics Ltd. Sahibabad, Ghaziabad (U.P) to estimate biological effects of microwave radiation [Figure 1a and b]. GTEM cell is a pyramidal tapered, dual terminated section with its outer cell dimension as L: 220 cm × B: 120 cm × H: 80 cm. Microwaves are generated from microwave generator SMC 100 (Rohde and Schwarz GmbH and Co, Germany). The microwave source consists of a signal generator operating at frequency range from 9 KHz to 3.2 GHz, an amplifier, a direct current DC regulator and a power meter. During the exposure rats were restrained in a L: 30 cm × B: 15 cm × H: 20 cm closed box divided into four compartments with holes of 1 cm diameter to facilitate easy movement and breathing, kept at a distance of 100 cm from source. One box can hold four rats and two such boxes can be placed within the GTEM cell, thus, 8 rats can be exposed at a time in GTEM cell. The microwave chamber is lined with absorbers which minimize the possibility of any reflections. Electric field has been experimentally checked by using an E-field probe inserted into the transverse electromagnetic (TEM) cell through a slit wall. The GTEM cell was placed in a temperature controlled room under constant lighting conditions. SAR distribution was calculated by Power Balance Method using the equation:[13]
Figure 1.
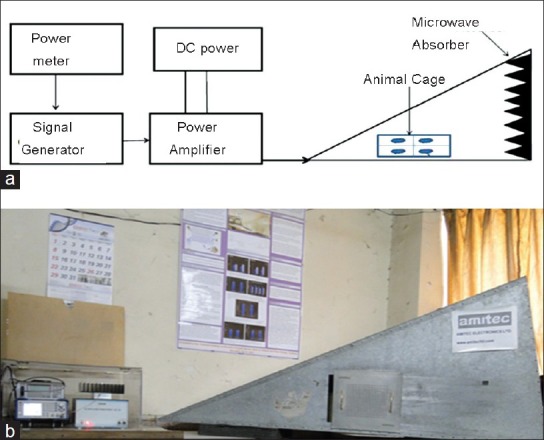
(a) Schematic diagram of microwave exposure setup (b) Photograph of gigahertz transversal electromagnetic cell
Pabs per mouse = 1/n (Pin − Pout − Prefl)
Where, Pabs = radiofrequency RF power in watt absorbed per animal, n = number of animals within the cell, Pin = input power (Watt), Pout = output power (Watt) and Prefl = reflected power (Watt).
Animal exposure
Male Fischer-344 rats weighing 150-200 g were obtained from central animal house facility of the institute and placed in individual raised, galvanized wired cages, kept under standard conditions (temperature 22 ± 2°C) under alternating 12 h light and dark cycle. They were provided with nutritionally adequate standard diet obtained from Nutrilab (Bangalore, India) and water ad libitum. Animals were divided into four groups (6 animals in each group): Group I (sham exposed): Animals kept under same conditions as that of other groups except microwave exposure, Group II: Animals exposed to microwave radiation at 900 MHz, SAR 5.953 × 10−4 W/kg, Group III: Animals exposed to 1800 MHz, SAR 5.835 × 10−4 W/kg and Group IV: Animals exposed to 2450 MHz at SAR 6.672 × 10−4 W/kg. Animals were exposed to microwave radiation in a TEM cell (Amitech Electronics Ltd, India) at above mentioned frequencies for 2 h/day, 5 days/week (power level 0.00 dBm) during light period and every day at the same time for 30 days. The sham exposed group was subjected to similar conditions except the microwave exposure. Appropriate permission was taken from Institutional Animal Ethics Committee, University College of Medical Sciences, Delhi and care of the animals was undertaken as per guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals India for laboratory animal facilities.
