Abstract
The restorative potential of trans-resveratrol (RV) was investigated in a rat neuronal cell line (PC12) exposed to organophosphate pesticide-monocrotophos (MCP). RV shows significant protection against MCP-induced alterations in PC12 cells by restoration of oxidative stress-mediated apoptosis and cytotoxicity. RV treatment significantly reduced reactive oxygen species (ROS) production and lipid peroxidation, and also restored glutathione levels and mitochondrial membrane potential, in cells receiving MCP. Restoration of markers such as cytochrome c, Bax, Bcl-2 and caspase-3 also confirms the effectiveness of RV against MCP-induced, mitochondria-mediated apoptosis in PC12 cells. The data identify the protective/restorative potential of RV against MCP-induced neuronal damages by affecting ROS production and the level of antioxidant defence enzymes.
Keywords: Monocrotophos, oxidative stress, PC12 cells, trans-resveratrol
INTRODUCTION
The plethora of literature has supported the vulnerability of neural cells to environmentally existing toxicants. Among such toxicants, pesticides, especially organophosphorus, have been reported to affect the brain severely.[1,2,3] We and others have reported earlier that pesticide-monocrotophos (MCP) induces neurotoxicity in PC12 cells via mitochondria-mediated apoptosis and induction of oxidative stress, lipid peroxidation and changes the in glutathione disulfide ratio.[4] Recently, it has been demonstrated that MCP-induced apoptosis in PC12 cells has an association with oxidative stress and the specific xenobiotic metabolizing cytochrome P450s.[5] Other in vitro as well as in vivo studies have also suggested the involvement of various apoptotic signaling pathways in MCP-exposed neuronal cells.[6,7,8]
MCP-induced apoptotic damages were found to be reversible in nature if treated with antioxidants. The present investigations were carried out to study the protective effects of trans-resveratrol (RV) in PC12 cells receiving MCP. RV is a strong antioxidant compound present in grapes and mulberries. Recent studies have shown that it could protect from severe brain injuries and Alzheimer's disease.[9] Several studies have shown the protection ability of RV from toxic compounds. Recently Huang et al.,[10] showed the neuroprotective effects of RV from Aβ peptide-induced neurotoxicity in rats. They have also found that RV suppresses nitric oxide synthase (iNOS) expression, which further reduces lipid peroxidation and apoptotic cell death. Pretreatment with RV has shown to protect PC12 cells from 4-hydroxynonenal-induced cell damage.[11] Recently, we have shown that RV protected PC12 cells under oxygen glucose deprivation insult by inhibiting hypoxia-associated transcription factors and increased the level of antioxidant defense enzymes.[12,13] The present investigations were carried out to study the protective potential of RV in MCP-induced toxic responses in PC12 cells.
MATERIALS AND METHODS
Cell culture
PC12 cells were procured from National Centre for Cell Sciences, Pune, India, and maintained at In Vitro Toxicology Laboratory, Indian Institute of Toxicology Research, Lucknow, India, as per the standard protocols described earlier.[4] Briefly, cells were cultured in a nutrient mixture (F-12 Hams) supplemented with 2.5% fetal bovine serum, 15% horse serum, 0.2% sodium bicarbonate (NaHCO3), 100 U/ml penicillin-G sodium, 100 mg/ml streptomycin sulfate and 0.25 mg/ml amphotericin-B. The cultures were maintained at 37°C under a 5% CO2-95% atmosphere under high humid conditions. The culture medium was changed twice weekly and the cultures were passaged at a ratio of 1:6 once a week. Prior to experiments, cells were also checked for viability using trypan blue dye exclusion assay, and batches showing more than 95% viability only were used in the experimentation.
Reagents and consumables
All specified chemicals, primers, probes and reagents, viz., MCP (dimethyl (E)-1-methyl-2-methyl carbanoyl vinyl phosphate (IUPAC) C7H14NO5-P.; catalog no. PS-609; purity 99.5%), trans-RV and diagnostic kits were purchased from Sigma (USA), unless otherwise stated. Culture medium nutrient mixture F-12 Hams, antibiotics/antimycotics, fetal bovine and horse sera were purchased from Gibco BRL (USA).
Experimental design
Biologically safe doses of MCP and RV were identified for PC12 cells prior to start of all experiments using standard endpoints of cytotoxicity assay, that is, MTT (3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazoliumbromide). Selection of safe dose of RV and MCP for the present investigation was based on our previous studies with the same cell line under identical conditions.[4,13] Four experimental groups were designed: (1) control cells (group-I), (2) cells treated with MCP (10 μM) for 24 h (group-II), (3) cells treated with RV (10 μM) for 24 h (group-III) and cells co-treated with RV (10 μM) h) and MCP (10 μM) for 24 h (group-IV).
