Abstract
Background:
In the present study, cigarette smoke contains more than four thousand chemicals, many of which are known to be carcinogen or cancer promoter. Many epidemiological reports suggest that cigarette smokers are at a greater risk of other cancers such as oropharynx, stomach, pancreas, liver, kidney, urinary bladder, colon, and breast, however, the few epidemiological reports are available on the role of cigarette smoke in the development of prostate cancer. In this study, we investigated the effects of farnesol against cigarette smoke extract (CSE)-induced oxidative stress in prostate.
Materials and Methods:
Farnesol was administered by gavage (50 mg/kg and 100 mg/kg b.wt. in corn oil) one time daily for 7 days. On day 7, rats were exposed to cigarette smoke via intratracheal instillation of aqueous CSE. CSE enhanced prostatic xanthine oxidase activity and lipid peroxidation (LPO) along with decrease in prostatic glutathione content, antioxidant enzymes activities, viz., glutathione peroxidase, glutathione reductase, and catalase.
Results:
Pre-treatment of rats with farnesol (50 mg/kg and 100 mg/kg b.wt. orally) resulted in significant decreased in xanthine oxidase activity and LPO at both the doses. The level of reduced glutathione, the activities of glutathione dependent enzymes and antioxidant enzymes were also augmented to significant level with pre-treatment with farnesol.
Conclusion:
Thus, our data suggests that farnesol is a potent defense against CSE induced prostatic oxidative damage in rodent model of experiment.
Keywords: Cigarette smoke extract, farnesol, intratracheal instillation, prostate, testis
INTRODUCTION
Prostate cancer (PCa) is the second most frequently diagnosed cancers and the sixth leading cause of cancer death world-wide.[1] Despite the fact that the incidences of PCa are growing at an alarming rate, the etiology of PCa is not yet clearly understood. Epidemiological studies have shown the involvement of genetic, environmental factors, race, old age, and life-style patterns as the risk-factors for PCa.[2,3,4] Few epidemiological studies have been carried out to elucidate the role of cigarette smoke on the onset of PCa however, the results are inconclusive.[5,6,7,8] Oxidative stress results from the imbalance between reactive oxygen species ROS production and detoxification enzymes when the level of ROS increases, they target cells leading to oxidative damage from the interaction of reactive oxygen with critical cellular macromolecules.[9] ROS may interact with and modify cellular proteins, lipids, and deoxyribonucleic acid DNA that results in altered target cell functioning.[10] Oxidative stress can lead to various chronic diseases including cancer.[11] Cigarette smoke extract (CSE) contains many known potent carcinogens, which increases the oxidative burden of the cell, which when persisted may lead to many pathological conditions.[12]
It has been shown that arylamine N-acetyltransferase play a major role in bioactivation of carcinogenic amines that are present in cigarette smoke consequently causes lung, urinary tract, prostate, breast, oral, and liver cancers.[13] Studies have shown that certain nitrosamines, which are responsible for PCa in rats are found to be present in the urine samples of smokers and secondly their serum showed increased levels of testosterone and androstenedione, which are responsible for PCa progression. The increased level of testosterone generates oxidative stress and may lead to PCa over a long period.[2,14]
Natural products/compounds are gaining much attention of several investigators for the prevention of various human diseases. Farnesol (trans-3,7-11-trimethyl-2,6-10-dodecatrien-1-ol) is a natural compound and it is a 15-carbon naturally occurring sesquiterpene/isoprenoid. It may be endogenously generated in the cells by enzymatic dephosphorylation of farnesyl pyrophosphate, a metabolic precursor of squalene yielding sterols and other isoprenoid compounds.[15,16] Dietary sources of farnesol are the plant products including, fruits and berries such as apricots, peaches, plums, blueberries, cranberries, raspberries, and strawberries, vegetables such as tomatoes, herbs such as lemon grass and chamomile and it is also obtained from the essential oils of ambrette seeds and citronella.[17,18,19,20]
Studies from our laboratory have revealed that farnesol is a potent antioxidant and protects the kidneys and lungs against oxidative damage induced by Ferric-Nitrilo Acetate (Fe-NTA) and CSE respectively.[12,21] Previous studies have also been shown that farnesol exhibits significant anti-tumor and anti-carcinogenesis effects in vivo that might be due to the inhibition of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase.[17,22,23,24,25]
Prevention of cancer by the natural products is called chemoprevention and it has no/minimal side effect and hence gaining much popularity. A number of naturally occurring products from vegetables and herbs exert their chemopreventive properties against various diseases including carcinogenesis. Therefore, chemoprevention is a logical strategy to reduce the mortality from cancer because numerous chemopreventive agents are naturally present in the plants.