Deoxyribonucleic acid damage analysis using alkaline comet assay
DNA damage was evaluated using the alkaline comet assay with some minor modifications.[14,15,16] Slides were prepared in duplicates per sample. For the comet assay experiment brain was taken out immediately from the sacrificed rats and washed with cold phosphate buffer saline (PBS). Briefly, brain was placed in 1 ml chilled mincing solution (Hank's balanced salt solution, with 20 mM Ethylene diamine tetra acetic acid EDTA and 10% dimethyl sulfoxide DMSO) in a petri dish and chopped into small pieces with a pair of scissors to get a uniform cell suspension. Slides were pre-coated with 600 μl of low melting agarose (LMA, 1.0%) prepared in PBS. On this first layer, 600 μl of diluted sample (50 μl cell suspension mixed with 600 μl of 0.75% LMA) was added to form the second layer. The slides were kept on ice for 5 min to allow the gel to solidify. The slides were immersed in freshly prepared chilled lysing solution containing 2.5 M NaCl, 100 mM EDTA, 10 mM Tris (pH 10) with 10% DMSO and 1% Triton X-100 added just before use. The slides remained in the lysing solution for 1 h at 4°C, followed by electrophoresis in a horizontal gel electrophoresis tank with agarose ends nearest to the anode. Fresh and chilled electrophoresis buffer (1 mM Na2 EDTA and 300 mM NaOH, pH > 13) was poured into the tank up to a level of approximately 2.5 mm above the slides. The slides were left in this solution for 25 min to allow DNA unwinding and expression of alkali-labile sites as DNA strand breaks. Electrophoresis was conducted at 0.9 V/cm for 60 min at 4°C. All these steps were performed under dim light and the electrophoresis tank was covered with black paper to avoid additional DNA damage due to stray light. After electrophoresis, the slides were drained and placed horizontally in a tray. Tris buffer (0.4 M; pH 7.5) was added drop-wise and left for 5 min to neutralize excess alkali. Neutralization of slides was repeated three times and subsequently slides were dried and stored.
Dried slides were rehydrated and each slide was stained next day with 100 μl of Ethidium Bromide (20 mg/ml) for 5 min. Slides were randomized and coded to blind the scorer. All slides were scored by one person to avoid inter-scorer variability. Slides were scored using an image-analysis system (Kinetic Imaging, Liverpool, UK) attached to a fluorescence microscope (BX 51, Olympus Japan). The microscope was connected to a computer through a charge-coupled device camera to transport images to software (Komet 5.0) for analysis. Images from 100 cells (50 from each replicate slide) were analyzed. Undamaged cells had an intact nucleus without a tail and damaged cells had the appearance of a comet. To quantify DNA damage, following parameters were evaluated: Percent of DNA content in head and tail, Olive tail moment (OTM) and tail length (TL) using Komet 5.0 software (Kinetic Imaging, Liverpool, UK) as described by Tice et al.[17]
Statistical analysis
All values were expressed as mean ± standard error. Statistical analysis was performed with SPSS (version 16.0). Significance of differences among groups was determined by one way analysis of variance (ANOVA) followed by Tukey's test. Statistical significance was accepted at P value < 0.05.
RESULTS AND DISCUSSION
The results of the present study indicate increase in single strand DNA breaks in brain tissue of rats chronically exposed to microwave radiation. In comparison with control the comet assay performed in brain tissue established a significant increase in the percent of DNA in tail, OTM and TL [Figures 2–4]. The percent of DNA migrating in to tail region was significantly enhanced (P < 0.001) in all the three groups i.e., 900 MHz, 1800 MHz and 2450 MHz. Correspondingly, the percentage of DNA in head was significantly decreased in all the microwave exposed groups. The % of DNA in head and tail of group exposed at 2450 MHz was found significantly different from the 900 MHz exposed group [Figure 2]. The OTM also increased significantly (P < 0.05) in the group of animals exposed to 900 MHz as well as in animals exposed to 1800 and 2450 MHz [P < 0.01; Figure 3]. The TL of comet was also increased significantly (P < 0.05) in animals exposed to 900 and 1800 MHz as well as in the animals exposed to 2450 MHz [P < 0.001; Figure 4]. However, there was no significant difference in the body weight of the rats exposed to microwave radiation as compared to controls or its initial weight.
Figure 2.
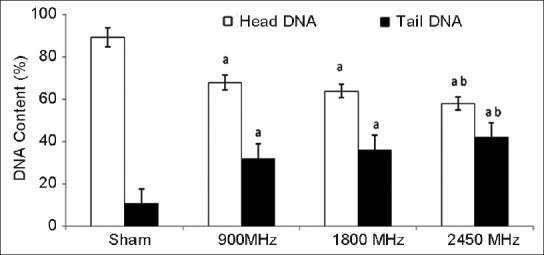
Percent deoxyribonucleic acid in head and tail after 30 days of exposure to microwave radiation. Values are expressed as mean±SE (6 animals per group) Significantly different from asham exposed (P<0.001) and b900 MHz (P<0.001)
Figure 4.