MTT assay
MTT assay was performed by following the protocol of Agarwal et al.,[12] In brief, cells (1 × 104) were seeded in poly-L-lysine pre-coated 96-well culture plates and allowed to adhere properly for 24 h at 37°C in a CO2 incubator (5% CO2/95% atmosphere under humid conditions). After the respective exposure, MTT (5 mg/ml of stock in phosphate-buffered saline (PBS)) was added (10 μl/well in 100 μl of cell suspension) and plates were incubated for 4 h. At the end of the incubation period, the reaction mixture was carefully taken out and 200 μl of dimethyl sulfoxide was added to each well by pipetting up and down several times until the contents became solubilized. After 10 min, absorbance was taken at 550 nm, using a multi-well microplate reader (Synergy HT; Bio-Tek, USA). Untreated sets were run simultaneously under identical conditions and served as basal control.
Estimation of reactive oxygen species
Estimation of MCP-induced reactive oxygen species (ROS) generation was carried out following the standard protocol of Srivastava et al.,[14] In brief, cells (104 per well) were seeded in poly-L-lysine pre-coated 96-well black-bottom culture plates and allowed to adhere for 24 h under a 5% CO2–95% atmosphere at 37°C. Cells were exposed to MCP (10 μM), RV (10 μM) and a combination of both (10 μM) for 24 h. Following exposure, cells were re-incubated with 2,7-dichloro-dihydrofluorescein-diacetate (DCFH-DA) (20 μM) for 30 min at 37°C. The reaction mixture was then replaced with 200 μl of PBS per well. The plates were kept on a rocker shaker platform for 10 min at room temperature in dark and fluorescence intensity was measured using a multi-well microplate reader (Synergy HT; Bio-Tek) at an excitation wavelength of 485 nm and emission wavelength of 528 nm. The data are expressed as percent of unexposed control. Intracellular ROS generation was also confirmed by image analysis. In brief, cells (5 × 104 per well) were seeded in poly-L-lysine pre-coated tissue culture slide flasks and allowed to adhere. The adhered cells were then exposed to MCP (10 μM), RV (10 μM) and a combination of both (10 μM) for 24 h. Following MCP exposure, cells were washed twice with PBS and re-incubated for 30 min in the dark in an incomplete culture medium containing DCFH-DA (20 μM). The slides were washed twice again with PBS and mounted for microscopy analysis. Images were taken using Nikon Eclipse 80i equipped with a Nikon DS-Ri1 12.7 megapixel camera. Cells exposed to H2O2 (100 μM) for 2 h under identical conditions served as positive control.
Estimation of glutathione
Glutathione (GSH) levels were assessed following exposure of PC12 cells to MCP (10 μM), RV (10 μM) and the combination of both (10 μM) for 24 h using a commercially available kit (glutathione detection kit, catalog no. APT250; Chemicon, USA). In brief, following respective MCP, RV and combination exposures, cells were collected by centrifugation at 7006 × g for 2 min at 4°C and lysed in lysis buffer. The samples were centrifuged again at 120,006 × g for 10 min at 4°C and the supernatant was collected. To estimate GSH levels, the lysed samples (90 ml/well) were transferred to 96-well black-bottom plates and mixed with a freshly prepared assay cocktail (10 ml) and read at an excitation wavelength of 380 nm and emission wavelength of 460 nm using a multi-well microplate reader (Synergy HT; Bio-Tek) after incubation for 1-2 h. A standard curve was plotted using the glutathione standard supplied in the kit and used to calculate experimental values. Cells exposed to H2O2 (100 μM) for 2 h under identical conditions served as positive control.
Estimation of lipid peroxidatio
Lipid peroxidation (LPO) was performed using the thiobarbituric acid reactive substances (TBARS) protocol.[11] In brief, cells (104 per well) were seeded in poly-L-lysine pre-coated 96-well, black-bottom culture plates and allowed to adhere for 24 h under a 5% CO2-95% atmosphere at 37°C. Cells were exposed to MCP (10 μM), RV (10 μM) and a combination of both (10 μM) for 24 h. After completion of the exposure time period, the cells were collected by centrifugation and sonicated in ice-cold potassium chloride (1.15%) and centrifuged for 10 min at 3000 × g. The resulting supernatant (1 ml) was added to 2 ml of thiobarbituric acid reagent (15% TCA, 0.7% TBA, and 0.25 N HCl) and heated at 100°C for 15 min in a water bath. The samples were then placed in cold and centrifuged at 1000 × g for 10 min. Absorbance of the supernatant was measured at 535 nm.