The present study was designed to investigate the anticipatory effects of farnesol against CSE induced oxidative stress in prostate and histological alterations in the testes and prostate of Wistar rats.
MATERIALS AND METHODS
Chemicals
Reduced glutathione (GSH), oxidized glutathione (GSSG), nicotinamide adenine dinucleotide phosphate reduced (NADPH), flavin adenine dinucleotide, ethylene diamine tetra-acetic acid (EDTA), nicotinamide adenine dinucleotide reduced, xanthine, di-nitrophenyl hydrazine, thiobarbituric acid (TBA), trichloroacetic acid (TCA), bovine serum albumin (BSA), 1,2-dithio-bis-nitrobenzoic acid (DTNB), glutathione reductase (GR) were obtained from Sigma (Sigma Chemical Co., St Louis, MO), farnesol (Fluka chemika, Buchs, Switzerland), ferric nitrate, ammonium thiocyanate, hydrogen peroxide, magnesium chloride, di-sodium hydrogen phosphate, sodium di-hydrogen phosphate, and sodium hydroxide were purchased from E. Merck Limited. All other chemicals and reagents were of the highest purity grade commercially available. Filter tipped cigarettes (69 mm.) of a popular international brand in India were purchased from the local market in New Delhi, India.
Animals
A 4-6-week-old male albino rats (130-150 g) of Wistar strain were obtained from Central Animal House of Hamdard University, New Delhi, India. They were housed in polypropylene cages in groups of six rats per cage and were kept in a room maintained at 25°C ± 2°C with a 12 h light/dark cycle. They were allowed to acclimatize for 1 week before the experiments and were given free access to standard laboratory feed (Hindustan Lever Ltd., Bombay, India) and water ad libitum.
Preparation of aqueous cigarette smoke extract
CSE was prepared by a modification of previously published method.[26] Cigarettes (69 mm filtered) were smoked to 0.5 cm above the filter, through an experimental apparatus with a constant airflow driven by a vacuum pump. Airflow was adjusted in such a way that a 69-mm cigarette took 1 min in combustion. CSE was prepared by bubbling smoke from three cigarettes into 30 ml sterilized deionized distilled water (DDW), at a rate of one cigarette per minute. This preparation was considered to be 100% CSE.[27,28] CSE was prepared just 20 min before the animal exposure, and stored at 4°C in airtight glass bottles. Differences in CSE content from one preparation to another were monitored for its efficacy by its absorbance at 320 nm.[29] and 524 nm.[28] Further phenolic content of the CSE was evaluated using Folin–Ciocalteu reagent.[30] There were slight variations in absorbance at 320 nm and 524 nm (0.630 ± 0.05 and 0.057 ± 0.002 Abs. Units respectively) and phenolic content in CSE prepared at different time intervals was found to be 26.018 μg/ml ± 1.86 μg/ml CSE and there were no significant difference. Freshly prepared CSE was administered to animal groups from same preparation on the same day. For control group air bubbled deionized water was prepared with the same procedure.
Intratracheal (i.t.) instillation of CSE
Animals were exposed to CSE by i.t. instillation method described previously.[31] Briefly, trachea of the animals was exposed by a minor surgery on the skin on ventral side of the neck, under anesthesia and the CSE then injected into the trachea by piercing a sterile syringe needle in between two cartilaginous tracheal rings. Control group was treated in the same manner with the injection of air bubbled DDW. The dose of CSE or DDW was taken as a volume injected intratracheally and the dose was 1.3 ml/kg b.wt.[32]
Treatment regimen
To study, the effect of pre-treatment of animals with farnesol on CSE induced oxidative damage to prostate and testis, 25 male Wistar rats were randomly allocated to five groups of five rats in each. The animals of Groups I served as control received corn oil orally (5 ml/kg b.wt. once daily, for 7 days). Group III received pre-treatment with farnesol (FS) by gavages once daily for 7 days at a dose of 50 mg/kg b.wt. (FS I). Groups IV and V received farnesol once daily for seven consecutive days at a dose level of 100 mg/kg b.wt. (FS II). On day 7, 1 h after the last treatment with farnesol or corn oil the animals of Groups II, III and IV were administered with a single i.t dose of CSE (1.3 ml/kg b.wt.), Group I (control group) and Group V (only FS II group) were administered with DDW i.t.(1.3 ml/kg b.wt.). All the animals were killed 24 h after last treatment.