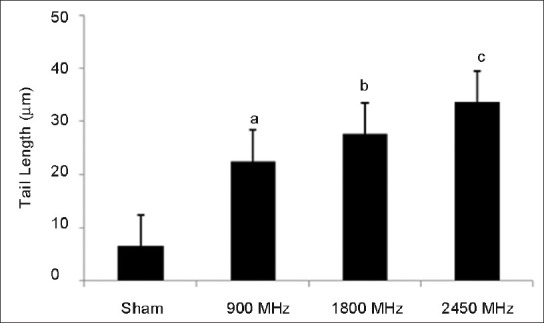
Tail length (in μm) after 30 days of exposure to microwave radiation. Values are expressed as mean±SE (6 animals per group) Significantly different from sham exposed group aP <0.05, bP <0.01 and cP <0.001
Figure 3.
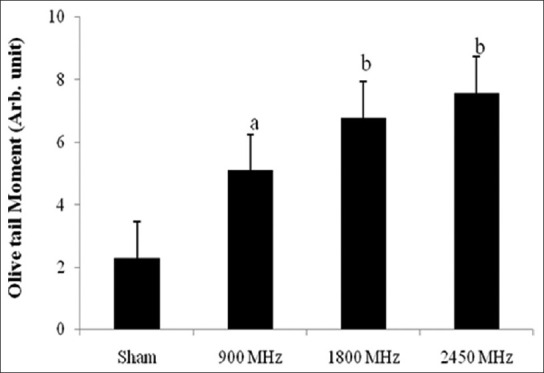
Olive tail moment after 30 days of exposure to microwave radiation. Values are expressed as mean±SE (6 animals per group) Significantly different from sham exposed group aP <0.05 and bP <0.01
The present study aimed to find out the possible effects of chronic low-level microwave exposure on DNA damage in brain tissue of experimental rats. This study provides evidence of DNA damage after 30 days of exposure to rats with three different frequencies of microwaves, i.e., 900 MHz, 1800 MHz and 2450 MHz and at a whole body SAR value of 5.953 × 10−4 W/kg, 5.835 × 10−4 W/kg, and 6.672 × 10−4 W/kg respectively in a GTEM Cell. An earlier study has reported that the low intensity electromagnetic field is capable of interacting with cellular processes associated with carcinogenesis.[18] The integrity of genetic material is a prerequisite for the well-being of living system and its damage is closely related to the pathophysiological condition of cells. Investigation of the biological effects of radiofrequency electromagnetic field requires the assessment of direct and indirect effects on genomic DNA and is one of the most active areas in this field. Cells are unusually sensitive to electromagnetic fields and are the primary targets of radiation.[19,20] The weak radiofrequency electromagnetic field may accelerate electron transfer and thereby destabilize the cellular macromolecules.[21,22] However, the weak electromagnetic fields are not sufficient directly to break a chemical bond in DNA. Therefore it can be concluded, that genotoxic effects are mediated by indirect mechanisms.
The comet assay also called Single Cell Gel Electrophoresis assay is a very sensitive and established genotoxicity assay for the estimation of DNA damage at the individual cell level in vivo and in vitro. The damage usually appears as single stranded and/or double stranded breaks in DNA. It is also used to measure the presence of different types of DNA altering lesions. The alkaline comet assay used in this study is based on the alkaline lysis of labile DNA at sites of damage.[23] The results of the present study established that prolonged exposure of microwave radiation to rats causes DNA strand breaks in brain cells. Paulraj and Behari[24] reported single strand DNA breaks in Wistar rat brain exposed to low intensity microwave radiation exposure for 35 days (2.45 and 16.5 GHz, SAR 1.0 and 2.01 W/kg respectively). However, the present study shows similar effects at even lower frequencies of microwave exposure in Fischer rats.
