Abstract
Battery manufacturing workers are occupationally exposed to lead (Pb), which is a highly toxic heavy metal. The aim of this study was to investigate the blood lead levels (BLL) of 30 battery manufacturing workers and find the correlation between BLL, micronucleated cell (MNC) frequency, binucleated cell (BNC) frequency in buccal mucosal cells and malondialdehyde concentrations in serum. 30 subjects of the BMW group, exposed to lead, and 30 control subjects, matched with the exposed subjects with respect to age, socio-economic status, sex, diet, smoking and drinking habits, were monitored for this study. BLL was found to have highly significant difference between both the groups (P < 0.001). The serum MDA levels were observed at significantly higher levels (6.76 ± 3.26) for the exposed group as compared to the control group (2.10 ± 1.02; P < 0.001). Buccal micronucleus test showed that both MNC and BNC frequencies were higher among the workers, in comparison to the control subjects. A positive correlation has been found between BLL and all the parameters. Our results indicate an increased health associated risk for workers occupationally exposed to lead.
Keywords: Battery manufacturing workers, blood lead levels, buccal micronucleus test, genotoxicity, lead, lipid peroxidation, malondialdehyde
INTRODUCTION
Lead (Pb) is a toxic heavy metal. It is a common environmental and occupational contaminant, widely distributed around the world.[1] It is widely used in the manufacture of batteries, varnishes and paints. Lead is toxic even at very low exposure levels and it interferes with a number of body processes, affecting multiple body systems. Exposure to levels of lead higher than 1.20 μM/l may pose a genetic risk.[2] Children absorb and retain more lead than adults for a given exposure. Lead impairs the development of the nervous system, resulting in severe damage, particularly in children under the age of six. Lead has been classified as a possible human carcinogen by the International agency for research on cancer.[1] Stoia et al.,[3] showed an increased micronucleus mean score of a non-ferrous metallurgical plant workers, compared to controls (6.0 ± 2.328% vs. 2.7 ± 0.978%; P < 0.001). Exposure to lead can be through soil, water and air. Although, acute lead poisoning is rare but a long-term low environmental exposure to lead is still a public issue.
Occupational exposure to lead poses a serious threat to the health of industrial workers. Battery manufacturing industries are one of the major sources of occupational exposure to lead. Most of the lead used in these industries is of the ‘secondary’ type, which has been recycled from lead-acid batteries.[4] Lead smelting and manufacture of lead alloy battery grids is a major source of lead oxides.[5] Whenever ingested via food, lead is absorbed by the gastro-intestinal tract. Lead inhibits the activities of several enzymes of heme biosynthesis. d-aminolevulinic acid dehydratase (ALAD) is one of the genes that gets inhibited, leading to alterations in heme biosynthesis.[6] Contaminated air inside the battery manufacturing units poses a high risk of air-borne lead exposure. According to the World Health Organization (WHO), for each 1 mg/m3 increase in the concentration of lead in air, the blood lead value increases by approximately 1.6 mg/dl. After absorption, lead enters the blood stream and almost 95% of it binds to the red blood corpuscles.[7] It then reaches almost all the organs of the body, including the kidneys, heart, intestines, bones, reproductive system and nervous system causing severe damage.
Various studies have established the use of the micronuclei test to find out the genotoxicity of different substances. Micronuclei (MN) and other nuclear anomalies such as nucleoplasmic bridges (NPBs) and nuclear buds (NBUDs) are biomarkers of genotoxic events and chromosomal instability.[8] Roth et al.,[9] found significantly higher micronucleated cell counts than controls in workers exposed to car paints and lead. The micronuclei test is a sensitive, non-invasive, cost efficient and reliable test to locate chromosomal mutations.[3,10]
There has been a growing interest in the levels of the reactive oxygen species in various occupational studies. Lead causes oxidative stress by stimulating the production of reactive oxygen species (ROS) and reducing the antioxidant defence system of the cells.[11] Malondialdehyde (MDA) is a widely accepted biomarker for lipid peroxidation.[12] MDA is a major reactive aldehyde resulting from the lipid peroxidation and its measurement in clinical samples is useful in the evaluation of oxidative stress. The workers of battery repair garages belong to an increased risk group and should be continuously monitored for health problems.[9] The present study was carried out to assess the genotoxicity and oxidative stress due to lead in battery manufacturing workers, using the buccal micronucleus test and estimation of serum malondialdehyde.