Post-mitochondrial supernatant preparation and estimation of different parameters
Prostate was removed quickly, cleaned with ice-cold saline (0.85% sodium chloride). The prostate (10% w/v) was homogenized in chilled phosphate buffer (0.1 M, pH 7.4) using a Potter Potter Elvehjen homogenizer. The homogenate was filtered through muslin cloth, and were centrifuged at 3000 rpm for 10 min at 4°C by Eltek Refrigerated Centrifuge (model RC 4100 D) to separate the nuclear debris. The aliquot so obtained was centrifuged at 12,000 rpm for 20 min at 4°C to obtain PMS, which was used as a source of various enzymes.
Measurement of xanthine oxidase activity
The activity of xanthine oxidase was assayed by the method of Stirpe and Della Corte (1969).[33] The reaction mixture consisted of 0.2 ml PMS, which was incubated for 5 min at 37°C with 0.8 ml phosphate buffer (0.1 M, pH 7.4). The reaction was started by adding 0.1 ml xanthine (9 mM) and kept at 37°C for 20 min and the reaction was terminated by the addition of 0.5 ml ice-cold perchloric acid (10% [v/v]). After 10 min, 2.4 ml of distilled water was added and centrifuged at 4000 rpm for 10 min and μg uric acid formed/min/mg protein was recorded at 290 nm.
Measurement of lipid peroxidation
The assay for membrane LPO was carried out by the method of Wright et al. (1981) with some modifications.[34] The reaction mixture in a total volume of 3.0 ml contained 1.0 ml tissue homogenate, 1.0 ml of TCA (10%), and 1.0 ml TBA (0.67%). All the test tubes were placed in a boiling water bath for a period of 45 min. The tubes were shifted to ice bath and then centrifuged at 2500 × g for 10 min. The amount of malondialdehyde (MDA) formed in each of the samples was assessed by measuring the optical density of the supernatant at 532 nm. The results were expressed as the nmol MDA formed/gram tissue by using a molar extinction co-efficient of 1.56 × 105/M/cm.
Measurement of Reduced glutathione (GSH) levels
The GSH content in prostate was determined by the method of Jollow et al. (1974) in which 1.0 ml of PMS fraction (10%) was mixed with 1.0 ml of sulphosalicylic acid (4%).[35] The samples were incubated at 4°C for at least 1 h and then subjected to centrifugation at 1200 × g for 15 min at 4°C. The assay mixture contained 0.4 ml filtered aliquot, 2.2 ml phosphate buffer (0.1 M, pH 7.4), and 0.4 ml DTNB (10 mM) in a total volume of 3.0 ml. The yellow colour developed was read immediately at 412 nm on spectrophotometer (Milton Roy Model-21 D). The GSH content was calculated as μmol DTNB conjugate formed/gram tissue using molar extinction co-efficient of 13.6 × 103/M/cm.
Measurement of glutathione peroxidase activity
Glutathione peroxidase activity was assayed by the method of Mohandas et al., (1984).[36] The reaction mixture consisted of 1.49 ml phosphate buffer (0.1 M, pH 7.4), 0.1 ml EDTA (1 mM), 0.1 ml sodium azide (1 mM), 0.05 ml GR (1 IU ml1), 0.05 ml GSH (1 mM), 0.1 ml NADPH (0.2 mM), 0.01 ml H2 O2 (0.25 mM) and 0.1 ml 10% PMS in a total volume of 2 ml. The disappearance of NADPH at 340 nm was recorded at 25°C. Enzyme activity was calculated as nmol NADPH oxidized/min/mg protein using molar extinction co-efficient of 6.22 × 103/M/cm.
Measurement of Glutathione reductase (GR)activity
The GR activity was measured by the method of Carlberg and Mannervik (1975).[37] The assay system consisted of 1.65 ml phosphate buffer (0.1 M, pH 7.6), 0.1 ml EDTA (0.5 mM), 0.05 ml GSSG (1.0 mM), 0.1 ml NADPH (0.1 mM), and 0.1 ml of 10% PMS in a total volume of 2.0 ml. The enzyme activity was assessed at 25°C by measuring the disappearance of NADPH at 340 nm and was calculated as nmol NADPH oxidized/min/mg protein using molar extinction co-efficient of 6.22 × 103/M/cm.
Measurement of catalase activity
Catalase activity was assayed by the method of Claiborne (1985).[38] The reaction mixture consisted of 1.95 ml phosphate buffer (0.1 M, pH 7.4), 1.0 ml hydrogen peroxide (0.019 M), and 0.05 ml 10% PMS in a final volume of 3 ml. Changes in absorbance were recorded at 240 nm. Catalase activity was calculated as nmol H2 O2 consumed/min/mg protein.