The present study demonstrates significant (P < 0.001) increase in percentage of DNA in tail and tail moments in all groups exposed to microwave radiation. The SAR values in this study are far below the limit of 2 W/Kg for possible exposure to head in human as per the International Commission on Non-Ionizing Radiation Protection guidelines.[25] Significant percentage of tail DNA in microwave exposed animals indicates that these radiations may act as genotoxic agents. Our earlier findings suggest that microwave exposure at low-levels causes alteration in cognitive function and heat shock protein levels in rat brain.[26] Our another study also suggests that there is a role of microwaves in inflammation and oxidative stress.[27] The results of present study are in accordance with some earlier studies suggesting that DNA damage is indeed caused by microwave exposure in humans.[22,28,29,30] Reports obtained so far on microwave radiation induced DNA damage are controversial and intriguing, and the precise mechanism of DNA strand breaks due to microwave radiation still remains unknown. However, DNA strand breaks may occur due to residual damage resulting from impairment of DNA repair or due to DNA-DNA and DNA-protein cross links and/or DNA adduct formation. Perhaps, the magnetic field created by microwave radiation could lead to generation of free radicals in the brain cells that may induce DNA strand breaks.[8,31,32] The magnetic field components of an electromagnetic field can delay the recombination rate of free radical pairs which causes radical to stay free longer and gives them more potential to do more damage.
The results of the present study showed a significant OTM due to low level microwave exposure in the group exposed to 900 MHz (P < 0.05) as well as 1800 and 2450 MHz (P < 0.01) [Figure 4]. The concept of tail moment as a metric for DNA migration was introduced by Olive et al.[33] and called as OTM. Tail moment is the product of the TL and the fraction of total DNA in the tail. The increase in the OTM may be due to indirect induction of DNA strand breaks and/or generation of such modifications in DNA, which can be transformed into DNA strand breaks, because it is obvious that intrinsic quantum energy (E = hu) of microwaves is too low to dislodge an electron from macromolecules.
In this study, we observed significant migration in length of tail DNA in groups exposed to 900 (P < 0.05), 1800 (P < 0.01) MHz and in group exposed to 2450 MHz (P < 0.001). It has been reported that exposure to 1800 MHz, SAR 1.2 W/kg induces DNA strand breaks in human fibroblasts and rat granulosa cells.[33] The results of the present study are in accordance with earlier studies, where it has been reported that chronic exposure to microwave radiation for 35 days at 2.45 and 16.5 GHz, SAR 1.0 and 2.01 W/kg respectively, causes single strand DNA breaks in Wistar rat brain cells.[24,34] DNA strand breaks, if not properly repaired, are known to lead cell death. Since nerve cells do not divide and the damage accumulates, the more likely consequences of DNA damage in nerve cells may be cell death, which could either lead to or accelerate the development of neurodegenerative diseases.[32] Factors that can influence DNA strand damage in a particular tissue include cell type heterogeneity, cell turnover frequency, cell cycle etc., Different cell types may have different background levels of DNA strand breaks due to variation in its metabolic activities.[35] Through a homeostatic mechanism, cells maintain a delicate balance between spontaneous and induced DNA damage. DNA damage accumulates if such a balance is altered, which may in turn affect cell functions.
CONCLUSION
Based on the present findings, it is concluded that chronic microwave radiation exposure at low-level induces DNA damage. Prolonged exposure may lead to neurodegenerative disorders. Although, the energy of microwaves is not sufficient directly to break a chemical bond in DNA, genotoxic effects may be mediated by indirect mechanisms such as generation of oxygen free radicals or a disturbance in DNA-repair processes. In order to minimize the exposure, safe limits with respect to frequency and duration of microwave radiation must also be prescribed in view of its increased use in society. However, knowledge of the possible health effects of microwave radiation is still inadequate and inconclusive. Though, the present study seems preliminary, this study is providing the evidence of the effect of microwave radiation at very low-level of exposure and at the lower, middle and upper frequency used in mobile telecommunication. Further exploration is needed to reach any concrete conclusion.
ACKNOWLEDGMENT
Authors are grateful to Indian Council of Medical Research (ICMR), New Delhi for the grant, in form of the extramural research project vide sanction letter No. 5/8/4-4(env) 07-NCD-I dated 03-08-09. One of the authors Pravin Deshmukh is grateful to ICMR for senior research fellow-ship (SRF) support. Mr. Digvijay Singh is duly acknowledged for his technical help during the animal experiments.
Footnotes
Source of Support: ICMR, New Delhi.
Conflict of Interest: None declared.