MATERIALS AND METHODS
Subjects
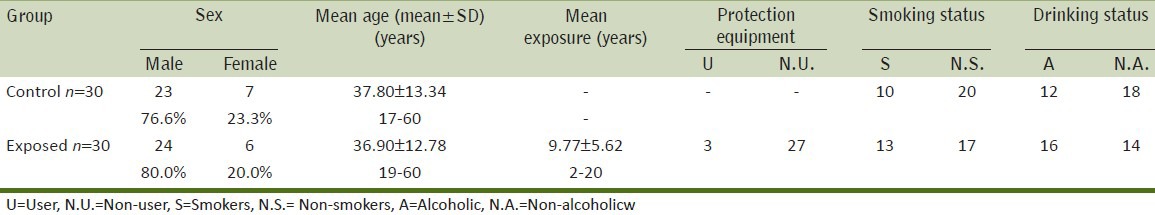
24 male and 6 female workers, of the age group 19-60 years (36.90 ± 12.78 yrs.), occupationally exposed to lead in a battery manufacturing industry were accepted as subjects for the present study. Administrators or transporters were not included in this study. Another group of 30 individuals (23 males and 7 females), of age group 17-60 years (37.80 ± 13.34 years), matched to the exposed subjects with respect to sex, socio-economic status, age, diet, smoking and drinking habits was considered as the control. The subjects were divided into 4 groups on the basis of their age. They were also grouped according to their smoking and drinking habits to find the effect of these confounding factors on genotoxicity and oxidative stress levels. The subjects consuming more than 5 cigarettes per day were considered as smokers and subjects taking more than 125 ml of alcohol per day were considered to be drinkers.
Data collection
Prior to sampling, the workers and controls were asked to complete a questionnaire, with information about their name, age, sex, smoking and drinking habits, diseases, drug intake, duration of exposure in the present industry, exposure history, working conditions and use of personal protection equipment, along with the consent to participate in this study. The information collected from the subjects was recorded for coding the samples.
Blood sampling and serum separation
Blood sampling was done on the last working day of the week to allow maximum exposure for the exposed subjects. 4 ml of venous blood samples from both exposed and control subjects were collected in fresh, clean glass tubes. Out of each sample, 1 ml of the whole blood was separated with heparin for the blood lead analysis and was kept at 4°C in a chiller box till further processing. Rest of the blood (3 ml) was allowed to clot for half an hour and then centrifuged at 3,000 rpm for 15 min. The clear supernatant was collected by micropipettes and used for MDA estimations. All the required precautions were taken during this procedure to avoid any kind of sample contamination. The serum samples were sent to the lab within 3 hours for subsequent MDA estimations.
Buccal cell sampling
After rinsing the mouth twice, buccal epithelial cells were collected by scraping the inner lining of the mouth with a wooden spatula, for analysis of micronucleated cells. Samples of the exfoliated cells of the buccal mucosa were obtained from both exposed and control subjects and were smeared on clean glass microscope slides. The samples from the exposed group were taken at the end of their work shift on the last working day of the week.
Determination of serum malondialdehyde
Lipid peroxidation in the form of serum MDA levels was measured according to the method described by Satoh.[13] To 0.5 ml serum, 2.5 ml of 20 mg/dl trichloroacetic acid was added and the tube was allowed to stand for 10 mins at room temperature. After centrifugation at 3,500 rpm for 10 min, the supernatant was decanted. The precipitate was washed with 0.05 M sulphuric acid. Then, 2.5 ml of 0.05 M sulfuric acid and 3.0 ml of 0.2 mg/dl thiobarbituric acid (TBA) in 2 M sodium sulfate were added to this precipitate and the solution was heated in a boiling water bath for 30 min. After cooling, the resulting chromogen was extracted with 4.0 ml of n-butyl alcohol by vigorous shaking. The organic phase was separated by centrifugation at 3,000 rpm for 10 min and absorbance of the supernatant was measured spectrophotometrically (UV-Visible-117 Spectrophotometer; Systronics) at 530 nm, using 1, 1, 3, 3-tetraethoxy-propane as standard. MDA was expressed as nmol/ml.