Estimation of mitochondrial membrane potential
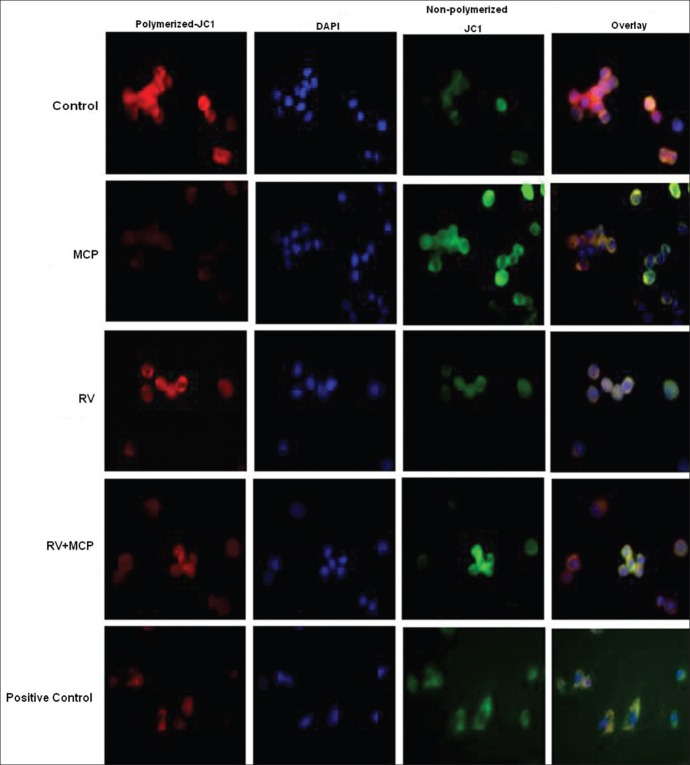
Mitochondrial membrane potential (MMP) was assessed using the JC1 dye. After completion the respective exposure time periods, cells were washed with PBS and incubated with the JC1 dye (10 μM) for 15 min at 37°C. The cells were then washed with PBS and counterstained with ****DAPI. The cells were visualized under an upright microscope (Nikon Eclipse 80i equipped with Nikon DS-Ri1 12.7 megapixel camera; Japan).
Transcriptional changes (real-time analysis)
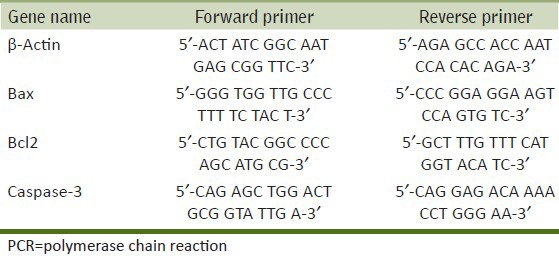
Transcriptional changes in the selected genes (Bax, caspase-3 and Bcl2) were studied in PC12 cells exposed to MCP or/and RV. Alterations in mRNA expression level were expressed in relative quantification by comparing data obtained from unexposed cells. Quantitative real-time polymerase chain reaction (PCR) analysis was done by following the protocol described earlier by us.[13] In brief, total RNA was isolated from both experimental and unexposed control sets using a Gene Elute mammalian total RNA Miniprep kit (catalog no. RTN-70; Sigma). Total RNA (1 μg) was reverse-transcribed to cDNA by using a Super Script III first strand cDNA synthesis kit (catalog no. 18080-051; Invitrogen Life Science, USA). Quantitative real-time PCR assay reactions were carried out using the 2X SYBR Green PCR master mix (Applied Biosystems, USA) using ABI PRISM 7900HT Sequence Detection System version 2.2.1 (Applied Biosystems). Results were expressed relative to the housekeeping gene, β-actin. Real-time reactions were carried out in triplicate wells for each sample. Details of primers sequences are listed in Table 1.
Table 1.
Nucleotide sequences of the primers used for SYBR Green real-time PCR analysis
Translational changes (western blot analysis)
Translational changes in the selected genes (cytochrome c, Bax, Bcl2 and caspase-3) were studied in PC12 cells exposed to various concentrations of MCP and RV. Western blot analysis was done by following the protocol described earlier by us.[12] In brief, cells were pelleted and lysed using the CelLytic™ M Cell Lysis Reagent (catalog no. C2978; Sigma) in the presence of a protease inhibitor cocktail (catalog no. P8340; Sigma). Protein estimation was done using a BCA Protein Assay kit (catalog no. G1002; Lamda Biotech Inc., St Louis, MO, USA). Then denatured proteins (100 μg/well) were loaded and electrophoresed using 10% Tricine–SDS (sodium dodecyl sulfate) gel.[12] The proteins were transferred to a polyvinylidene fluoride membrane (Millipore, USA; catalog no. IPVH00010) by the wet transfer method under 180 mA current for 3 h. Nonspecific binding was blocked with 5% non-fat dry milk powder in TBST (20 mM Tris–HCl (pH 7.4), 137 mM NaCl and 0.1% Tween-20] for 2 h at 37°C. After blocking, the membranes were incubated overnight at 4°C with anti-protein primary antibodies specific for cytochrome c, Bax, Bcl2 and caspase-3 (1:1000; Chemicon International, USA), in blocking buffer (pH 7.5). The membranes were then incubated for 2 h at room temperature with a secondary anti-primary antibody conjugated with horseradish peroxidase (Chemicon International). Then the blots were developed using the Super Signal West Fempto Chemiluminescent Substrate™ (Thermo Fisher Scientific, USA) and Bio-Rad Versa Doc™ Imaging System 4000 (Bio-Rad, PA, USA). Densitometry for protein-specific bands was done using a gel documentation system (Alpha Innotech, USA) with the help of the Alpha Ease™ FC Stand Alone V.4.0 software. β-Actin was used as internal control to normalize data. Xenobiotics-induced alterations were expressed in relative term fold change in expression by comparing the data with respective unexposed controls.