Histology
The prostate and testes were excised flushed with saline, fixed in 10% neutral buffered formalin for at least 24 h and after fixation, the specimens were dehydrated in ascending grades of ethanol, cleared in benzene, and embedded in paraffin wax. Blocks were made and 5 μm thick sections were cut from the prostate and testes. The paraffin embedded tissue sections were deparaffinized using xylene and ethanol. The slides were washed with phosphate buffered saline and permeabilized with permeabilization solution (0.1 M citrate, 0.1% Triton X-100). These sections stained with haematoxylin and eosin and were observed under light microscope at × 40 magnifications to investigate the histo-architecture of prostate and testes of Wistar rats.
Measurement of protein
The protein concentration in all samples was determined by the method of Lowry et al.[39] using BSA as standard.
Statistical analysis
The data from individual experiments are presented as the means ± SD. Differences between groups were analyzed using ANOVA followed by Dunnett's multiple comparisons test and minimum criterion for statistical significance was set at P < 0.05 for all comparisons.
RESULTS
Effect of CSE on antioxidant enzymes in prostate of Wistar rats and its amelioration by FS
The i.t. instillation of CSE (Group II) caused a significantly decreased the activities of glutathione metabolizing enzymes such as GR and glutathione peroxidase GPx (P < 0.001), and catalase activity (P < 0.001) when compared with control (Group I) animals. Lower dose of farnesol (FS I 50 mg/kg b.wt. + CSE) (Group III) caused a significant augmentation in the activities of GR (P < 0.05), GPx (P < 0.001) and catalase (P < 0.001) as compared to Group II animals. Higher dose of farnesol (FS II 100 mg/kg b.wt. + CSE) (Group IV) also significantly increased the activities of GR (P < 0.001), GPx (P < 0.001) and catalase (P < 0.001) as compared to CSE-treated animals (Group II). Whereas only farnesol treated group (Group V) animals did not showed any significant change in the activities of different enzymes as compared to control (Group I) animals [Table 1].
Table 1.
Effects of farnesol and cigarette smoke extract on the activities of glutathione reductase, glutathione peroxidase, catalase and xanthine oxidase
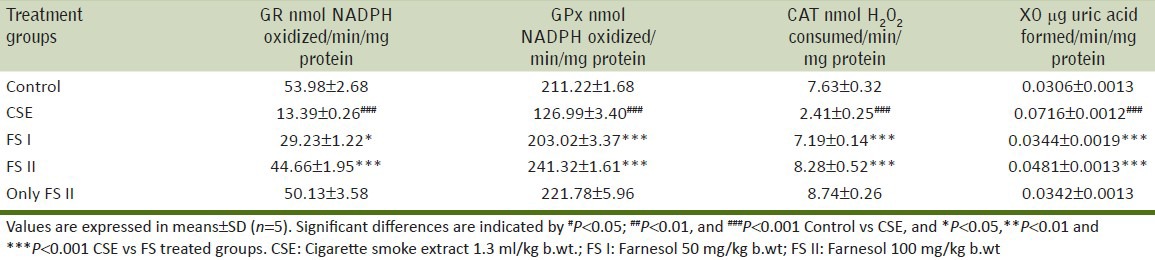
Effect of CSE on reduced glutathione level in prostate of Wistar rats and its amelioration by FS
GSH levels were decreased significantly (P < 0.001) in CSE treated animals (Group II) as compared to control (Group I) animals. Lower dose of farnesol (FS I 50 mg/kg b.wt. + CSE) (Group III) and higher dose of farnesol (FS II 100 mg/kg b.wt. + CSE) (Group IV) showed a significant increase in GSH level i.e. (P < 0.05) and (P < 0.001) when compared with Group II animals. Only FS II (farnesol 100 mg/kg b.wt.) (Group V) did not show any significant change in the GSH levels when compared with Group I [Figure 1].
Figure 1.
Effect of farnesol and cigarette smoke extract (CSE) on reduced glutathione level in prostate of Wistar rats. Values are represented in means ± S.D. (n = 5). Significant differences are indicated by #P <0.05, ##P <0.01, and ###P <0.001 when compared with control animals (Group I), and *P<0.05, **P<0.01 and ***P<0.001 when compared with CSE treated animals (Group II). CSE = Cigarette smoke extract 1.3 ml/kg b.wt.; FS I = Farnesol 50 mg/kg b.wt; FS II = Farnesol 100 mg/kg b.wt
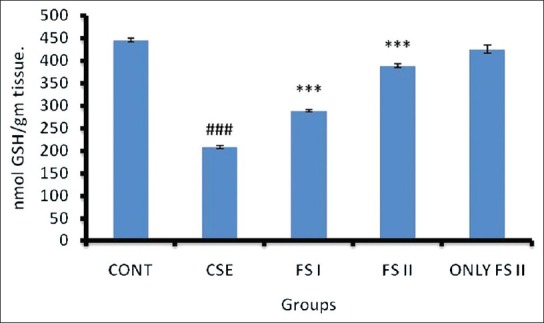
Effect of CSE on MDA level and xanthine oxidase activity in prostate of Wistar rats and its amelioration by FS
CSE administration (Group II) resulted in increased MDA formation (P < 0.001) [Figure 2] and XO (P < 0.001) [Table 1] when compared to control (Group I). FS I (farnesol 50 mg/kg b.wt.) + CSE (Group III) and dose FS II (farnesol 100 mg/kg b.wt.) + CSE (Group IV) (P < 0.001) treated groups restored the levels to normal when compared with Group II animals. Only FS II (farnesol 100 mg/kg b.wt.) (Group V) did not show any significant change in the MDA levels and XO activity when compared with Group I.