Buccal micronucleus test
The buccal cell smears were fixed in a solution of methanol and acetic acid (3:1) on clean microscope slides. The slides were placed in 1 M HCl at room temperature for 5 min. Then they were incubated at 60°C for 5 min. After cooling, the slides were rinsed in deionized water at room temperature for 3 mins. The slides were stained with aceto-orecein and counterstained with fast green. The slides were rinsed three times in absolute ethanol (2 mins), air dried and examined under light microscope. The criterion of scoring the cells with MN was applied as described by Tolbert et al.[14] Two thousand cells per subject were analyzed for recording the number of micronucleated cells (MNC). Binucleated cells (BNC) were also observed and counted.
Blood lead levels
Whole blood samples from the BMW group were procured during morning hours on the last working day of the week for analyzing lead levels with atomic absorption spectroscopy. Preparation of the blood samples for blood lead levels (BLL) were done as per the method given by Palmer et al.[15] The concentrations of blood lead were quantified as μg/dl.
Statistical analysis
Mann Whitney U test was used to find out the significance of the differences in the means of all the parameters in BMW and control groups. Analysis of variance (1 way and 2 way GLM) was used to find out the effect of confounding factors and the interactions of the confounders and the sub-groups on the results. Pearson correlation was used to find out the correlation between BLL and all the parameters. P < 0.05 was considered as the significant level for the statistical analysis. All statistical analyses were performed using the program Minitab v. 16.1.0 for windows.
RESULTS
In the present study, the BMW group consisted of 30 subjects (24 males and 6 females) having a mean lead exposure of 9.77 ± 5.62 years. Only 3 subjects used handkerchiefs on their faces as a protective measure. No particular equipments like masks or gloves were used by any of the subjects. General characteristics of control and exposed population are given in Table 1.
Table 1.
General characteristics of subjects of control and exposed groups
Our results indicate a significant increase in the number of MNC and BNC in the buccal mucosa cell smears of BMW group as compared to controls (4.20 ± 2.69 and 1.70 ± 1.51 vs. 1.26 ± 1.48 and 0.70 ± 0.87 respectively; P < 0.001). Table 2 shows the highly significant increase in blood lead levels (BLL) in the BMW group as compared to controls. We found a positive correlation in between BLL and MNC frequency (r = 0.591; P < 0.001) as well as BLL and BNC frequency (r = 0.460; P < 0.01) among exposed subjects [Figures 1 and 2]. The results also indicate a highly significant increase in the values of MDA concentrations in the BMW group as compared to controls (6.76 ± 3.26 vs. 2.10 ± 1.02; P < 0.001). The higher standard deviation values may be attributed to the varied dietary habits of the exposed subjects. [Figure 3] shows the positive correlation (r = 0.895; P < 0.001) between BLL and MDA concentrations in blood among the exposed subjects. The positive correlation clearly justifies the higher mean values of MNC and MDA and the harmful effects of lead on the health of the workers. In controls, the correlation between BLL and all the parameters was insignificant.
Table 2.
Average number of micronucleated cells, binucleated cells (in 2000 cells per individual), malondialdehyde values and blood lead levels in control and exposed groups
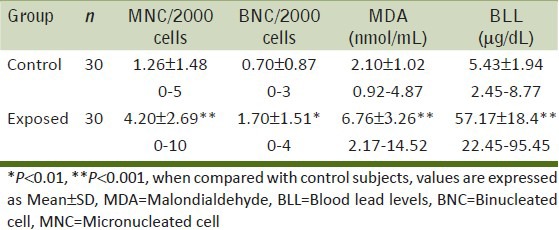
Figure 1.
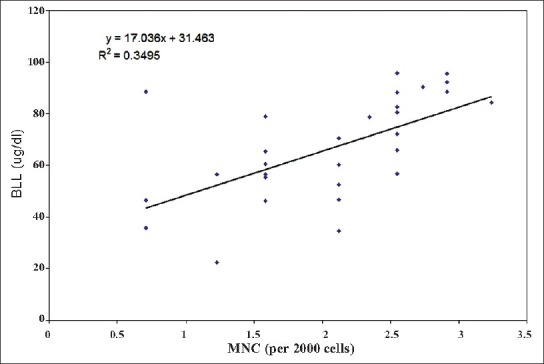
Relationship between blood lead concentration and micronucleated cell frequency among lead exposed workers (Correlation coefficient, r = 0.591; P < 0.001)
Figure 2.