REFERENCES
- 1.Croft RJ, Chandler JS, Burgess AP, Barry RJ, Williams JD, Clarke AR. Acute mobile phone operation affects neural function in humans. Clin Neurophysiol. 2002;113:1623–32. doi: 10.1016/s1388-2457(02)00215-8. [DOI] [PubMed] [Google Scholar]
- 2.Avendaño C, Mata A, Sanchez Sarmiento CA, Doncel GF. Use of laptop computers connected to internet through Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertil Steril. 2012;97:39–45. doi: 10.1016/j.fertnstert.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Vijayalaxmi, Obe G. Controversial cytogenetic observations in mammalian somatic cells exposed to radiofrequency radiation. Radiat Res. 2004;162:481–96. doi: 10.1667/rr3252. [DOI] [PubMed] [Google Scholar]
- 4.Verschaeve L. Genetic effects of radiofrequency radiation (RFR) Toxicol Appl Pharmacol. 2005;207:336–41. doi: 10.1016/j.taap.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Vijayalaxmi, Leal BZ, Szilagyi M, Prihoda TJ, Meltz ML. Primary DNA damage in human blood lymphocytes exposed in vitro to 2450 MHz radiofrequency radiation. Radiat Res. 2000;153:479–86. doi: 10.1667/0033-7587(2000)153[0479:pddihb]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 6.McNamee JP, Bellier PV, Gajda GB, Lavallée BF, Marro L, Lemay E, et al. No evidence for genotoxic effects from 24 h and exposure of human leukocytes to 1.9 GHz radiofrequency fields. Radiat Res. 2003;159:693–7. doi: 10.1667/0033-7587(2003)159[0693:nefgef]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Vijayalaxmi, Sasser LB, Morris JE, Wilson BW, Anderson LE. Genotoxic potential of 1.6 GHz wireless communication signal: in vivo two-year bioassay. Radiat Res. 2003;159:558–64. doi: 10.1667/0033-7587(2003)159[0558:gpogwc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Paulraj R, Behari J. Radio frequency radiation effects on protein kinase C activity in rats’ brain. Mutat Res. 2004;545:127–30. doi: 10.1016/s0027-5107(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 9.Collins A, Dusinská M, Franklin M, Somorovská M, Petrovská H, Duthie S, et al. Comet assay in human biomonitoring studies: Reliability, validation, and applications. Environ Mol Mutagen. 1997;30:139–46. doi: 10.1002/(sici)1098-2280(1997)30:2<139::aid-em6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10.European Standards Committee on Oxidative DNA Damage (ESCODD) Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic Biol Med. 2003;34:1089–99. doi: 10.1016/s0891-5849(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 11.Kumaravel TS, Vilhar B, Faux SP, Jha AN. Comet Assay measurements: A perspective. Cell Biol Toxicol. 2009;25:53–64. doi: 10.1007/s10565-007-9043-9. [DOI] [PubMed] [Google Scholar]
- 12.Garaj-Vrhovac V, Orescanin V. Assessment of DNA sensitivity in peripheral blood leukocytes after occupational exposure to microwave radiation: The alkaline comet assay and chromatid breakage assay. Cell Biol Toxicol. 2009;25:33–43. doi: 10.1007/s10565-008-9060-3. [DOI] [PubMed] [Google Scholar]
- 13.Ardoino L, Lopresto V, Mancini S, Marino C, Pinto R, Lovisolo GA. A radio-frequency system for in vivo pilot experiments aimed at the studies on biological effects of electromagnetic fields. Phys Med Biol. 2005;50:3643–54. doi: 10.1088/0031-9155/50/15/011. [DOI] [PubMed] [Google Scholar]
- 14.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 15.Grover P, Danadevi K, Mahboob M, Rozati R, Banu BS, Rahman MF. Evaluation of genetic damage in workers employed in pesticide production utilizing the Comet assay. Mutagenesis. 2003;18:201–5. doi: 10.1093/mutage/18.2.201. [DOI] [PubMed] [Google Scholar]
- 16.Chandna S. Single-cell gel electrophoresis assay monitors precise kinetics of DNA fragmentation induced during programmed cell death. Cytometry A. 2004;61:127–33. doi: 10.1002/cyto.a.20071. [DOI] [PubMed] [Google Scholar]