Figure 2.
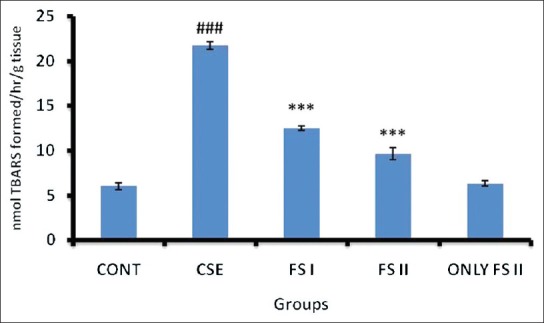
Effect of farnesol and cigarette smoke extract (CSE) on MDA level of prostate. Values are expressed in means ± SD (n = 5). Significant differences are indicated by #P <0.05, ##P <0.01, and ###P <0.001 when compared with control animals (Group I), and *P<0.05, **P<0.01 and ***P<0.001 when compared with CSE treated animals (Group II). CSE = Cigarette smoke extract 1.3 ml/kg b.wt.; FS I = Farnesol 50 mg/kg b.wt; FS II = Farnesol 100 mg/kg b.wt
Effect of CSE on histology of prostate of Wistar rats and its amelioration by FS
CSE-treated rats show normal histo-architecture for the prostate tissue with thin lining of small columnar cells, large lumen, and with less prostatic secretions. Figure 2 shows broad and irregular columnar cell lining with nuclei stratification and prostatic secretions were also elevated. Farnesol treated Groups III and IV showed comparatively restored histological findings similar to that of control. Only Farnesol treated group were found to be devoid of any pathological histology [Figure 3].
Figure 3.
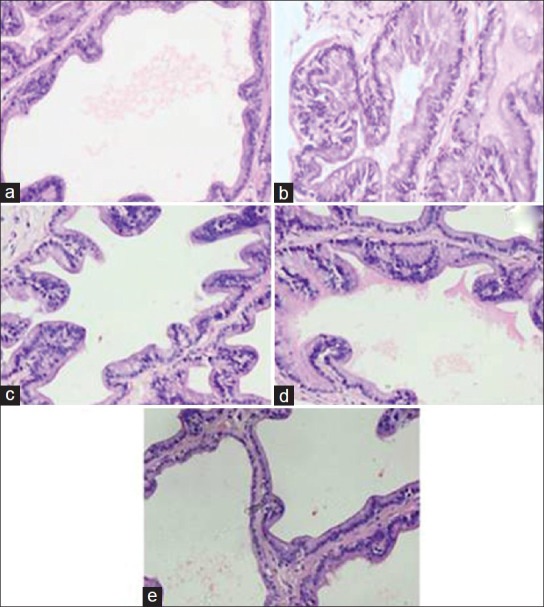
Effect of farnesol and cigarette smoke extract (CSE) on histology of prostate in Wistar rats. Slide a shows normal histo-architecture with minimal amount of acini secretions represents control (Group I), slide b represents CSE treated Group II showed prostatic acini with nucear stratification and increased secretion. Slide c and d represent FS treated Group III and IV respectively showed almost normal prostate histology. Slide e represents only FS II (Group V) showed normal histology
Effect of CSE on rat testis and its amelioration by FS
CSE treatment resulted in decrease of Leydig cells (Group II) when compared to control (Group I). FS treatment at both the doses (Group III and IV) resulted in the normal number of leydig cells. Only FS II dose (Group V) did not show and significant difference when compared to control (Group I) [Figure 4].
Figure 4.