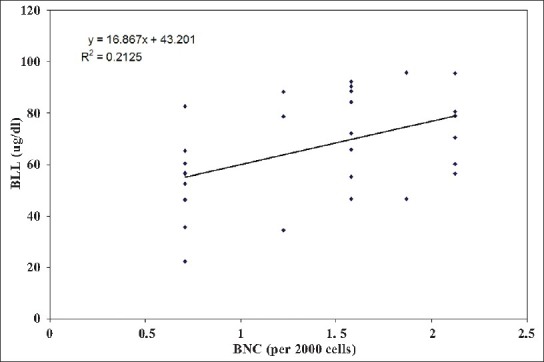
Relationship between blood lead concentration and binucleated cell frequency among lead exposed workers (Correlation coefficient, r = 0.460; P < 0.01)
Figure 3.
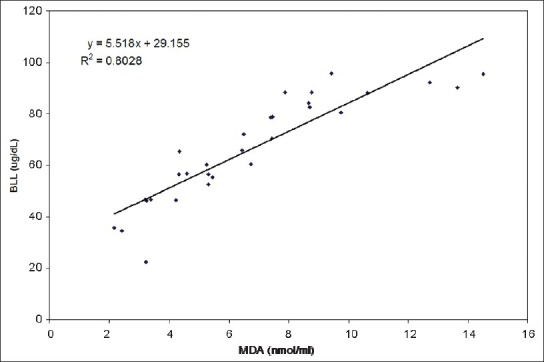
Relationship between blood lead and serum malondialdehyde concentrations among lead exposed workers (Correlation coefficient, r = 0.895; P < 0.001)
When the effect of duration of exposure was studied on the BMW group, the highest mean values for MNC, BNC and MDA were found out to be in the highest exposure duration group of 16-20 years (5.60 ± 3.29, 3.00 ± 1.00 and 10.86 ± 2.70 respectively). Values of MNC and MDA were found to increase (P < 0.001) with the increasing duration of exposure [Figure 4] except for BNC (1 way ANOVA, P = 0.127).
Figure 4.
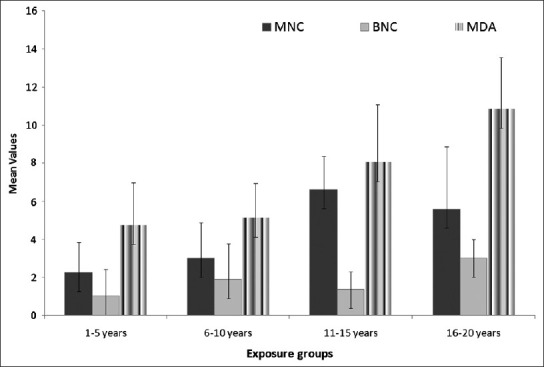
Average values of micronucleated cells, binucleated cells and malondialdehyde in different exposure groups of exposed population
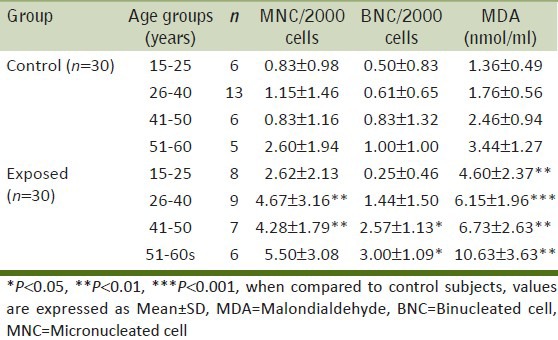
To study the effect of age, both the control and exposed groups were further divided into 4 age groups [Table 3]. The results showed a significant increase in the MNC frequency only in the exposed sub-groups (26-40 and 41-50 years) as compared to the controls (P < 0.01). Age groups of 41-50 and 51-60 years showed a significant increase in BNC frequency (P < 0.05). MDA was significantly increased in all the age groups (P < 0.01). In BMW group, age had a significant effect on the values of BNC and MDA (1 way ANOVA; P < 0.01) whereas MNC were not found to increase with age (P > 0.05). The interaction between age groups and group was insignificant for MNC frequency and MDA values. The interaction was significant only for BNC (2 way ANOVA; P = 0.03).
Table 3.