Statistical analysis
Results are expressed as mean and standard error of means (mean ± SE) for at least three experiments. One-way analysis of variance followed by post hoc Dunnett's test was employed to detect differences between the treated and control groups.
RESULTS
MTT assay
The cytoprotective effects of RV against MCP in PC12 cells observed by MTT assays are summarized in Figure 1. A significant (P < 0.001) decrease in percent cell viability was observed in PC12 cells following 24-h exposure to MCP (10 μM) as compared with the control. In the co-treatment group, RV (10 μM) significantly (P < 0.01) decreased the toxic effect of MCP and attenuated loss of cell viability.
Figure 1.
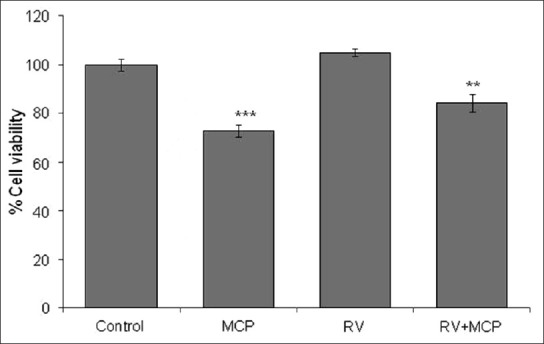
Protective potential of RV (10 mM) on percent cell viability against MCP (10 mM): MTT assay in PC12 cells following exposure to MCP, RV and co-treatment with RV + MCP. The values are the mean ± SEM of three experiments each carried out in triplicate. The data are expressed as the mean of the percent of the unexposed control ± SEM, n= 8. *P< 0.05, **P< 0.01, ***P< 0.001
ROS generation
Significant induction in ROS production (146 ± 2.9%) was noted in MCP (10 μM)-treated cells when compared with the controls [Figure 2a], although ROS generation reduced significantly (119 ± 2.3%) following co-treatment with RV (10 μM). For cells exposed to H2O2 (100 μM) for 24 h, which were considered positive control, the value was recorded was 179 ± 2.87%. Fluorescence microscopy analysis using the DCFH-DA fluorescence dye maintained linearity with the data obtained by spectrofluorometric analysis [Figure 2b].
Figure 2.
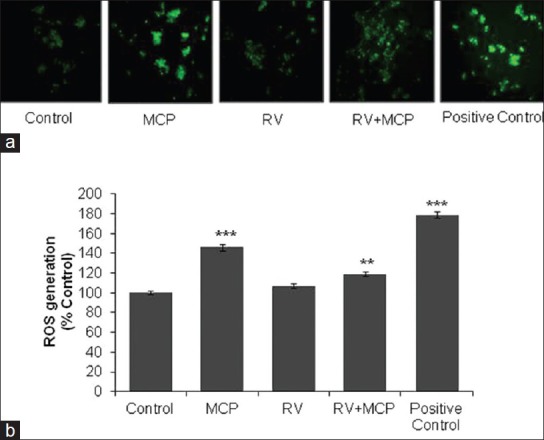
ROS generation in PC12 cells exposed to MCP, RV and RV + MCP: Representative microphotographs showing MCP-, RV- and MCP + RV-induced ROS generation in PC12 cells. ROS generation was studied using the DCFH-DA dye. Images were captured by a Nikon phase-contrast cum fluorescence microscope (model 80i) attached with a 12.7 Megapixel Nikon DS-Ri1 digital CCD cool camera (a). Percent change in ROS generation following 24-h exposure to MCP, RV and RV + MCP of PC12 cells assessed by spectrofluorometric analysis. Fluorescence intensity was measured using a multi-well microplate reader (Synergy HT; Bio-Tek) using an excitation wavelength of 485 nm and emission wavelength of 528 nm. The data are expressed as the mean of the percent of unexposed control ± SEM, n= 8. *P< 0.05, **P< 0.01, ***P< 0.001 (b)
Glutathione level
The data of MCP-induced changes in the levels of GSH are presented in Figure 3a. A statistically significant (P < 0.001) decrease in GSH level was observed at 24-h exposures, that is, 80 ± 2.2% following exposure to MCP (10 μM), and a significant increase in the level of GSH (91 ± 1.7%) was observed when the cells were exposed to the combination of MCP + RV in comparison with unexposed control cells. Thus, RV has shown most effective restoration in the levels of glutathione in MCP-treated cells.