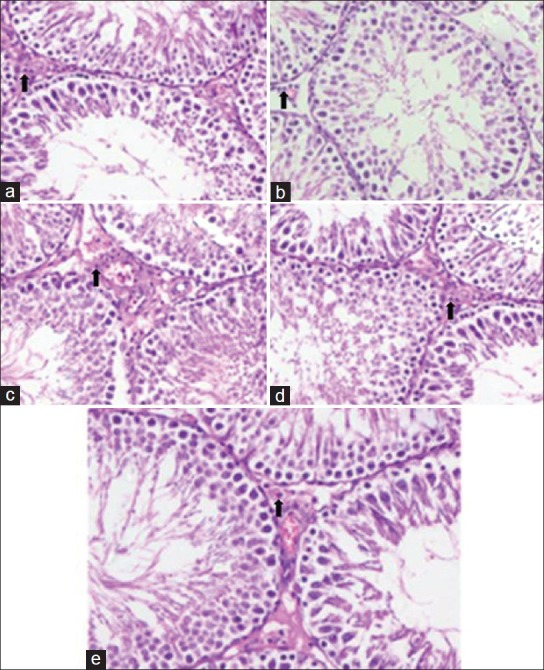
Effect of farnesol and cigarette smoke extract (CSE) on histology of testis in Wistar rats. Slide a represents control Group I shows normal testis histology. Slide b represents CSE treated Group II showed decreased number of Leydig cells. Slide c and d represent FS treated Group III and IV respectively showed increase in number of Leydig cells. Slide e represents only FS II (Group V) showed normal histology of testis
DISCUSSION
In this study, we have examined the protective effects of farnesol against CSE-induced prostate oxidative stress in Wistar rats. According to some epidemiological studies, cigarette smoke plays a role in the development of prostate cancer and it is a major health hazard.[5,6,7,8] The exact mechanism of cigarette smoke induced prostate cancer is not known however, it may be because of oxidative burden induced by cigarette smoke. Cigarette smoke contains billions of free radicals and chemicals that generate ROS in vivo[40] and this makes cigarette smoke highly oxidative and is responsible for most of the damages. The present data has demonstrated causal role of cigarette smoke oxidants in prostate and testes injury and farnesol is minimizing it significantly. Free radicals and ROS generated by cigarette smoke may play a key role in the instigation of membrane LPO. LPO is a marker of oxidative stress and several studies have reported that remarkable elevation in the level of MDA, a LPO product, was observed after CSE treatment.[12,41] Our results also corroborated with the above mentioned findings, which showed that there is increase in the MDA level in rats treated with CSE and pre-treatment with farnesol significantly reduced the MDA level. It was also observed that CSE-induced enhanced activity of xanthine oxidase further strengthen the involvement of oxidative stress, which resulted in CSE-induced oxidative damage in the prostate of Wistar rats. Farnesol pre-treatment significantly attenuated the CSE induced XO activity. XO is an enzyme that reduces O2 to superoxide anion radical (O2•−) and consequently produce oxidative stress.[42]
GSH is a low-molecular weight tripeptide cellular antioxidant, which protects against the peroxidation of lipid membrane by conjugating with the electrophile such as 4-Hydroxy-3-nonenal, formed during LPO and thus, GSH gets depleted in this conjugation reaction.[43] In this study, it was observed that the level of GSH is depleted in CSE treated group as compared to control group. Farnesol supplementation significantly attenuated the level of GSH at both the doses of farnesol. Our findings are in agreement with the previous reports from our laboratory, which showed farnesol significantly alleviated the Fe-NTA, CSE, and DMH-induced depletion of GSH level.[9,12,21] According to present findings, farnesol significantly attenuated the activities of glutathione dependent enzymes i.e. GPx and GR against CSE in the prostate of Wistar rats. It also attenuated the activity of catalase, an antioxidant enzyme responsible for the break-down of hydrogen peroxide into water and oxygen.[21]
The above mentioned findings corroborated with the histological data, which exhibited the protective effects of farnesol against CSE-induced nuclei stratification with papilli formation and closely packed gland lined by tall columnar cells and an increase in prostatic secretions. Pre-treatment with farnesol showed normal prostate architecture, minimal secretion in the prostate acini, and no nuclei stratification is seen. It was also demonstrated that CSE treatment resulted in decrease of leydig cells. Farnesol pre-treatment showed significant protective effects against CSE-induced reduction in leydig cells in the testes of Wistar rats.
The precise mechanism of protective action of farnesol against CSE is still unknown but it can be concluded from the findings of the present study that farnesol exhibits the protective effect against CSE-induced oxidative damages in prostate probably through the attenuation of CSE-induced oxidative stress. Further studies are still warranted to explicate the exact protective mechanism of farnesol.