Average number of micronucleated cells, binucleated cells and malondialdehyde values in control and exposed groups with respect to different age groups
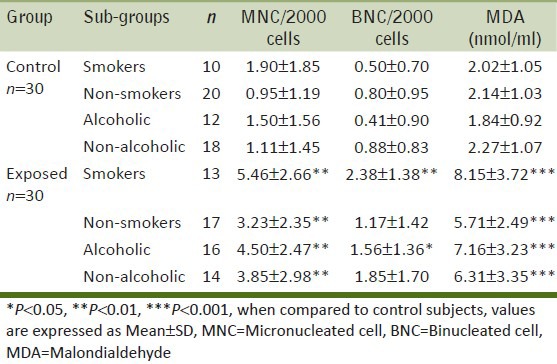
Exposed smokers and drinkers had a significantly higher mean MNC, BNC and MDA values as compared to the controls [Table 4]. The smoking-group interaction is significant for BNC and MDA (P < 0.05) but not for MNC (P = 0.246) as analyzed by the 2 way ANOVA (GLM). In our studies, drinking habits were not found to significantly increase any of the parameters (2 way ANOVA; P > 0.05).
Table 4.
Average number of micronucleated cells, binucleated cells and malondialdehyde values in control and exposed groups with respect to smoking and drinking habits
DISCUSSION
In the present study, the exposed group showed a significantly increased frequency of micronucleated buccal cells as compared to the control group (4.20 ± 2.69 vs. 1.26 ± 1.48; P < 0.001) and a positive correlation with BLL [Figure 1]. This clearly proves the genotoxic effect of lead on the workers of battery manufacturing industry. The results are in conformity with the findings of Roth et al. and Khan et al.,[9,16] while working on car and battery repair workers exposed to paints and lead. Kasuba et al.,[17] found similar results in battery-manufacturing workers. Significant higher numbers of micronuclei in lymphocytes were also reported by Minnozzo et al., Palus et al., Stoia et al., Grover et al.,[3,18,19,20,21] in lead exposed workers. On the contrary Cassini et al.,[22] reported no differences among frequency of micronuclei in lymphocytes between workers exposed to lead containing paints and control subjects.
We found a significant increase in the mean values of BNC in BMW group as compared to the controls. Similarly, Vaglenov et al.,[2,23] reported significantly increased numbers of binucleated cells with MN (BNMN) in peripheral lymphocytes in lead exposed workers. Increased tail lengths in comet assay were found by Grover et al. and Minozzo et al.,[19,21](Z = 7.7056; P < 0.0001) in lead exposed workers. Danadevi et al.,[24] reported significantly higher percentage of DNA damaged cells (44.58%) in secondary lead recovery plant workers versus control subjects (21.14%). Zuhair et al., Shaik and Jamil[5,25] reported significantly higher frequencies of chromosomal aberrations among lead exposed workers.
Our results also indicated a highly significant increase in the values of serum MDA concentration in the BMW group as compared to controls (6.76 ± 3.26 vs. 2.10 ± 1.02; P < 0.001) which is in conformity with Patil et al.,[6] as found in battery manufacturing workers, Mohammad[26] in painters, Permpongpaiboon et al.,[27] in lead exposed workers, Oktem et al.,[28] in adolescent auto repair workers and Xu et al.,[29] in lead exposed mice. Our study indicates a positive correlation (r = 0.895) between BLL and serum MDA in exposed subjects. This result is in conformity with Patil et al.,[6] who found a positive correlation (r = 0.45, P < 0.02) between blood lead and serum MDA level in battery manufacturing workers group (Pb-B range 25.8-78.0 micrograms/dl). Kasperczyk et al.,[30] reported higher (56%) MDA concentrations in seminal plasma of workers highly exposed to lead.