Figure 3.
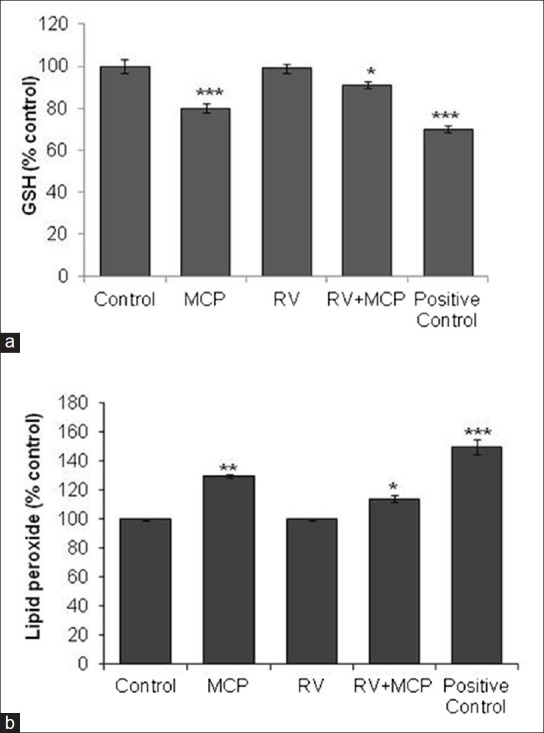
Glutathione (GSH) levels in PC12 cells exposed to MCP, RV and RV + MCP for 24 h assessed by a fluorescence-based glutathione detection kit. The data are expressed as percentage intracellular concentration of GSH ± SEM, n= 3. *P< 0.05, **P< 0.01, ***P< 0.001 (a). LPO levels in PC12 cells exposed to MCP, RV and RV + MCP for 24 h assessed by the thiobarbituric acid reactive substances (TBARS) protocol - The data are expressed as percentage LPO ± SEM, n= 3. *P< 0.05, **P< 0.01, ***P< 0.001 (b)
Lipid peroxidation level
A statistically significant (P < 0.01) increase in lipid peroxidation was observed in the MCP-exposed cells (130 ± 1.3% compared with the control). RV (10 μM) significantly (P < 0.05) restored the level of lipid peroxidation in the group exposed to the combination of RV + MCP, that is, 114 ± 2.4% with respect to the unexposed control after the 24-h time duration [Figure 3b].
Mitochondrial membrane potential
MMP was detected by using the JC1 dye using an upright phase-contrast microscope (Nikon 80i; Japan) at × 1000 magnification [Figure 4]. Control cells showed intense red color due to polymerization of the JC1 dye in mitochondria, indicative of healthy mitochondria, whereas MCP (10 μM)-exposed cells showed significant accumulation of non-polymerized dye (green color) in the cytoplasm. Moreover, in the co-treatment group, RV provided protection against MCP and MMP was restored as compared with the MCP-exposed cells. The positive control group (cells exposed to 100 μM H2O2) showed nuclear condensation and fragmentations. (Superimposed microphotograph showing apoptotic events. Nuclei were stained with DAPI.)
Figure 4.
MMP in PC12 cells exposed to MCP, RV and RV + MCP. MMP was detected by using the JC1 dye using an upright phase-contrast microscope (Nikon 80i; Japan) at × 10 × 100 oil-immersion magnification. The images were taken by a Nikon DS-Ri1 (12.7 megapixel) camera
Transcriptional changes
MCP significantly increased the expression of Bax and caspase-3, that is, 3.5 ± 0.30- and 2.1 ± 0.10-fold when compared with the normal control cells [Figures 5a and b]. A significant decrease in the expression of Bax and caspase-3 was observed in the co-treatment group exposed to RV + MCP, that is, 1.8 ± 0.10 and 1.6 ± 0.10. While in the case of Bcl2, MCP-exposed cells showed a significant decrease in expression, that is, 0.5-fold in comparison with the control. In the co-treatment group, expression of Bcl2 was increased, that is, 0.8-fold as compared with the MCP-treated cells. Moreover, RV-exposed cells showed 1.23-fold increase in the expression of Bcl2 in comparison with the control [Figure 5c].
Figure 5.