ACKNOWLEDGMENTS
The author is thankful to the Department of Biotechnology, Government of India, New Delhi for providing funding for the work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Abdulla M, Gruber P. Role of diet modification in cancer prevention. Biofactors. 2000;12:45–51. doi: 10.1002/biof.5520120108. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. CMAJ. 2000;163:739–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Kelloff GJ, Boone CW, Malone WF, Steele VE, Doody LA. Introductory remarks: Development of chemopreventive agents for prostate cancer. J Cell Biochem Suppl. 1992;16H:1–8. doi: 10.1002/jcb.240501203. [DOI] [PubMed] [Google Scholar]
- 5.Levi F, La Vecchia C. Tobacco smoking and prostate cancer: Time for an appraisal. Ann Oncol. 2001;12:733–8. doi: 10.1023/a:1011124523984. [DOI] [PubMed] [Google Scholar]
- 6.Hsing AW, McLaughlin JK, Hrubec Z, Blot WJ, Fraumeni JF., Jr Tobacco use and prostate cancer: 26-year follow-up of US veterans. Am J Epidemiol. 1991;133:437–41. doi: 10.1093/oxfordjournals.aje.a115910. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E, Rimm EB, Ascherio A, Colditz GA, Spiegelman D, Stampfer MJ, et al. Smoking and risk of total and fatal prostate cancer in United States health professionals. Cancer Epidemiol Biomarkers Prev. 1999;8:277–82. [PubMed] [Google Scholar]
- 8.Rodriguez C, Tatham LM, Thun MJ, Calle EE, Heath CW., Jr Smoking and fatal prostate cancer in a large cohort of adult men. Am J Epidemiol. 1997;145:466–75. doi: 10.1093/oxfordjournals.aje.a009129. [DOI] [PubMed] [Google Scholar]
- 9.Khan R, Sultana S. Farnesol attenuates 1,2-dimethylhydrazine induced oxidative stress, inflammation and apoptotic responses in the colon of Wistar rats. Chem Biol Interact. 2011;192:193–200. doi: 10.1016/j.cbi.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8:583–99. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- 11.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qamar W, Sultana S. Farnesol ameliorates massive inflammation, oxidative stress and lung injury induced by intratracheal instillation of cigarette smoke extract in rats: An initial step in lung chemoprevention. Chem Biol Interact. 2008;176:79–87. doi: 10.1016/j.cbi.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Wang CY, Jones RF, Debiec-Rychter M, Soos G, Haas GP. Correlation of the genotypes for N-acetyltransferases 1 and 2 with double bladder and prostate cancers in a case-comparison study. Anticancer Res. 2002;22:3529–35. [PubMed] [Google Scholar]
- 14.Dai WS, Gutai JP, Kuller LH, Cauley JA. Cigarette smoking and serum sex hormones in men. Am J Epidemiol. 1988;128:796–805. doi: 10.1093/oxfordjournals.aje.a115033. [DOI] [PubMed] [Google Scholar]
- 15.Crick DC, Andres DA, Waechter CJ. Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem Biophys Res Commun. 1997;237:483–7. doi: 10.1006/bbrc.1997.7145. [DOI] [PubMed] [Google Scholar]
- 16.Meigs TE, Simoni RD. Farnesol as a regulator of HMG-CoA reductase degradation: Characterization and role of farnesyl pyrophosphatase. Arch Biochem Biophys. 1997;345:1–9. doi: 10.1006/abbi.1997.0200. [DOI] [PubMed] [Google Scholar]
- 17.He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE. Isoprenoids suppress the growth of murine B16 melanomas in vitro and in vivo. J Nutr. 1997;127:668–74. doi: 10.1093/jn/127.5.668. [DOI] [PubMed] [Google Scholar]
- 18.Tatman D, Mo H. Volatile isoprenoid constituents of fruits, vegetables and herbs cumulatively suppress the proliferation of murine B16 melanoma and human HL-60 leukemia cells. Cancer Lett. 2002;175:129–39. doi: 10.1016/s0304-3835(01)00723-6. [DOI] [PubMed] [Google Scholar]
- 19.Horn TL, Long L, Cwik MJ, Morrissey RL, Kapetanovic IM, McCormick DL. Modulation of hepatic and renal drug metabolizing enzyme activities in rats by subchronic administration of farnesol. Chem Biol Interact. 2005;152:79–99. doi: 10.1016/j.cbi.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Zhao J, Nan P, Zhong Y, Zhao J, Nan P, Zhong Y. Chemical composition of the essential oils of Clausena lansium from Hainan Island, China. Z Naturforsch C. 2004;59:153–6. doi: 10.1515/znc-2004-3-401. [DOI] [PubMed] [Google Scholar]
- 21.Jahangir T, Khan TH, Prasad L, Sultana S. Farnesol prevents Fe-NTA-mediated renal oxidative stress and early tumour promotion markers in rats. Hum Exp Toxicol. 2006;25:235–42. doi: 10.1191/0960327106ht616oa. [DOI] [PubMed] [Google Scholar]