CONCLUSIONS
The battery manufacturing workers, occupationally exposed to lead, showed a significant increase in the mean buccal MNC frequency, BNC frequency and serum MDA concentrations as compared to the controls. All these parameters showed a positive correlation with BLL. The findings of the present study strongly points out the genotoxic exposure of the battery manufacturing workers to lead. This occupational lead exposure is in small amounts but accounts for serious health risks. Thus, exposure to lead should be avoided, as far as possible, using proper protection equipment.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Leston JG, Mendez J, Pasaro E, Laffon B. Genotoxic effects of lead: An updated review. Environ Int. 2010;36:623–36. doi: 10.1016/j.envint.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Vaglenov A, Creus A, Laltchev S, Petkova V, Pavlova S, Marcos S. Occupational exposure to lead and induction of genetic damage. Environ Health Perspect. 2001;109:295–8. doi: 10.1289/ehp.01109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoia M, Oancea S, Doina C Obreja. Comparative study of genotoxic effects in workers exposed to inorganic lead and low dose irradiation using micronucleus test. Rom J Leg Med. 2009;4:287–94. [Google Scholar]
- 4.Atlanta, Georgia, USA: US Department of health and human services; 2005. ATSDR. Draft toxicological profile for lead; pp. 102–225. [Google Scholar]
- 5.Al-Hakkak ZS, Hamamy HA, Murad AMB, Hussain AF. Chromosome aberrations in workers at a storage battery plant in Iraq. Mutat Res. 1986;171:53–60. doi: 10.1016/0165-1218(86)90008-x. [DOI] [PubMed] [Google Scholar]
- 6.Patil AJ, Bhagwat VR, Patil JA, Dongre NN, Ambekar JG, Jailkhani R. Effect of lead (Pb) exposure on the activity of superoxide dismutase and catalase in battery manufacturing workers (BMW) of Western Maharashtra (India) with reference to heme biosynthesis. Int J Environ Res Public Health. 2006;3:329–37. doi: 10.3390/ijerph2006030041. [DOI] [PubMed] [Google Scholar]
- 7.Cavalleri A, Minoia C, Pozzoli L. Determination of plasma lead levels in normal subjects and in lead exposed workers. Br J Ind Med. 1978;35:21–6. doi: 10.1136/oem.35.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenech M, Kirsch-Volders M, Natarajan A, Surralles J, Crott JW, Parry J, et al. Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis. 2011;26:125–32. doi: 10.1093/mutage/geq052. [DOI] [PubMed] [Google Scholar]
- 9.Martino-Roth MG, Viegas J, Amaral M, Oliveira L, Ferreira LS, Erdtmann B. Evaluation of genotoxicity through micronuclei test in workers of car and battery repair garages. Gen and Mol Bio. 2002;25:495–500. [Google Scholar]
- 10.Majer BJ, Laky B, Knasmuller S, Kassie F. Use of the micronucleus assay with exfoliated epithelial cells as a bio-marker for monitoring individuals at elevated risk of genetic damage and in chemoprevention trials. Mutat Res. 2001;489:147–72. doi: 10.1016/s1383-5742(01)00068-0. [DOI] [PubMed] [Google Scholar]
- 11.Hande G, Naran E. Can antioxidants be beneficial in the treatment of lead poisoning? Free Radic Biol Med. 2000;29:927–45. doi: 10.1016/s0891-5849(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 12.Munoz HS, Puga MP, Wrobel K, Sevilla EG, Wrobel K. Micro assay for malondialdehyde in human serum by extraction- spectrophotometry using an internal standard. Microchim Acta. 2004;148:285–91. [Google Scholar]
- 13.Satoh K. Plasma lipid peroxide in cerebrovascular disorder determined by a new colorimetric method. Clinica Chimica Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 14.Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: Methods development. Mutat Res. 1992;271:69–77. doi: 10.1016/0165-1161(92)90033-i. [DOI] [PubMed] [Google Scholar]
- 15.Palmer CD, Lewis ME, Geraghty CM, Barbosa F, Parsons PJ. Determination of lead, cadmium and mercury in blood for assessment of environmental exposure: A comparison between inductively coupled plasma–mass spectrometry and atomic absorption spectrometry. Spectrochimica Acta Part B. 2006;61:980–90. [Google Scholar]