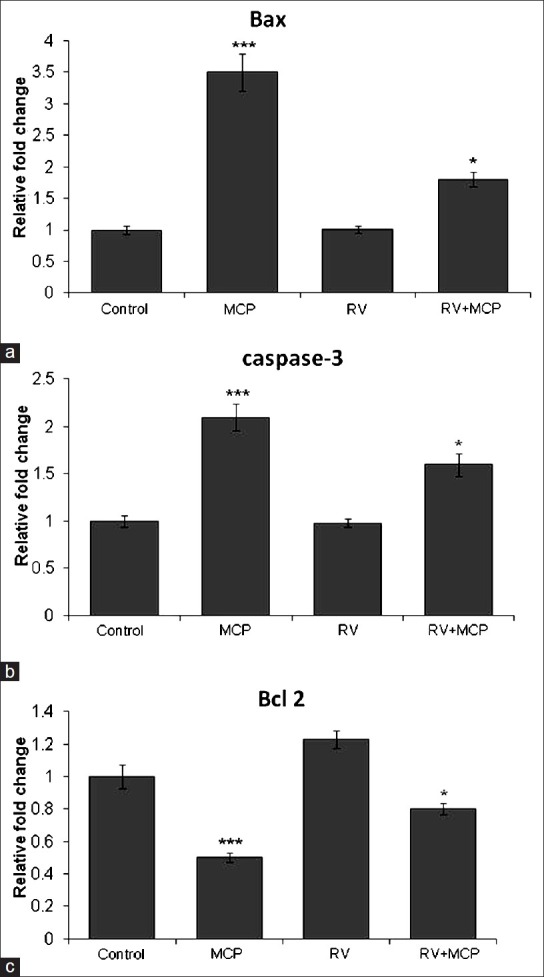
Transcriptional changes in the levels of Bax, caspase-3 and Bcl2 in PC12 cells exposed to MCP, RV and RV + MCP: MCP-, RV- and RV + MCP-induced alterations in the mRNA expression of Bax, caspase-3 and Bcl2 in PC12 cells [Figure 5a, b and c]. Quantitative real-time PCR was performed in triplicate using SYBER Green using the ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). β-Actin was used as an internal control to normalize the data and MCP-, RV- and RV + MCP-induced alterations in mRNA expression are expressed in relative quantity compared with the respective unexposed control groups. The data are expressed as relative fold change of the unexposed control ± SEM. *P< 0.05, **P< 0.01***P< 0.001
Translational changes
In western blot analysis, a significant increase in protein expression of cytochrome c (1.24), Bax (1.39) and caspase-3 (5.26) was observed in cells exposed to MCP (10 μM) for 24 h. The cells also showed a significant increase in the expression of cytochrome c, Bax and caspase-3 when co-exposed to RV + MCP for 24 h, that is, 1.08, 1.17 and 3.47, respectively; however the elevated levels of Bax, caspase-3 and cytochrome c were restored in comparison with the MCP-exposed cells [Figures 6a–c]. While in the case of Bcl2, MCP-exposed cells showed a significant decrease in expression, that is, 0.76-fold in comparison with the control. While in the co-treatment group, the RV + MCP-exposed cells showed 0.89-fold expression of Bcl2 in comparison with the control [Figure 6d].
Figure 6.
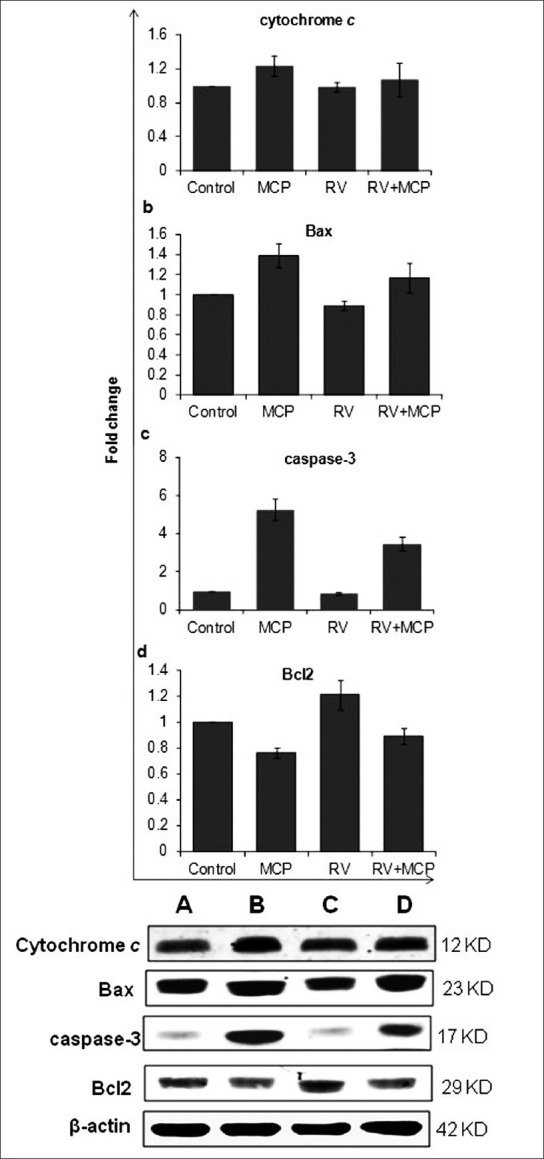
Alterations in the expression of the proteins of cytochrome c, Bax, Bcl2 and caspase-3 were studied in PC12 cells exposed to MCP, RV and RV + MCP for a 24-h time period: Results are shown in a, b, c and d. Lane (a): Unexposed;(b): Cells exposed to MCP; (c) RV + MCP; (d) RV for 24 h. Relative quantification of alterations in the expression of different proteins, viz., Bax (23 kDa), Bcl2 (29 kDa), activated caspase-3 (17 kDa), cytochrome c (12 kDa) and β-actin (42 kDa) in PC12 cells. β-Actin was used as an internal control to normalize the data. Quantification was done using a gel documentation system (Alpha Innotech) with the help of the Alpha Ease™ FC Stand Alone V.4.0 software.