- 17.Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, et al. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–21. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Juutilainen J, Lang S. Genotoxic, carcinogenic and teratogenic effects of electromagnetic fields. Introduction and overview. Mutat Res. 1997;387:165–71. doi: 10.1016/s1383-5742(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 19.Brusick D, Albertini R, McRee D, Peterson D, Williams G, Hanawalt P, et al. Genotoxicity of radiofrequency radiation. DNA/Genetox Expert Panel. Environ Mol Mutagen. 1998;32:1–16. doi: 10.1002/(sici)1098-2280(1998)32:1<1::aid-em1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Blank M, Goodman R. Initial interactions in electromagnetic field-induced biosynthesis. J Cell Physiol. 2004;199:359–63. doi: 10.1002/jcp.20004. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Johnson D, Dunbar K, Dong H, Ge X, Kim YC, et al. 2.45 GHz radiofrequency fields alter gene expression in cultured human cells. FEBS Lett. 2005;579:4829–36. doi: 10.1016/j.febslet.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 22.Nylund R, Leszczynski D. Mobile phone radiation causes changes in gene and protein expression in human endothelial cell lines and the response seems to be genome-and proteome-dependent. Proteomics. 2006;6:4769–80. doi: 10.1002/pmic.200600076. [DOI] [PubMed] [Google Scholar]
- 23.Singh S, Kumar V, Thakur S, Banerjee BD, Rautela RS, Grover SS, et al. Paraoxonase-1 genetic polymorphisms and susceptibility to DNA damage in workers occupationally exposed to organophosphate pesticides. Toxicol Appl Pharmacol. 2011;252:130–7. doi: 10.1016/j.taap.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Paulraj R, Behari J. Single strand DNA breaks in rat brain cells exposed to microwave radiation. Mutat Res. 2006;596:76–80. doi: 10.1016/j.mrfmmm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 25.ICNIRP guidelines for limiting exposure to time varying electric, magnetic and electromagnetic field (up to 300 GHz) Health Phys. 1998;74:494–522. [PubMed] [Google Scholar]
- 26.Deshmukh PS, Kanu M, Banerjee BD, Abegaonkar MP, Ahmed RS, Tripathi AK, et al. Modulation of heat shock protein level and cognitive impairment in fischer rats exposed to low level microwave radiation. Asiatic J Biotech Res. 2012;03:1391–99. [Google Scholar]
- 27.Kanu M, Deshmukh PS, Banerjee BD, Tripathi AK, Abegaonkar MP. Microwave radiation induced oxidative stress, cognitive impairment and inflammation in brain of Fischer rats. Indian J Exp Biol. 2012;50:889–96. [PubMed] [Google Scholar]
- 28.Garaj-Vrhovac V. Micronucleus assay and lymphocyte mitotic activity in risk assessment of occupational exposure to microwave radiation. Chemosphere. 1999;39:2301–12. doi: 10.1016/s0045-6535(99)00139-3. [DOI] [PubMed] [Google Scholar]
- 29.Moustafa YM, Moustafa RM, Belacy A, Abou-El-Ela SH, Ali FM. Effects of acute exposure to the radiofrequency fields of cellular phones on plasma lipid peroxide and antioxidase activities in human erythrocytes. J Pharm Biomed Anal. 2001;26:605–8. doi: 10.1016/s0731-7085(01)00492-7. [DOI] [PubMed] [Google Scholar]
- 30.Yadav AS, Sharma MK. Increased frequency of micronucleated exfoliated cells among humans exposed in vivo to mobile telephone radiations. Mutat Res. 2008;650:175–80. doi: 10.1016/j.mrgentox.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Paulraj R, Behari J. The effect of low level continuous 2.45 GHz waves on enzymes of developing rat brain. Electromagnetic Biol Med. 2002;21:221–31. [Google Scholar]
- 32.Lai H, Singh NP. Single-and double-strand DNA breaks in rat brain cells after acute exposure to radiofrequency electromagnetic radiation. Int J Radiat Biol. 1996;69:513–21. doi: 10.1080/095530096145814. [DOI] [PubMed] [Google Scholar]
- 33.Olive PL, Banáth JP, Durand RE. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the «comet» assay. Radiat Res. 1990;122:86–94. [PubMed] [Google Scholar]
- 34.Diem E, Schwarz C, Adlkofer F, Jahn O, Rüdiger H. Non-thermal DNA breakage by mobile-phone radiation (1800 MHz) in human fibroblasts and in transformed GFSH-R17 rat granulosa cells in vitro. Mutat Res. 2005;583:178–83. doi: 10.1016/j.mrgentox.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Tice RR. Applications of the single cell gel assay to environmental biomonitoring for genotoxic pollutants. In: Butterworth FM, Corkum LD, Guzman-Rincon J, editors. Biomonitors and Biomarkers as Indicators of Environmental Change. New York: Plenum Press; 1995. pp. 69–79. [Google Scholar]