- 16.Khan MI, Ahmad I, Mahdi AA, Akhtar MJ, Islam N, Ashquin M, et al. Elevated blood lead levels and cytogenetic markers in buccal epithelial cells of painters in India: Genotoxicity in painters exposed to lead containing paints. Environ Sci Pollut Res Int. 2010;17:1347–54. doi: 10.1007/s11356-010-0319-x. [DOI] [PubMed] [Google Scholar]
- 17.Kasuba V, Rozgaj R, Milic M, Zeljezic D, Kopjar N, Pizent A, et al. Evaluation of lead exposure in battery-manufacturing workers with focus on different biomarkers. J Appl Toxicol. 2010;30:321–8. doi: 10.1002/jat.1497. [DOI] [PubMed] [Google Scholar]
- 18.Minozzo R, Deimling LI, Gigante LP, Santos-Mello R. Micronuclei in peripheral blood lymphocytes of workers exposed to lead. Mutat Res. 2004;565:53–60. doi: 10.1016/j.mrgentox.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Minozzo R, Deimling LI, Santos-Mello R. Cytokinesis-blocked micronucleus cytome and comet assays in peripheral blood lymphocytes of workers exposed to lead considering folate and vitamin B12 status. Mutat Res. 2010;697:24–32. doi: 10.1016/j.mrgentox.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Palus J, Rydzynski K, Dziubaltowska E, Wyszynska K, Natarajan AT, Nilsson R. Genotoxic effects of occupational exposure to lead and cadmium. Mutat Res. 2003;540:19–28. doi: 10.1016/s1383-5718(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 21.Grover P, Rekhadevi PV, Danadevi K, Vuyyuri SB, Mahboob M, Rahman MF. Genotoxicity evaluation in workers occupationally exposed to lead. Int J Hyg Environ Health. 2010;213:99–106. doi: 10.1016/j.ijheh.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Cassini C, Calloni C, Bortolini G, Garcia SC, Dornelles MA, Henriques JA, et al. Occupational risk assessment of oxidative stress and genotoxicity in workers exposed to paints during a working week. Int J Occup Med Environ Health. 2011;24:308–19. doi: 10.2478/s13382-011-0030-2. [DOI] [PubMed] [Google Scholar]
- 23.Vaglenov A, Carbonell E, Marcos A. Biomonitoring of workers exposed to lead. Genotoxic effects, its modulation by polyvitamin treatment and evaluation of the induced radioresistance. Mutat Res. 1998;418:79–92. doi: 10.1016/s1383-5718(98)00111-9. [DOI] [PubMed] [Google Scholar]
- 24.Danadevi K, Rozati R, Saleha BB, Hanumanth RP, Grover P. DNA damage in workers exposed to lead using comet assay. Toxicology. 2003;187:183–93. doi: 10.1016/s0300-483x(03)00054-4. [DOI] [PubMed] [Google Scholar]
- 25.Shaik AP, Jamil K. Individual susceptibility and genotoxicity in workers exposed to hazardous materials like lead. J Hazar Mat. 2009;168:918–24. doi: 10.1016/j.jhazmat.2009.02.129. [DOI] [PubMed] [Google Scholar]
- 26.Mohammad IK, Mahdi AA, Raviraja A, Najmul I, Iqbal A, Thuppil V. Oxidative stress in painters exposed to low lead levels. Arh Hig Rada Toksikol. 2008;59:161–9. doi: 10.2478/10004-1254-59-2008-1883. [DOI] [PubMed] [Google Scholar]
- 27.Permpongpaiboon T, Nagila A, Pidetcha P, Tuangmungsakulchai K, Tantrarongroj S, Porntadavity S. Decreased paraoxonase 1 activity and increased oxidative stress in low lead-exposed workers. Hum Exp Toxicol. 2011;30:1196–203. doi: 10.1177/0960327110388536. [DOI] [PubMed] [Google Scholar]
- 28.Oktem F, Arslan MK, Dundar B, Delibas N, Gultepe M, Ergurhan I. Renal effects and erythrocyte oxidative stress in long-term low-level lead-exposed adolescent workers in auto repair workshops. Arch Toxicol. 2004;78:681–7. doi: 10.1007/s00204-004-0597-5. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Lian LJ, Wu C, Wang XF, Fu WY, Xu LH. Lead induces oxidative stress, DNA damage and alteration of p53, Bax and Bcl-2 expressions in mice. Food Chem Toxicol. 2008;46:1488–94. doi: 10.1016/j.fct.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Kasperczyk A, Kasperczyk S, Horak S, Ostalowska A, Grucka-Mamczar E, Romuk E, et al. Assessment of semen function and lipid peroxidation among lead exposed men. Toxicol Appl Pharmacol. 2008;228:378–84. doi: 10.1016/j.taap.2007.12.024. [DOI] [PubMed] [Google Scholar]