- 22.Crowell PL. Prevention and therapy of cancer by dietary monoterpenes. J Nutr. 1999;129:775S–8. doi: 10.1093/jn/129.3.775S. [DOI] [PubMed] [Google Scholar]
- 23.Belanger JT. Perillyl alcohol: Applications in oncology. Altern Med Rev. 1998;3:448–57. [PubMed] [Google Scholar]
- 24.Coleman PS, Chen LC, Sepp-Lorenzino L. Cholesterol metabolism and tumor cell proliferation. Subcell Biochem. 1997;28:363–435. doi: 10.1007/978-1-4615-5901-6_13. [DOI] [PubMed] [Google Scholar]
- 25.Edwards PA, Ericsson J. Sterols and isoprenoids: Signaling molecules derived from the cholesterol biosynthetic pathway. Annu Rev Biochem. 1999;68:157–85. doi: 10.1146/annurev.biochem.68.1.157. [DOI] [PubMed] [Google Scholar]
- 26.Carp H, Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978;118:617–21. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Kohyama T, Kobayashi T, Abe S, Kim HJ, Reed EC, et al. Cigarette smoke extract inhibits chemotaxis and collagen gel contraction mediated by human bone marrow osteoprogenitor cells and osteoblast-like cells. Osteoporos Int. 2003;14:235–42. doi: 10.1007/s00198-002-1350-7. [DOI] [PubMed] [Google Scholar]
- 28.Martey CA, Pollock SJ, Turner CK, O’Reilly KM, Baglole CJ, Phipps RP, et al. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: Implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol. 2004;287:L981–91. doi: 10.1152/ajplung.00239.2003. [DOI] [PubMed] [Google Scholar]
- 29.Baglole CJ, Bushinsky SM, Garcia TM, Kode A, Rahman I, Sime PJ, et al. Differential induction of apoptosis by cigarette smoke extract in primary human lung fibroblast strains: Implications for emphysema. Am J Physiol Lung Cell Mol Physiol. 2006;291:L19–29. doi: 10.1152/ajplung.00306.2005. [DOI] [PubMed] [Google Scholar]
- 30.Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- 31.Thrall RS, Peterson LB, Linehan JH, Abramoff P, Moore VL. The effect of immunization on the uptake of intratracheally administered antigen. Clin Immunol Immunopathol. 1978;10:136–47. doi: 10.1016/0090-1229(78)90021-1. [DOI] [PubMed] [Google Scholar]
- 32.Porter DW, Hubbs AF. Application of intratracheal instillation exposure to the etiological determination of a pulmonary disease outbreak: Nylon flock as an example. In: Salem H, Kartz AS, editors. Inhalation Toxicology. Boca Raton, FL: CRC Press, Taylor and Francis Group; 2006. pp. 109–17. [Google Scholar]
- 33.Stirpe F, Della Corte E. The regulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O) J Biol Chem. 1969;244:3855–63. [PubMed] [Google Scholar]
- 34.Wright JR, Colby HD, Miles PR. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys. 1981;206:296–304. doi: 10.1016/0003-9861(81)90095-3. [DOI] [PubMed] [Google Scholar]
- 35.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–69. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 36.Mohandas M, Marshall JJ, Duggin JS, Horvath D. Differential distribution of glutathione and glutathione related enzymes in rabbit kidney. Cancer Res. 1984;44:5086–91. [PubMed] [Google Scholar]
- 37.Carlberg I, Mannervik B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem. 1975;250:5475–80. [PubMed] [Google Scholar]
- 38.Claiborne A. Catalase activity. In: Greenwald RA, editor. CRC Handbook of Methods for Oxygen Radical Research. Boca Raton, FL: CRC Press; 1985. pp. 283–4. [Google Scholar]
- 39.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 40.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–26. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qamar W, Sultana S. Polyphenols from Juglans regia L. (walnut) kernel modulate cigarette smoke extract induced acute inflammation, oxidative stress and lung injury in Wistar rats. Hum Exp Toxicol. 2011;30:499–506. doi: 10.1177/0960327110374204. [DOI] [PubMed] [Google Scholar]
- 42.Heunks LM, Dekhuijzen PN. Respiratory muscle function and free radicals: From cell to COPD. Thorax. 2000;55:704–16. doi: 10.1136/thorax.55.8.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawanishi S, Yamamoto K. Mechanism of site-specific DNA damage induced by methylhydrazines in the presence of copper (II) or manganese (III) Biochemistry. 1991;30:3069–75. doi: 10.1021/bi00226a013. [DOI] [PubMed] [Google Scholar]