DISCUSSION
Oxidative stress induced by increased generation of ROS has been known to cause cell injury and apoptotic cell death.[15] Naturally occurring compounds have been extensively used as an alternative therapeutic strategy for various illnesses. Among them RV has emerged as a promising compound with immense properties of an antioxidant. In the present study, we explored the antioxidant properties of RV in the case of MCP toxicity. Several in vivo and in vitro studies have shown the protective effects of RV in neurodegenerative diseases. It is also known to protect against oxidative DNA damage in other disease such as cancer.[16] RV has found to protect the olfactory cortex and the hippocampus of rat brain from kainic acid toxicity.[17] We have also shown the ability of RV in attenuating the toxic effects of 4-hydroxynonenal in PC12 cells.[11] RV has also been shown to reduce the anti-apoptotic effects of the anticancer drug paclitaxel when administered to the human neuroblastoma cell line SH-SY5Y, by inhibiting the activation of caspase-7;[18] collectively these studies suggest that RV can relieve cells from oxidative stress caused by toxicants, thus preventing apoptosis.
In the present study, we used a 10-μM dose of MCP as well as RV for treatment either separately or combined. We observed that percent cell survival increased in the case of RV + MCP treatment in comparison with MCP alone, but decreased in comparison with RV alone. We inferred that the 10-μM dose of RV is biologically safe and simultaneously reduces the toxicity of MCP. Therefore, for further experimentation we used 10 μM RV in combination with 10 μM MCP.
We and others have shown previously that MCP induces mitochondria-mediated apoptosis via induction of ROS, which leads to lipid peroxidation and decrease in glutathione levels, causing apoptotic cell death.[5,6,8,19] Thus, we evaluated the potential of RV in reversing the cytotoxic effects of MCP. We observed that after 24 h of treatment of MCP, ROS were increased to 146% as compared with the control and this induced ROS generation reduced to 119% under the combined dose of RV + MCP; thus 10 μM RV was found to inhibit ROS generation effectively in PC12 cells treated with 10 μM MCP. Further, RV was also found to decrease the level of LPO and elevate the level of GSH in the MCP-treated PC12 cells. Thus collectively we are able to say that RV can effectively protect PC12 cells from oxidative stress caused by MCP exposure.
To evaluate the ability of RV to protect cells from apoptosis, we first checked the level of MMP, as a decreased level of MMP induces apoptotic cell death by release of cytochrome c and further caspase activation. As MCP is already reported to decrease MMP, we found that RV restored the MMP of the MCP-exposed PC12 cells and brought it back to the control level. To further verify the anti-apoptotic potential of RV, we determined the expression of the apoptotic markers cytochrome c, Bax and caspase-3, and the anti-apoptotic marker Bcl2 at both mRNA and protein level. As assumed, combined treatment with MCP and RV decreased the expression of the apoptotic markers and restored the expression of the anti-apoptotic marker Bcl2 to the level of the control, thus protecting the cells from apoptotic cell death.
In conclusion, we presented that RV can effectively protect PC12 cells from MCP-induced cytotoxicity by averting oxidative DNA damage, which is the key mechanism of the neurodegenerative effects of many genotoxic compounds.
ACKNOWLEDGMENT
The authors are grateful to the Director of Indian Institute of Toxicology Research, Lucknow India, for his keen interest in the present work. Indian Council of Medical Research (ICMR), New Delhi, is acknowledged for providing a fellowship to Mr. Vivek Kumar.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ray DE, Richards PG. The potential for toxic effects of chronic, low-dose exposure to organophosphates. Toxicol Lett. 2001:343–51. doi: 10.1016/s0378-4274(01)00266-1. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Kobayashi M, Kawada T. Chlorpyrifos induces apoptosis in human T cells. Toxicology. 2009;255:53–7. doi: 10.1016/j.tox.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Kashyap MP, Singh AK, Siddiqui MA, Kumar V, Tripathi VK, Khanna VK, et al. Caspase cascade regulated mitochondria mediated apoptosis in monocrotophos exposed PC12 cells. Chem Res Toxicol. 2010;23:1663–72. doi: 10.1021/tx100234m. [DOI] [PubMed] [Google Scholar]
- 5.Kashyap MP, Singh AK, Kumar V, Tripathi VK, Srivastava RK, Agrawal M, et al. Monocrotophos induced apoptosis in PC12 cells: Role of xenobiotic metabolizing cytochrome P450s. PLoS One. 2011;6:e17757. doi: 10.1371/journal.pone.0017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashyap MP, Singh AK, Kumar V, Yadav DK, Khan F, Jahan S, et al. Pkb/Akt1 mediates Wnt/GSK3b/b-catenin signaling induced apoptosis in human cord blood stem cells exposed to organophosphate pesticide-monocrotophos. Stem Cells Dev. 2013;22:224–38. doi: 10.1089/scd.2012.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh AK, Kashyap MP, Jahan S, Kumar V, Tripathi VK, Siddiqui MA, et al. Expression and inducibility of cytochrome P450s (CYP1A1, 2B6, 2E1, 3A4) in human cord blood CD34(+) stem cell-derived differentiating neuronal cells. Toxicol Sci. 2012;129:392–410. doi: 10.1093/toxsci/kfs213. [DOI] [PubMed] [Google Scholar]
- 8.Kazi AI, Oommen A. Monocrotophos induced oxidative damage associates with severe acetylcholinesterase inhibition in rat brain. Neurotoxicology. 2012;33:156–61. doi: 10.1016/j.neuro.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Li F, Gong Q, Dong H, Shi J. Resveratrol, a neuroprotective supplement for Alzheimer's disease. Curr Pharm Des. 2012;18:27–33. doi: 10.2174/138161212798919075. [DOI] [PubMed] [Google Scholar]
- 10.Huang TC, Lu KT, Wo YY, Wu YJ, Yang YL. Resveratrol protects rats from Ab-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS One. 2011;6:29102. doi: 10.1371/journal.pone.0029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqui MA, Kashyap MP, Kumar V, Al-Khedhairy AA, Musarrat J, Pant AB. Prophylactic potential of trans-resveratrol against 4-hydroxynonenal induced damages in PC12 cells. Toxicol In Vitro. 2010;24:1592–8. doi: 10.1016/j.tiv.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal M, Kumar V, Kashyap MP, Randhawa GS, Pant AB. Ischemic insult induced apoptotic changes in PC12 cells: Protection by trans-resveratrol. Eur J Pharmacol. 2011;666:5–11. doi: 10.1016/j.ejphar.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal M, Kumar V, Singh AK, Kashyap MP, Yadav S, Khanna VK, et al. trans Resveratrol protects ischemic PC12 cells by inhibiting the expression of hypoxia associated transcription factors and increasing the levels of antioxidant defense enzymes. ACS Chem Neurosci. 2013;4:285–94. doi: 10.1021/cn300143m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srivastava RK, Pant AB, Kashyap MP, Kumar V, Lohani M, Jonas L, et al. Multi-walled carbon nanotubes induce oxidative stress and apoptosis in human lung cancer cell line – A549. Nanotoxicology. 2011;5:195–207. doi: 10.3109/17435390.2010.503944. [DOI] [PubMed] [Google Scholar]
- 15.Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr Res. 2009;66:121–7. doi: 10.1203/PDR.0b013e3181a9eafb. [DOI] [PubMed] [Google Scholar]
- 16.Sgambato A, Ardito R, Faraglia B, Boninsegna A, Wolf FI, Cittadini A. Resveratrol, a natural phenolic compound, inhibits cell proliferation and prevents oxidative DNA damage. Mutat Res. 2001;496:171–80. doi: 10.1016/s1383-5718(01)00232-7. [DOI] [PubMed] [Google Scholar]
- 17.Virgili M, Contestabile A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine antioxidant agent, trans-resveratrol in rats. Neurosci Lett. 2000;281:123–6. doi: 10.1016/s0304-3940(00)00820-x. [DOI] [PubMed] [Google Scholar]
- 18.Nicolini G, Rigolio R, Miloso M, Bertelli AA, Tredici G. Anti-apoptotic effect of trans-resveratrol on paclitaxel-induced apoptosis in the human neuroblastoma SH-SY5Y cell line. Neurosci Lett. 2001;302:41–4. doi: 10.1016/s0304-3940(01)01654-8. [DOI] [PubMed] [Google Scholar]
- 19.Moser VC. Age-related differences in acute neurotoxicity produced by mevinphos, monocrotophos, dicrotophos, and phosphamidon. Neurotoxicol Teratol. 2011;33:451–7. doi: 10.1016/j.ntt.2011.05.012. [DOI] [PubMed] [Google Scholar]